AbstractPurposeWe aimed to determine the current application and survival trends of hematopoietic stem cell transplantation (HSCT) among Korean children and adolescents with cancer.
Materials and MethodsData of patients aged < 20 years with KCD-10 (Korean Classifications of Diseases, 10th revision) C codes and specific designation codes were collected from the National Health Insurance Service database. Thirty claim codes for HSCT were included, and data from 2009 to 2019 were analyzed.
ResultsThe operational definition of pediatric cancer yielded an annual average of 2,000, with annual cases decreasing. In 2019, 221 HSCTs were performed, a decrease from the ten-year average of 276. Allografts outnumbered autografts with a ratio of 1.5:1. The source of allograft was bone marrow in 15% of patients in 2009; however, it substantially decreased to 3.3% in 2019. Furthermore, 70.5% of allogeneic HSCT used peripheral blood stem cell (PBSC) grafts, which increased to 89.3% by 2015. Cord blood utilization markedly decreased to 2.7% in 2018. The 5-year overall survival (OS) rate of all patients was 85.1%. Overall mortality decreased among patients who underwent recent HSCT, and they exhibited a higher 5-year OS rate.
ConclusionIn Korea, the number of pediatric patients with cancer is declining; however, the ratio of transplants to all patients remains constant. Patients who recently underwent transplantation showed better survival rates, possibly due to HSCT optimization. Korea showed a substantially greater PBSC utilization in pediatric HSCT. An in-depth examination encompassing donor relations and cause of death with a prospective registry is required in future studies.
IntroductionHematopoietic stem cell transplantation (HSCT) is a curative treatment for patients with hematologic malignancies, including various leukemias and lymphomas, as well as is used in children with high-risk solid tumors [1]. HSCT is only beneficial for children who have a high risk of relapse from conventional chemotherapy [2]. Therefore, therapy plans that precisely identify these high-risk patients and perform HSCT when suitable allogeneic donors are available are the preferred treatment for various disorders. In addition, the indication for HSCT has changed over time because of the introduction of new drugs or changes in HSCT-related mortality, and the use of HSCT donor or stem cell sources has been influenced by advancements in transplant-related techniques [1].
Recent advances in HSCT have contributed to improved patient outcomes for diseases curable by HSCT. A human leukocyte antigen (HLA)–identical sibling donor is preferred for effective allogeneic HSCT (allo-HSCT), and an HLA-matched unrelated donor is the next best option. However, finding an HLA-matched donor for patients who require HSCT is not always possible.
Alternative transplantation techniques have been developed in response to the demand for alternative donors, including HLA-haploidentical family member transplants and umbilical cord blood transplants [3]. Recent advances in the successful ex vivo reduction of T cells or unmanipulated in vivo regulation of T cells with post-transplant cyclophosphamide, improved supportive care, and appropriate conditioning regimens have significantly enhanced the outcomes of haploidentical HSCT [4]. Conversely, the use of umbilical cord blood as an alternative stem cell source declined as the demand for haploidentical HSCT increased. Allo-HSCT using an HLA-haploidentical family donor is currently accepted for patients who cannot find a suitable HLA-matching donor.
Autologous HSCT (auto-HSCT) has been developed using advancements in blood separation and cell freezing technology and the process of collecting hematopoietic stem cells from peripheral blood [5]. Auto-HSCT has emerged as a viable treatment option for several conditions, such as malignancies that exhibit sensitivity to chemotherapy or radiotherapy. The indication has expanded to high-risk neuroblastoma and brain tumors in several years [6,7].
Notably, the patterns and results of HSCT exhibit temporal variation, necessitating comprehensive, long-term investigations nationwide. The Korean National Health Insurance Service (NHIS) is the sole government insurer in Korea that provides universal health insurance to approximately 97% of the Korean population [8]. NHIS has built and operated large-capacity healthcare big data systems and provides researchers with various secure healthcare big data for research purposes [9]. Additionally, it provides longitudinal patient data suitable for longitudinal temporal studies, such as the analysis of the long-term survival rate after HSCT.
In this study, we determined the current use and survival trends of HSCT among Korean children and adolescents with cancer using the National Health Insurance claims data.
Materials and Methods1. Data source and study populationData collected from the NHIS database between 2002 and 2020 included patients aged < 20 years who first received the KCD-10 (Korean Classifications of Diseases, 10th revision) code for cancer and concomitant specific symbolized type, indicating selective insurance benefits for patients with cancer. We identified 29,386 patients from the NHIS database aged < 20 years and who had cancer codes (C code–based on the International Classification of Diseases-10) from 2002 to 2020 (Fig. 1). Additionally, we designated the main diagnosis for every patient as some patients received more than two different C codes. The most commonly received C code for each patient was designated as the principal diagnosis. However, the primary diagnosis for 87 patients could not be selected since the frequency of several C codes was comparable. Fifty-seven patients with fewer than 10 hospital billing codes associated with the C code were excluded from the analysis. The primary diagnosis of the remaining 30 patients was found by individually examining the C code and claims pattern. Consequently, 29,329 patients with primary diagnoses were selected. Finally, patients diagnosed between 2002 and 2008 and 2020 (n=10,829) were excluded from the study because the claims pattern was irregular during these periods. This is because claims data were predominantly processed retrospectively at the beginning of the system (2002-2008). In addition, the claim for HSCT in 2020 had not been finalized at the time of data collection for this study in 2021; hence, the data from 2020 was excluded. Finally, 18,500 individuals who received diagnoses between 2009 and 2019 were included in the analysis. The first billing date of the main diagnosis (C code) was identified as the date of diagnosis (Fig. 1).
2. Diagnostic group and codes for stem cell transplantationKCD-C codes were classified as the clinically representative diagnostic groups for the analysis. According to the categorization of children’s cancer based on the KCD-10 code used in the “Annual report of cancer statistics in Korea”, the principal classification of diseases included brain tumor, lymphoma, leukemia, malignant neoplasms of the bone and joint, soft tissue sarcoma, and others (S1 Table). All medical treatments administered to a patient were coded with the date and type and recorded in the claims data. Therefore, the claim code and date of execution were noted in the claim record for HSCT.
HSCT codes were selected for the analysis, including the terminated codes for previous data: seven codes for allogeneic bone marrow (BM), five for allogeneic peripheral blood, five for umbilical cord transplantation, and ten for auto-HSCT (S2 Table). Allo-HSCT comprised allogeneic BM, peripheral blood, cord blood, and allogeneic cord blood, whereas auto-HSCT comprised autologous BM, peripheral blood, and autologous cord blood.
3. Statistical analysisThe annual number of HSCTs were expressed as frequencies and proportion according to HSCT year. As the follow-up period differs among study populations based on the time of cancer diagnosis (defined as the first billing date of the main diagnosis), the 5-year overall survival (OS) was calculated only for patients observed for more than 5 years. In the case of patients who undergo multiple HSCT, the follow-up period was calculated from the last date of HSCT. Survival probabilities were estimated using Kaplan-Meier plots and the log-rank test for testing the equality of survival functions between groups. p-values < 0.05 were considered significant. SAS ver. 9.4 (SAS Institute Inc., Cary, NC) was used for the statistical analyses.
Results1. Annual cancer incidence in the claims dataNumber of patients with cancer falling under the C code and aged < 20 years was 1,916 in 2009 and 2,081 in 2011, maintaining a national average of approximately 2000 (S3 Table). Subsequently, the population progressively declined to 1,501 in 2017 and 1,420 in 2019. With an average of 266 annual patients (range, 212 to 328), lymphoid leukemia was the most prevalent disease, followed by brain tumors with an average of 262 cases (range, 182 to 358). An average of 229 patients (range, 156 to 486 patients) were diagnosed with non-Hodgkin’s lymphoma (NHL). The total number of pediatric patients with cancer declined; however, the disease ranking by the number of cases remained constant.
2. Annual numbers and types of total HSCTOver the 11-year period from 2009 to 2019, an average of 276 HSCTs have been performed annually; 221 transplants were performed in 2019, representing a gradual decline in the overall number (Fig. 2A). Allogeneic peripheral blood stem cell transplantation (PBSCT) accounts for approximately 50% of all HSCT performed annually, while autologous PBSCT accounts for approximately 25%. In 2009, 183 HSCTs were performed; in 2017, the number had dropped to less than 150 cases, and in 2019, only 121 HSCTs had been performed (Fig. 2B). The ratio of allograft to autograft was about 1.5:1. Among the patients, the rate of allo-HSCT was the highest in 2009 and 2015, at 9.6% and 11.2%, respectively; however, it decreased to 8.5% in 2019. More than 100 auto-HSCT were performed from 2009 to 2016, with 127 cases in 2009 and 136 cases in 2016, followed by 97 cases in 2017 and 100 cases in 2019. During the entire duration, a range of 13.6% to 17.4% of pediatric cancer patients received at least one HSCT (Fig. 2B).
In 2009, BM was used as a source of graft in 15.9% of alloHSCT cases; however, this gradually decreased thereafter, and only 3.3% used BM in 2019 (Fig. 2C). Conversely, peripheral blood stem cell (PBSC) was used in 70.5% of allo-HSCT cases in 2009; however, it gradually increased to 89.3%. Furthermore, the use of cord blood gradually declined, from 12.6% in 2009 to 3.9% in 2015. In 2019, it showed a slight increase to 7.4%.
3. HSCT trends according to disease typesDifferent patterns were observed for each condition when HSCT trends were confirmed according to disease type (S4 Fig.). Leukemia cases exhibited a modest decline, from 177 in 2009 to 111 in 2019 (S5 Fig). Allogeneic PBSCT (allo-PBSCT) has been mostly used for leukemia treatment since 2009. In contrast, the use of allogeneic bone marrow transplantation (allo-BMT) and allogeneic cord blood transplantation (CBT) decreased from 28 and 22 in 2009 to 2 and 7 in 2019, respectively (S4A Fig.). In brain tumors, there were 1-2 instances of allo-PBSCT usage, which were mostly auto-HSCT, and 38 and 45 HSCT in 2009 and 2019, respectively (S4B Fig.). The number of lymphoma cases decreased from 43 in 2009 to 14 in 2019, and most patients underwent autologous PBSCT (S4C Fig., S6 Fig). With the exception of 2018, 10-14 transplants for malignant neoplasms of the bone and joints were performed yearly, which were mostly autologous PBSCT (S4D Fig.). Approximately 10 transplants were performed annually for soft tissue sarcoma (S4E Fig.), and 30 transplants were performed annually for other cancers (S4F Fig.).
4. Number of patients who underwent multiple HSCTThe number of patients who underwent two transplants was 70 in 2009 and 71 in 2019. Most patients who underwent two transplants had brain tumors, leukemia, or other disorders (S7 and S8 Tables). The average number of patients with leukemia who underwent more than three HSCTs per year was six (range, 0 to 11) (S9 Table).
5. Survival outcomes after HSCTThe mortality rate for all pediatric patients with cancer has markedly decreased since 2009. The mortality rate reduced from 20% in 2009 to 10% in 2019. From 2009 to 2013, the mortality rate of transplanted patients was higher than that of all patients or non-transplanted patients, surpassing 35%. Subsequently, it reduced steadily to 25% in 2019. Patients who did not undergo transplantation died at a rate of 16% in 2009 and 7% in 2019 (S10 Fig.).
The 5-year OS rate for all patients was 85.1% (Fig. 3A); for brain tumors, lymphomas, and leukemia it was 74%, 92% (Hodgkin lymphoma 95.3%, NHL 91.5%), and 81.6% (lymphoid leukemia 85.1%, myeloid leukemia 75.0%), respectively (S11 Fig.). The 5-year OS rates were 80.6% and 76.5% for malignant neoplasms of the bone and joint and soft tissue sarcoma, respectively (S10 Fig.). In all disease groups, the 5-year OS rates for transplant recipients were lesser than that of nontransplant recipients (Fig. 3B).
According to the analyses of 5-year OS rates at 4-year intervals from 2009 to 2012, 2013 to 2016, and 2017 to 2020 based on the year of diagnosis, patients in all disease groups who are more recently diagnosed and those with leukemia had a higher chance of survival (Fig. 3C, S12 Fig.). However, when the 5-year survival rate was examined according to the time of transplantation, the patient group who recently underwent transplantation showed a better survival rate; however, the difference was not statistically significant (Fig. 3D, S12 Fig.).
When the survival rate was analyzed according to the type of donor and stem cell source by disease, allo-PBSCT showed comparable results with allo-BMT in patients with lymphoid leukemia (Fig. 4A). Patients who underwent autoHSCT had higher OS than those who underwent allo-PBSCT or allo-BMT; however, the number of patients was small and declined gradually (Fig. 4A). However, in myeloid leukemia, patients who underwent allo-BMT showed a higher 5-year OS of 77.8% than those who underwent allo-PBSCT, followed by those who underwent CBT (Fig. 4B). The 5-year OS of auto-HSCT was the highest in this analysis; however, the total number of transplantations was remarkably small for proper comparison (Fig. 4B). Among patients with brain tumors, those who underwent two auto-HSCT had the highest 5-year OS of 73.1%, better than those who underwent one or three HSCTs (Fig. 4C). In other leukemia, there was no statistically significant difference in OS according to the year or type of transplantation (S13 Fig.). Among patients with lymphoma, both in Hodgkin lymphoma and NHL, a higher survival probability without statistical significance of auto-HSCT over allo-PBSCT was observed (S14 Fig.).
DiscussionSince the first successful allo-HSCT was performed in Korea in 1983, there has been noticeable progress in the quantity and quality of HSCT. The annual number of HSCTs was 185 in 1995 and steadily increased to 1,314 in 2006 [10].
Few studies on pediatric HSCT have been published in Korea. The only 1-year data on pediatric HSCT published in 2006 was conducted in 2003 through a multi-center survey at the time [5]. HSCT was performed in 184 out of 766 pediatric and adolescent patients with cancer, with allo-HSCT in 140 patients (67%) and auto-HSCT in 44 patients. Hematologic malignancy was the target of allo-HSCT in 84.3% of cases, while neuroblastoma was the target of auto-HSCT in 50% of cases. BMT, CBT, and PBSCT accounted for 61%, 34%, and 5% of allo-HSCT cases, respectively. In auto-HSCT, PBSC was used as the graft source in all patients. In allo-HSCT, HLA-matched unrelated transplants accounted for 37%, sibling transplants accounted for 22.5%, and familial mismatched transplants accounted for 4%.
The 310 cases of transplants performed in 2009 and documented in this study revealed a significant increase in the number of transplants after 2006. Subsequently, the number of HSCT procedures performed in 2016 persisted at a comparable level until experiencing a significant decrease, with a total of 221 cases executed in 2019. Fewer transplants were performed among Korean children and adolescents annually, potentially attributed to fewer patients with cancer owing to the decline in birth rate [11]. In addition, the ratio of patients who underwent transplantation to the total annual number of pediatric patients with cancer was 16.2% in 2009 and 15.5% in 2019, and the difference was not significant. This showed no significant change in the proportion of patients with cancer to HSCTs performed over the past 10 years.
However, when transplants were analyzed by disease, the number of HSCT among patients with leukemia and lymphoma declined, while the number of HSCT in patients with brain tumors remained constant or slightly increased during the 10-year period. This suggests that the indications for transplantation in cases of brain tumors are increasing. Recently, among patients with high-risk neuroblastoma and medulloblastoma, tandem auto-HSCT and intensified chemotherapy combined with radiotherapy have helped improve survival rates [6,7,12-14]. In this study, patients with brain tumors who underwent tandem auto-HSCT had the highest 5-year OS rates, suggesting the survival benefits of the tandem approaches in these patients. True OS comparison might have certain limitations given that patients who underwent auto-HSCT thrice were at a higher risk than those who had transplantation once or twice, and a second HSCT might be performed following recurrence.
This study analyzed data for insurance claims following treatment; hence, there was a limitation in fully understanding all the statistics related to HSCT. This analysis showed that PBSC and BM were both used in a sizable number of auto-HSCT cases. However, in practice, PBSC is mostly used as a stem cell source for auto-HSCT, except for a few extremely poor mobilizers. Therefore, we assumed that there were many instances where BMT codes were retrospectively claimed even when PBSC was used. Another limitation of this study was that the donor relationship or HLA-matching information could not be identified from the data source.
Changes in donor sources have recently become more noticeable, and both transplantation using mismatched unrelated donors and haploidentical family donors are increasingly being optimized globally [3,4,15]. According to the summary from the Center for International Blood & Marrow Transplant Research (CIBMTR) [16], a decline in the absolute number of matched-related donor (MRD) transplants has been observed since 2013. The number of haploidentical donor transplants is increasing steadily; however, the number of transplants using cord blood or a mismatched unrelated donor is slightly declining.
In Korea, the donor relation trend exhibits similar trends to CIBMTR. According to the annual report of the Society of Hematopoietic Stem Cell Transplant Nurses [17], 8,431 HSCTs were performed on children from 1983 to 2021. Considering the annual trends, all transplants—apart from those performed using umbilical cord blood—are increasing. The most drastic increase was observed in haploidentical stem cell transplants, representing 17.5% of all transplants in 2021.
As a stem cell source, BM was originally used for HSCT; however, in the early 2000s, granulocyte colony-stimulating factor-mobilized PBSC was introduced as an alternative stem cell source [18]. Thereafter, the use of PBSC has increased, accounting for up to 70% and 30% of adult and pediatric hematopoietic cell transplants, respectively [19].
However, BM remains more frequent than PBSC among pediatric patients undergoing HSCT using MRD, according to the CIBMTR data [16]. PBSC is utilized less frequently than BM among pediatric patients undergoing HSCT from MUDs [19]. Nevertheless, according to the findings of this study, allo-BMT accounted for only 10% of all transplants in 2009, and this percentage decreased steadily until less than 10 instances, or 1.8%, used BM as a graft source in 2019. This is possibly attributed to the difficulties in collecting BM and reports that BMT and PBSCT have equivalent survival outcomes in treating various disorders.
According to a recent systematic analysis in children [19], the 5-year OS was comparable across the PBSCT and BMT groups (PBSCT, 56.2%; BMT, 63.5%; relative risk [RR], 1.17; 95% confidence interval [CI], 91 to 1.52), similar to event-free survival (PBSCT, 49.9%; BMT, 57.5%; RR, 1.14; 95% CI, 93 to 1.39). However, nonrelapse mortality (NRM) and chronic graft-versus-host disease (GVHD) were more prevalent in the PBSCT group than in the BMT group (RR, 1.73; 95% CI, 1.50 to 1.99 vs. RR, 1.55, 95% CI, 1.18 to 2.03). In this study, there was no difference in OS between allo-BMT and allo-PBSCT for lymphoid leukemia. However, in myeloid leukemia, the OS was higher with allo-BMT than with allo-PBSCT. This analysis could not identify the cause of death; hence, the reason for this survival difference is unknown. Future analysis of prospective registry data is required.
In this study, patients who underwent transplantation more recently had higher survival rates, which corroborates the findings of previous reports [20,21]. The HSCT procedures for pediatric patients have developed over time to prevent initial GVHD, recurrence, infections, graft failure, and debilitating late sequelae, particularly in allo-HSCT. These developments reduced treatment-related mortality (TRM), which explains the improvements in OS. Brissot et al. [21] reported that the incidence of grades 3-4 GVHD was comparable between the two periods. In contrast with the period from 1983 to 1999, the cumulative incidence of deaths attributable to GVHD was lower from 2000 to 2010. Notably, NRM was statistically reduced in recent times. However, there was no improvement in the mortality rate because of recurrence.
These findings correspond with the results of the study by Svenberg et al. [20]. There was clear evidence of OS over two decades because of a decline in TRM; however, the relapse did not diminish over time and was the leading cause of death after allo-HSCT.
This study has several limitations besides the aforementioned limitation on the donor relation data. First, since this dataset was established for claims and reimbursements, there may be discrepancies between the actual time of disease and the claimed diagnosis [8]. Furthermore, there could be differences between the actual time of HSCT and the claiming time. Second, because we could not obtain information on the stages of primary cancer or relapse, we could not verify whether HSCT was performed as a primary or salvage treatment after relapse. Additionally, the precise indication for HSCT could not be verified. Therefore, there was a limitation in survival comparison. Third, this study did not include HSCT for severe hematologic illnesses such as myelodysplastic syndrome and aplastic anemia because of the limited C code–based analysis for patients with cancer. Therefore, this report did not quantify the total number of HSCT procedures performed on children in Korea. Lastly, since only the ICD-10 codes reflecting the anatomical classification are provided in the claim data, accurately confirming the specific pathological diagnosis proves challenging; therefore, information could not be extracted for each specific common childhood cancer.
In conclusion, the number of pediatric patients with cancer is declining in Korea; however, the ratio of transplants to all patients remains constant. Patients who recently underwent transplantation showed better survival rates, possibly due to HSCT optimization. Korea showed a substantially greater PBSC utilization in pediatric HSCT. An in-depth examination encompassing donor relations and cause of death with a prospective registry is required in future studies.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study used anonymous, secondary data; hence, it was exempted from review by the Institutional Review Board of the National Cancer Center, Korea (AMC 2021-0940). Furthermore, the requirement for written informed consent was waived for the same reason. AcknowledgmentsThis work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C2046); the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Republic of Korea’s Ministry of Health Welfare (Grant HR21C0198); and the National Research Foundation of Korea (NRF), funded by the Korean government (MSIT) (No. NRF-2021R1A2C1095874).
Fig. 1.Patient selection flow. ICD-10, International Classification of Diseases, 10th revision; KCD-10, Korean Classifications of Diseases, 10th revision; NHIS, Korean National Health Insurance Service. 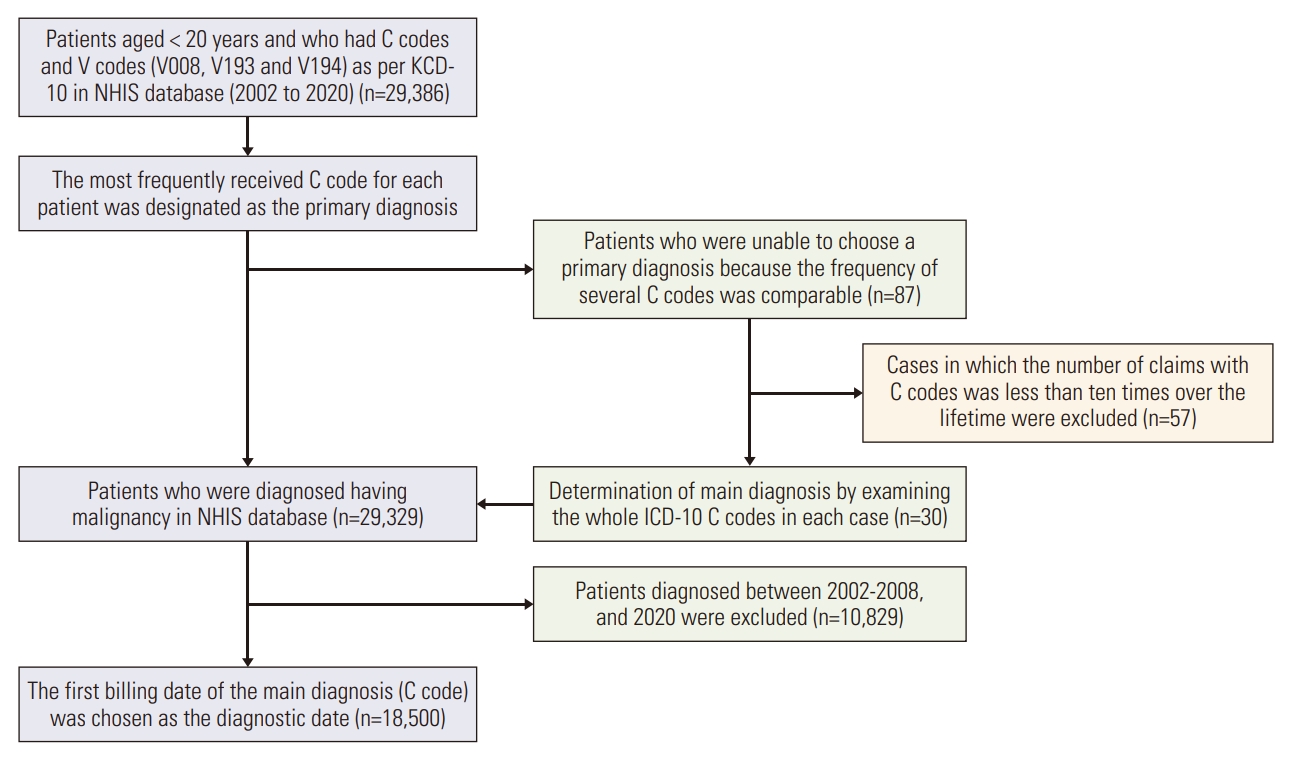
Fig. 2.(A) Annual number of hematopoietic stem cell transplantations (2009-2019), and types of hematopoietic stem cell transplantation (HSCT). (B) Annual number of HSCT according to the type of transplant. (C) Stem cell sources among the allogeneic stem cell transplantations. CBT, cord blood transplantation; BMT, bone marrow transplantation; HSCT, hematopoietic stem cell transplantation; PBSCT, peripheral blood stem cell transplantation. 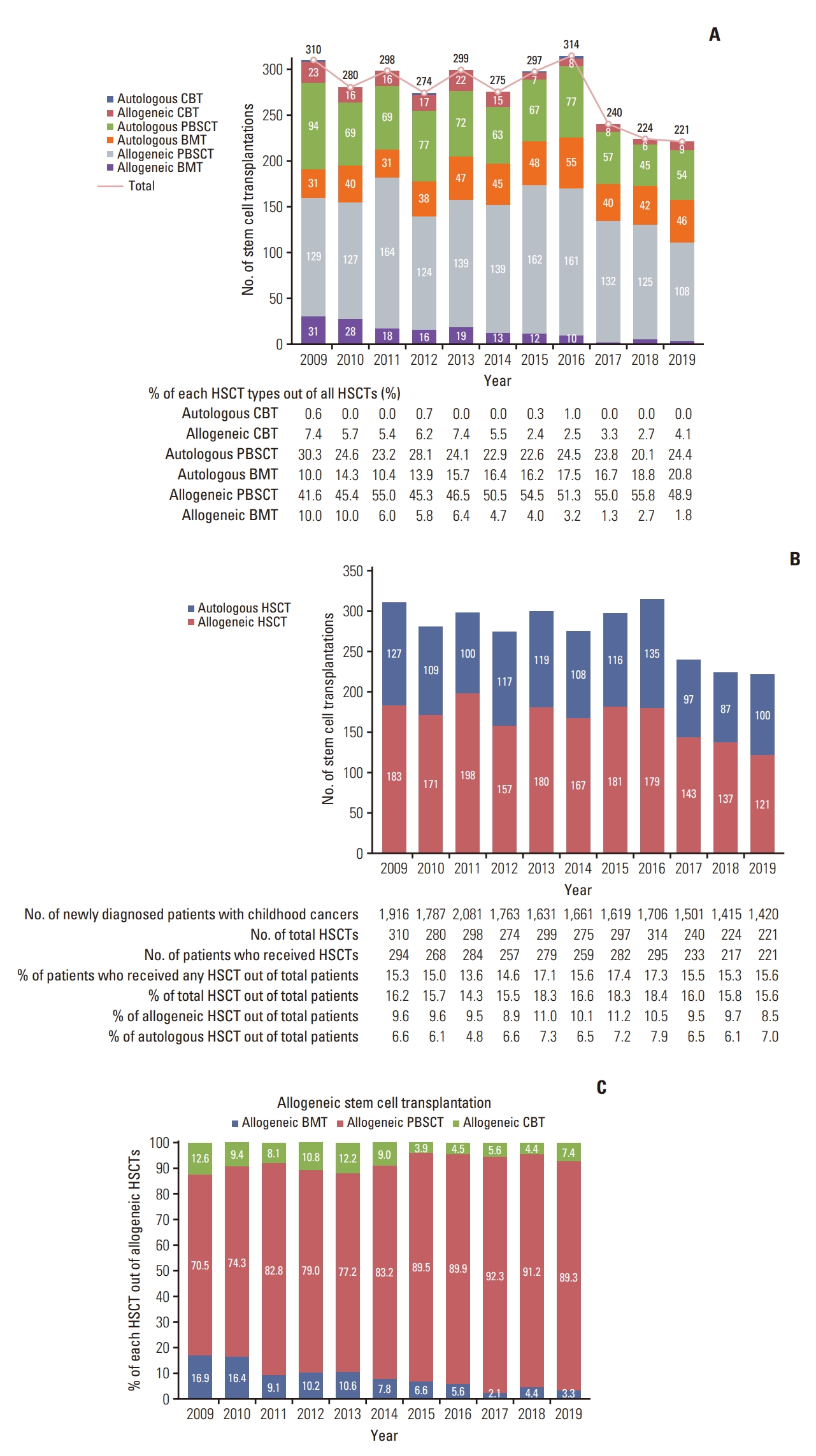
Fig. 3.(A) 5-Year overall survival (OS) rates from the diagnosis of all patients. (B) 5-Year OS rates of patients who underwent hematopoietic stem cell transplantation (HSCT) or not. (C) Based on the analyses of 5-year OS rates at 4-year intervals from 2009 to 2012, 2013 to 2016, and 2017 to 2020, according to the year of diagnosis, patients with more recent diagnoses showed a higher chance of survival. (D) Based on the analyses of 5-year OS rates at 4-year intervals from 2009 to 2012, 2013 to 2016, and 2017 to 2020, according to the year of transplantation, patients with more recent transplants had a higher chance of survival but without significance. 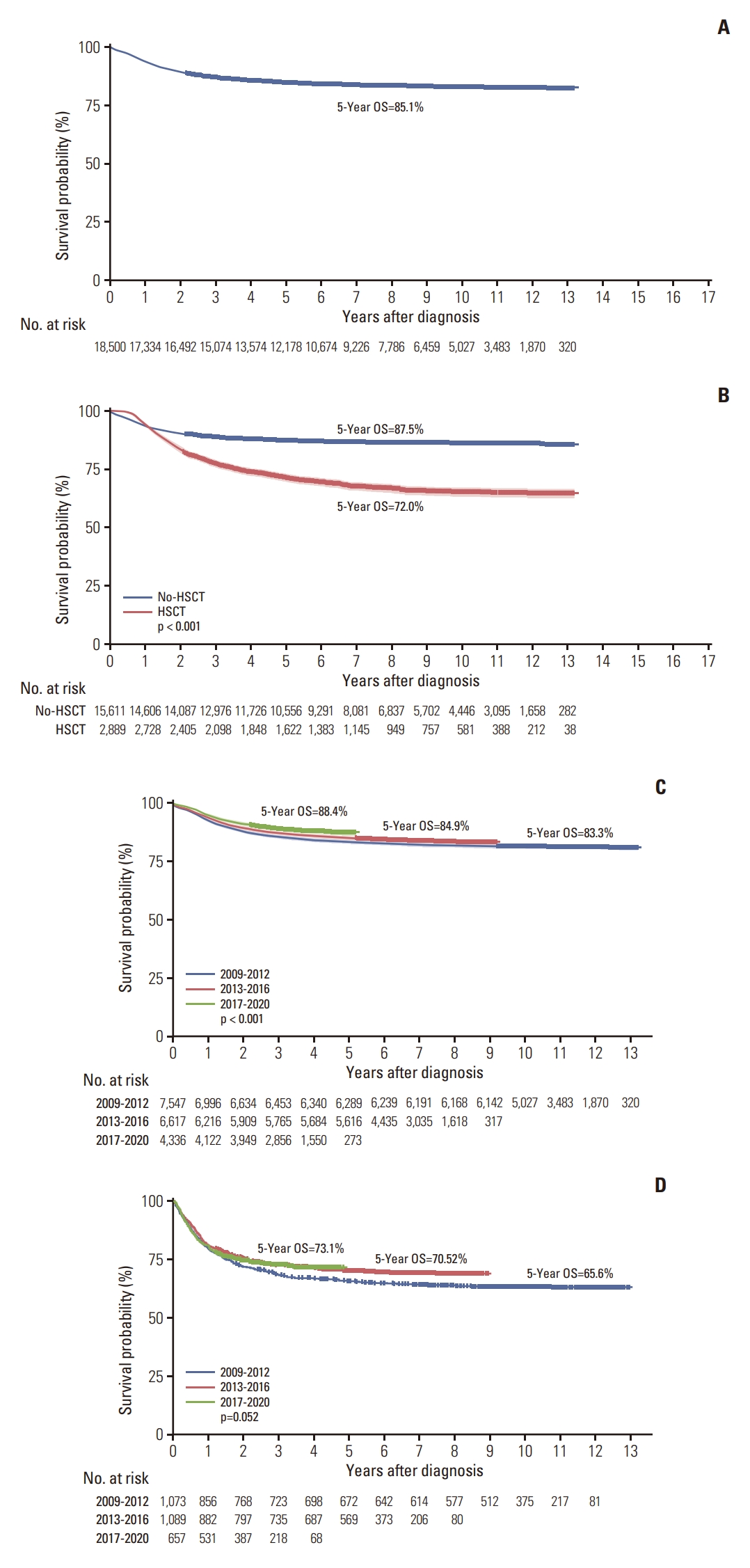
Fig. 4.Comparison of the 5-year overall survival (OS) rates based on transplant type and number for each disease. The 5-year OS rates of lymphoid leukemia (A), myeloid leukemia (B), and brain tumors (C) were compared according to the number of autologous HSCT (auto-HSCT): single HSCT, auto (1); tandem HSCT, auto (2); three or more HSCT, auto (3). Allo-BMT, allogeneic bone marrow transplantation; Allo-PBSCT, allogeneic peripheral blood stem cell transplantation; CBT, cord blood transplantation; HSCT, hematopoietic stem cell transplantation. 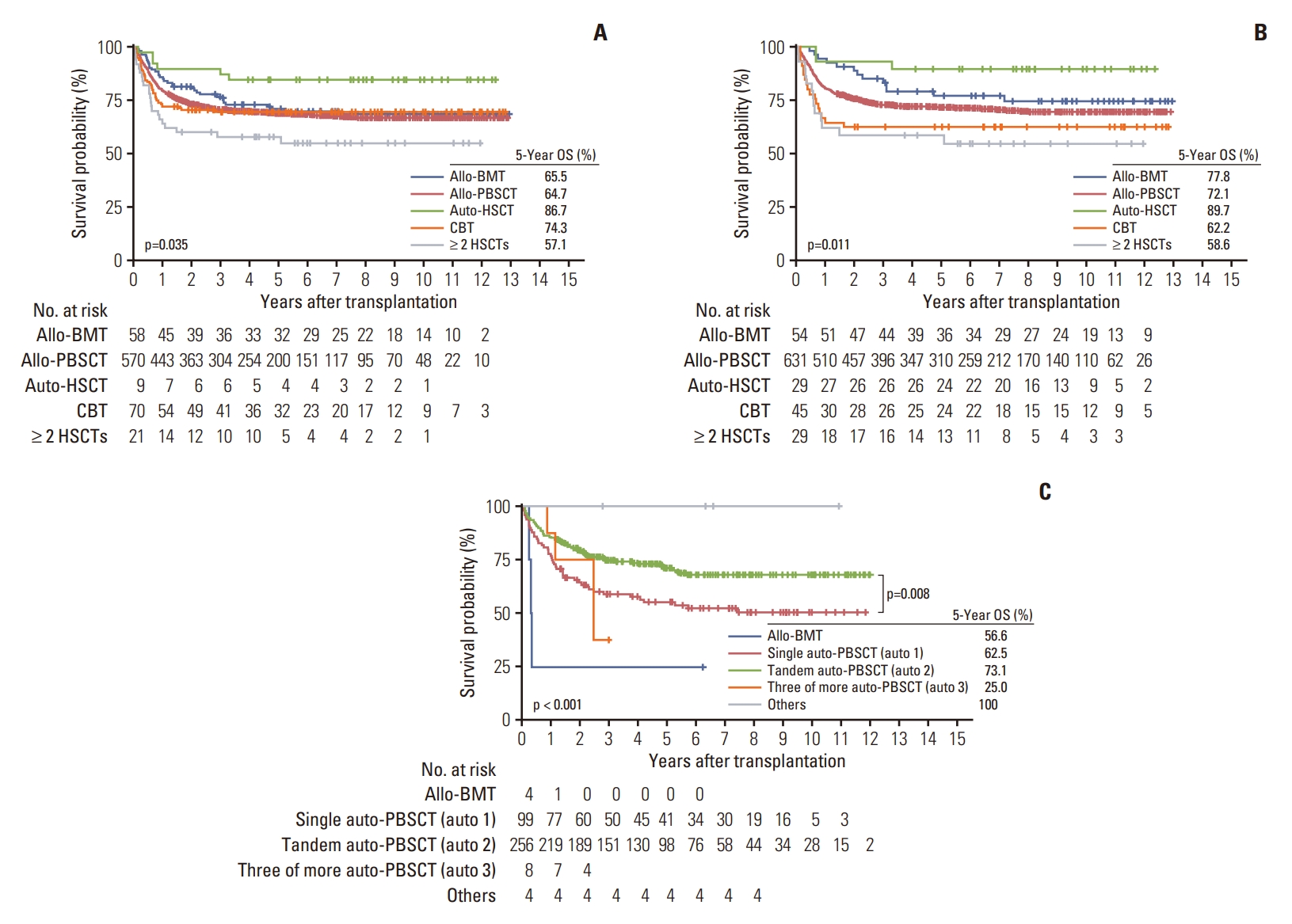
References1. Snowden JA, Sanchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57:1217–39.
2. Calvo C, Ronceray L, Dhedin N, Buechner J, Troeger A, Dalle JH. Haematopoietic stem cell transplantation in adolescents and young adults with acute lymphoblastic leukaemia: special considerations and challenges. Front Pediatr. 2021;9:796426.
3. Lee JW. Haploidentical family donor transplantation for pediatric hematologic malignancies. Clin Pediatr Hematol Oncol. 2021;28:67–74.
4. Im HJ, Koh KN, Seo JJ. Recent advances in haploidentical hematopoietic stem cell transplantation using ex vivo T cell-depleted graft in children and adolescents. Blood Res. 2016;51:8–16.
5. Jeong DC, Kang HJ, Koo HH, Kook H, Kim SY, Kim SK, et al. Current status of hematopoietic stem cell transplantation in Korean children. Korean J Hematol. 2006;41:235–42.
6. Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–73.
7. Sung KW, Ahn HS, Cho B, Choi YM, Chung NG, Hwang TJ, et al. Efficacy of tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma: the Korean Society of Pediatric Hematology-Oncology experience over 6 years (2000-2005). J Korean Med Sci. 2010;25:691–7.
8. Kim H. Pediatric cancer research using healthcare big data. Clin Pediatr Hematol Oncol. 2022;29:1–11.
9. Chung H, Kim SY, Kim HS. Clinical research from a health insurance database: practice and perspective. Korean J Med. 2019;463–70.
10. Lee JW, Kim CC. The activity of hematopoietic stem cell transplantation in Korea. Bone Marrow Transplant. 2008;42 Suppl 1:S92–5.
11. Park HJ, Moon EK, Yoon JY, Oh CM, Jung KW, Park BK, et al. Incidence and survival of childhood cancer in Korea. Cancer Res Treat. 2016;48:869–82.
12. Suh JK, Koh KN, Min SY, Kim YS, Kim H, Im HJ, et al. Feasibility and effectiveness of treatment strategy of tandem high-dose chemotherapy and autologous stem cell transplantation in combination with (131) I-MIBG therapy for high-risk neuroblastoma. Pediatr Transplant. 2020;24:e13658
13. Park JE, Kang J, Yoo KH, Sung KW, Koo HH, Lim DH, et al. Efficacy of high-dose chemotherapy and autologous stem cell transplantation in patients with relapsed medulloblastoma: a report on the Korean Society for Pediatric Neuro-Oncology (KSPNO)-S-053 study. J Korean Med Sci. 2010;25:1160–6.
14. Sung KW, Lim DH, Son MH, Lee SH, Yoo KH, Koo HH, et al. Reduced-dose craniospinal radiotherapy followed by tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk medulloblastoma. Neuro Oncol. 2013;15:352–9.
15. D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26:e177–82.
16. Auletta JJ, Kou J, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides. Milwaukee, WI: Center for International Blood and Marrow Transplant Research; 2021.
17. Korean Blood & Marrow Transplantation Nursing Society. Annual number of BMT in Korea [Internet]. Seoul: Korean Blood & Marrow Transplantation Nursing Society; 2022. [cited 2023 Sep 7]. Available from: http://www.bmtnurse.org/default/mp6/mp6_sub6.php?sub=01
19. Shimosato Y, Tanoshima R, Tsujimoto SI, Takeuchi M, Shiba N, Kobayashi T, et al. Allogeneic bone marrow transplantation versus peripheral blood stem cell transplantation for hematologic malignancies in children: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2020;26:88–93.
|
|
|||||||||||||||||||||||||||||||||||||||