AbstractPurposeThe matrix metalloproteinases (MMPs) are a family of proteolytic enzymes. MMPs are known to be involved in tumor invasion, and several have been implicated in tumor prognosis. The aim of this study was to evaluate the prognostic significances of the expressions of MMP-7 and -9 in rectal cancer.
Materials and MethodsThe tumor tissues of 87 patients with stage II or III rectal carcinoma that underwent potentially curative resection followed by postoperative adjuvant chemoradiation and 5-fluorouracil based chemotherapy, were investigated immunohistochemically using monoclonal antibodies against MMP-7 and MMP-9. Clinical information, including tumor grades, carcinoembryonic antigen (CEA) levels, and disease-free survival and overall survival were evaluated with respect to the expressions of MMP-7 and -9.
ResultsMedian follow-up duration was 53.2 months, and median patient age was 55±11 years (range 32~75). MMP-7 expression in tumor tissue was found to be significantly correlated with the presence of nodal metastasis (p=0.029), whilst MMP-9 expression correlated with depth of tumor invasion (p=0.019). No relationships were found between the expressions of MMP-7 or -9 and age, sex, tumor size, tumor grade, or CEA level. Univariate analysis showed that MMP-7 expression was associated with poor 5-year overall survival (12.8 months vs. 65.3 months, p=0.0405). Multivariate analysis confirmed that MMP-7 was independently associated with an adverse outcome (Relative risk: 1.415, p=0.027). However, MMP-9 expression was not found to be related to clinical outcome.
INTRODUCTIONThe incidence of colorectal cancer is higher in the West than in Korea, where it is the fourth most common cancer. However recently, its incidence in Koreans has shown a steep increase, from 6.6% of all malignancies in 1990 to 10.6% in 2001 in men, and from 7.2% to 10.5% in women (1). Thus, colorectal cancer has emerged to become one of the greatest public health problems in Korea.
Approximately, one quarter of colorectal cancers are located in the rectum, and surgical resection remains the best curative treatment option. Although adjuvant therapy is of benefit after potentially curative resection in patients with stage II or III rectal cancer (2), adjuvant radiotherapy alone decreases local recurrence without survival benefit (3). Only combined chemotherapy and radiotherapy has consistently demonstrated efficacy in terms of reducing the incidence of pelvic recurrence, and improving disease free survival and overall survival (4). Thus, it remains a priority to identify potential biomarkers capable of predicting recurrence, and disease free survival and overall survival. Moreover, the majority of prognostic factors provide no insight into the molecular events responsible for tumor invasion and/or metastasis (5,6).
More specifically, the proteolytic degradation of extracellular matrix by matrix metalloproteinases (MMP) has been shown to be one of the essential events in tumor invasion and metastasis (7). And, it has been shown that they are also involved in cell differentiation, apoptosis, angiogenesis, immune surveillance, and cancer cell growth (8).
MMP-7 is characterized by a broad strong proteolytic activity against a variety of extracellular matrix substrates, such as, collagens, proteoglycans, laminin, fibronectin, and casein (2). MMP-7 does not possess a C-terminal domain, and thus is not easily regulated by tissue inhibitor of metalloproteinases (TIMP) (9). The clinical impact of MMP-7 expression has been assessed by examining surgically resected colorectal cancer tissues (10). Moreover, there is almost unanimous agreement that enhanced MMP-7 expression in colorectal cancer, as measured by immunohistochemistry or by in situ hybridization, is correlated with the presence of nodal or distant metastasis (11). MMP-9 also plays an important role in cancer invasion and metastasis by degrading extracellular matrix components and basement membrane (12). Increased levels of MMP-9 in tumor tissue, as determined by mRNA measurements, have been found to be correlated with advanced stages of colorectal cancer (13). Moreover, MMP-9 appears to be differentially expressed in colonic and rectal tumors (14).
The aims of this study were to confirm if immunohistochemical assessments of the expressions of MMP-7 and -9 correlate with clinical features in patients with rectal cancer, and thus to determine whether such immunohistochemical studies could differentiate tumors with a high or low malignancy potential.
MATERIALS AND METHODS1) PatientsNinety patients that registered at our center between January 1995 and January 2001 were enrolled in this study. All patients had histologically confirmed stage II or III adenocarcinoma of the rectum, and had undergone a potentially curative resection, with neither gross nor microscopic evidence of residual disease. Tumor samples were obtained from these patients and each sample was fixed in formalin and embedded in paraffin wax. Of the 90, three patients were ineligible for the following reasons; 2 patients refused their assigned treatment, and one died due to a postoperative complication. Thus, the remaining 87 patients were eligible with follow-up and were included in the analyses. Written informed consent was obtained from each patient prior to study entry. The study was approved by the institutional review board of Dong-A University Medical Center. Median patient age was 55±12.3 years (range, 33~73).
2) Immunohistochemical Analysis for MMP-7 and MMP-9Immunohistochemical studies for MMP-7 and MMP-9 were performed on formalin-fixed, paraffin-embedded, 4 µm-thick tissue sections, using the avidin-biotin-peroxidase complex method. Mouse monoclonal antibody directed against MMP-7 (Neomarker, Fremont, CA) at a 1 : 25 dilution, and rabbit polyclonal antibody directed against MMP-9 (Neomarker, Fremont, CA) at a 1 : 25 dilution, were used as primary antibodies. All sections were deparaffinized through a series of xylene baths and then rehydrated through a series of graded alcohol solutions. After blocking endogenous peroxidase activity with 5% hydrogen peroxidase for 10 min, primary antibody incubation was performed for 30 min at room temperature. An Envision Kit (DakoCytomation, Carpinteria, CA) was used to incubate sections with secondary antibody at room temperature for 30 min. After washing sections in Tris buffered saline for 10 min, 3, 3'-diaminobenzidine was applied as a chromogen, and this was followed by Mayer's hematoxylin counterstain. Stainings for MMP-7 and MMP-9 were considered to be positive when >10% of tumor cells showed cytoplasmic reactivity at any intensity. Negative control sections were prepared by omitting the primary antibodies.
3) Statistical analysisThe associations between the expressions of MMP-7 or MMP-9 and the clinicopathologic parameters (sex, age, carcinoembryonic antigen (CEA), tumor size, histologic differentiation, depth of bowel wall invasion) were examined using χ2 (chi-squared) test or Fisher's exact test. Disease-free survival was defined as the length of time from surgery to initial disease recurrence. Overall survival was defined as the length of time from surgery to death. The Kaplan-Meier method was used to construct curves for disease-free survival and overall survival. Data on patients who died without evidence of disease recurrence were censored at the time of death for disease-free survival calculations. The log-rank test was used to compare distributions. To identify independent factors significantly related to patient prognosis we used Cox's proportional hazards model with a stepwise procedure. All tests were two-sided, and p values of <0.05 were considered statistically significant. Analyses were performed using SPSS version 10.0 (SPSS Inc, Chicago, IL).
RESULTS1) Expressions of MMP-7 and MMP-9Positively stained cancer cells were distributed heterogeneously in tumor nests. Carcinoma cell cytoplasm stained brown for MMP-7, but stromal cells (other than some monocytes or surrounding normal mucosa) were not stained (Fig. 1A, B). MMP-9 expression was observed in the cytoplasms of carcinoma cells, stromal fibroblasts, and vascular endothelial cells (Fig. 1C, D).
2) Correlations between the expressions of MMP-7 and MMP-9 and clinicopathologic parametersDetails of these relations are listed in Table 1. MMP-7 expression was observed in 19 of the 87 cases (21.8%), and was found to be significantly correlated with lymph node metas tasis (p=0.035), and an advanced stage (p=0.029). No significant correlations were observed between MMP-7 expression and sex, age, CEA, tumor size, histologic differentiation, or depth of bowel wall invasion (tumor stage). On the other hand MMP-9 expression was detected in 24 cases (27.6%), and found to be significantly correlated with depth of invasion (p=0.019).
3) Expressions of MMP-7 and MMP-9 and survivalMedian follow-up duration was 53.2 months. Univariate analysis showed that CEA (p=0.0056), tumor differentiation (p=0.0168), and stage (p=0.0003) were significantly associated with 5-year disease-free survival and overall survival (Table 2). Patients with an MMP-7 expressing cancer had a significantly shorter 5-year overall survival time than those without (p=0.0405) (Fig. 2). MMP-7 expression was also correlated with 5-year overall survival in stage II patients (86.4 months vs. 16.7 months, p=0.004), however, no similar significance correlation was found in stage III patients (31.3 months vs. 38.9 months, p=0.5743). On the other hand, MMP-9 expression was not found to be correlated with 5-year overall survival (p=0.7198) (Fig. 3), and the presence of MMP-9 expression was found to have no clinical significance in stage II or III rectal cancer patients (p=0.6705, p=0.9006, respectively). To examine the independent prognostic value of MMP-7 expression, we used a multivariate Cox model to control for other prognostic factors. Accordingly, CEA histologic differentiation, stage, and MMP-7 expression were identified as significant predictors of overall survival, after controlling for the other clinicopathologic parameters. The relative risks (RRs) of MMP-7 expression and stage were 1.42 (95% confidence interval [CI], 1.19 to 1.91; p=0.027) and 1.99 (95% CI, 1.35 to 2.96; p=0.001), respectively.
DISCUSSIONCurrently, there is no definitive way of predicting which rectal cancer patients with an intermediate risk of recurrence (stage II or III), will develop recurrent disease. The primary goal of this study was to determine whether the expressions of MMP-7 or MMP-9 in rectal cancer can predict disease outcome. In addition to affecting primary tumor growth, a substantially body of evidence indicates that MMPs also influence colorectal cancer (CRC) invasion and metastasis. Numerous studies have been performed in vitro and in vivo to determine how MMPs contribute to CRC metastasis (15). Because the expression of several MMPs have been correlated with invasion and metastasis, and because MMPs are capable of degrading ECM components, their roles in invasion and metastasis have been extensively examined (15).
Our study shows that immunohistochemical expression of MMP-7 in rectal cancer tissue is more common in stage III than in stage II and in those with nodal metastasis, thus indicating that its presence correlates with the presence of metastases. This result is supported by the findings of a previous report, in which MMP-7 mRNA expression was found to be greater in colorectal cancer than in paired normal colorectal mucosal tissues, and the expression of MMP-7 mRNA increased with increasing Dukes' stage (16). Analysis of MMP-7 as a potential prognostic indicator revealed that positive MMP-7 expression (>30% positive tumor cells at the invasive front) is related to the depth of invasion, lymph node metastasis, lymphatic invasion, an advanced Dukes' stage, and a poor outcome (17). Moreover, in this study, the Kaplan-Meier life table indicated that 5-year survival is 3.5 times greater in patients without an MMP-7 expressing tumor (77.3% vs. 21.8%, respectively) (17). In the present study, similar results were obtained. However, MMP-7 expression was found to be correlated with 5-year overall survival in stage II patients only, indicating that MMP-7 has important role in early stage rectal cancer. Moreover, we compared our immunohistochemical findings with the main clinicopathological prognostic factors, i.e., stage, CEA level, and tumor differentiation, and confirmed the highly significant independent prognostic significance of MMP-7 expression. These results further support the notion that MMP-7 plays an important role in the progression of colorectal cancer and that MMP-7 could be a reliable marker of cancer aggressiveness and a poor prognosis, independently of conventional clinicopathologic criteria. Therefore, we believe that the immunohistochemical analysis of MMP-7 expression in tumor biopsy specimens before treatment would be useful for predicting prognosis.
Several reports have been issued on the roles of MMP-9 in tumor invasion and metastasis, but these remain unresolved issues in colorectal cancer (18). However, MMP-9 overexpression has been correlated with synchronous metastasis from colorectal cancer and Dukes' stage (13). Moreover, a 5-fold increase or more in MMP-9 RNA expression in tumor versus normal tissue was found to be associated with increased recurrence, and patients with tumors expressing high levels of MMP-9 RNA were found to be six times more like experience recurrence than patients with low levels of MMP-9 RNA (13). However, it has also been reported that MMP-9 mRNA levels are not significantly different in colon tumors and healthy mucosa (19), and that the tumors of patients with or without metastases expressed similar levels of MMP-9 mRNA (20). In the present study, we detected 5-fold higher MMP-9 expression in colon carcinoma tissues than in normal tissues of the same individuals, whereas in rectal carcinoma no significant difference was found between normal and tumor tissues (14).
In the present study, we found that MMP-9 expression was only related to depth of invasion. These results suggest that different mechanisms are at play in rectal and colon cancer, and indicate the likelihood of their differential transcriptional activities.
In general, the results of phase III clinical trials on MMP inhibitors in advanced stage cancers have been disappointing (21), and to select patients likely to benefit from such therapies, examinations of MMP expression may be necessary. In this regard, our findings suggest that measurements of MMP-7 expression may help identify those likely to benefit from new therapeutic strategies based on selective MMP inhibitors.
References1. Shin HR, Won YJ, Jung KW, Park JG. 2001 Annual Report of the Korea Central Cancer Registry: based data from 134 hospitals. Cancer Res Treat. 2004;36:19–30.
2. Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. PMID: 1997835
3. Colorectal Cancer Collaborative GroupAdjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358:1291–1304. PMID: 11684209
4. O'Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion 5-FU with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. PMID: 8041415
5. Seong J, Chung EJ, Kim H, Kim GE, Kim NK, Sohn SK, et al. Assessment of biomarkers in paired primary and recurrent colorectal adenocarcinomas. Int J Radiat Oncol Biol Phys. 1999;45:1167–1173. PMID: 10613309
6. Garrity MM, Burgart LJ, Mahoney MR, Windschitl HE, Salim M, Wiesenfeld M, et al. Prognostic value of proliferation, apoptosis, defective DNA mismatch repair, and p53 overexpression in patients with resected Dukes' B2 or C colon cancer: a North Central Cancer Treatment Group Study. J Clin Oncol. 2004;22:1572–1582. PMID: 15117979
7. Crawford HC, Matrisian LM. Tumor and stromal expression of matrix metalloproteinases and their role in tumor progression. Invasion Metastasis. 1995;14:234–245. PMID: 7657516
8. McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. 2000;6:149–156. PMID: 10740253
9. Miyazaki K, Hattori Y, Umenishi F, Yasumitsu H, Umeda M. Purification and characterization of extracellular matrix-degrading metalloproteinase, matrin (pump-1), secreted from human rectal carcinoma cell line. Cancer Res. 1990;50:7758–7764. PMID: 2253219
10. Adachi Y, Yamamoto H, Itoh F, Hinoda Y, Okada Y, Imai K. Contribution of matrilysin (MMP-7) to the metastatic pathway of human colorectal cancers. Gut. 1999;45:252–258. PMID: 10403738
11. Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. PMID: 15000152
12. Liotta LA, Stetler-Stevenson WG. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990;1:99–106. PMID: 2103492
13. Zeng ZS, Huang Y, Cohen AM, Guillem JG. Prediction of colorectal cancer relapse and survival via tissue RNA levels of matrix metalloproteinase-9. J Clin Oncol. 1996;14:3133–3140. PMID: 8955659
14. Roeb E, Dietrich CG, Winograd R, Arndt M, Breuer B, Fass J, et al. Activity and cellular origin of gelatinases in patients with colon and rectal carcinoma differential activity of matrix metalloproteinase-9. Cancer. 2001;92:2680–2691. PMID: 11745204
15. Wagenaar-Miller RA, Gorden L, Matrisian LM. Matrix metalloproteinases in colorectal cancer: is it worth talking about? Cancer Metastasis Rev. 2004;23:119–135. PMID: 15000153
16. Mori M, Barnard GF, Mimori K, Ueo H, Akiyoshi T, Sugimachi K. Overexpression of matrix metalloproteinase-7 mRNA in human colon carcinomas. Cancer. 1995;75(Suppl 6):1516–1519. PMID: 7889484
17. Adachi Y, Yamamoto H, Itoh F, Arimura Y, Nishi M, Endo T, et al. Clinicopathologic and prognostic significance of matrilysin expression at the invasive front in human colorectal cancers. Int J Cancer. 2001;95:290–294. PMID: 11494227
18. Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705:69–89. PMID: 15588763
19. Collins HM, Morris TM, Watson SA. Spectrum of matrix metalloproteinase expression in primary and metastatic colon cancer: relationship to the tissue inhibitors of metalloproteinases and membrane type-1-matrix metalloproteinase. Br J Cancer. 2001;84:1664–1670. PMID: 11401321
20. Masuda H, Aoki H. Host expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in normal colon tissue affects metastatic potential of colorectal cancer. Dis Colon Rectum. 1999;42:393–397. PMID: 10223763
21. Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. PMID: 11923519
Fig. 1Immnohistochemical staining of rectal cancer for MMP-7 and MMP-9. (A) MMP-7 positive rectal adenocarcinoma;MMP-7 was stained in cancer cell cytoplasm, (B) MMP-7 negative rectal adenocarcinoma, (C) MMP-9 positive rectal adenocarcinoma; MMP-9 was stained in tumor cytoplasm, (D) MMP-9 negative rectal adenocarcinoma (×200). 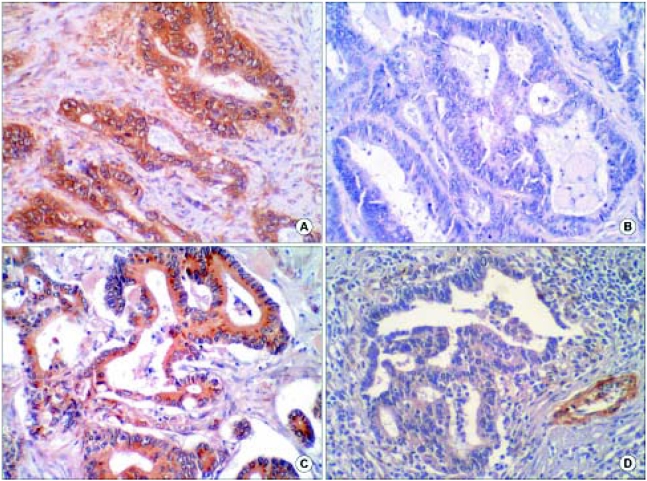
Fig. 2Comparison of overall survival curves for patients with MMP-7 positive and MMP-7 negative rectal cancer (p=0.0405). 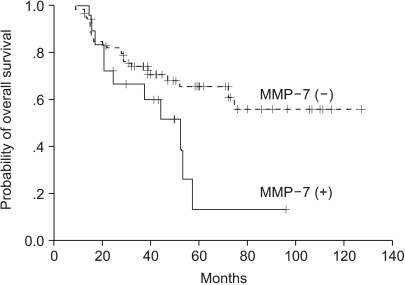
Fig. 3Comparison of overall survival curves for patients with MMP-9 positive and MMP-9 negative rectal cancer (p=0.7198). 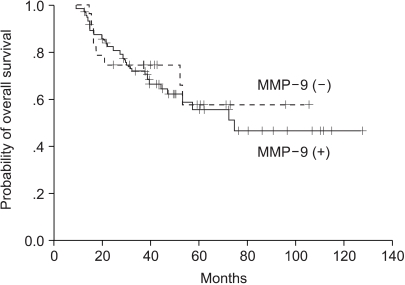
|
|
||||||||||||||||||||||||||||||||||||