AbstractPurposeIn the treatment of concurrent chemoradiotherapy (CCRT) in limited-stage small cell lung cancer, the optimal once-daily radiotherapy (RT) dose/fractionation remain unclear although it is the most frequently used. Therefore, this study aimed to compare the treatment outcomes and toxicities of modest dose RT (≤ 54 Gy) with those of standard dose RT (> 54 Gy) and investigate the benefit of the high dose based on patient factors.
Materials and MethodsSince 2004, our institution has gradually increased the thoracic RT dose. Among the 225 patients who underwent CCRT, 84 patients (37.3%) received > 54 Gy. Because the patients treated with RT > 54 Gy were not randomly assigned, propensity score matching (PSM) was performed.
ResultsThe proportion of patients treated with > 54 Gy increased over time (p=0.014). Multivariate analysis revealed that the overall tumor stage and dose > 54 Gy (hazard ratio, 0.65; p=0.029) were independent prognostic factors for overall survival (OS). PSM confirmed that thoracic RT doses of > 54 Gy showed significantly improved progression-free survival (3-year, 42.7% vs. 24.0%; p < 0.001) and OS (3-year, 56.2% vs. 38.5%; p=0.003). Sensitivity analysis also showed that 60 Gy resulted in better survival than 54 Gy. However, in patients with underlying lung disease, OS benefit from > 54 Gy was not observed but considerable rates of severe pulmonary toxicities were observed (p=0.001).
ConclusionOur analysis supports that the 60 Gy RT dose should be considered in the once-daily regimen of CCRT for limited-stage small cell lung cancer without underlying lung disease, but RT dose > 54 Gy did not seem to benefit for patients with chronic obstructive pulmonary disease or interstitial lung disease. Further study is needed to validate these results.
IntroductionConcurrent chemoradiotherapy (CCRT) is the treatment of choice in limited-stage small cell lung cancer (LS-SCLC). In thoracic radiotherapy (RT), although a twice-daily (BID) regimen is considered the standard of care after the Intergroup 0096 trial [1], the once-daily (QD) regimen is used more frequently. The U.S. National Cancer Database analysis between 1999 and 2012 revealed that the utilization of a BID regimen was only 11.3% [2]. Another recent survey analysis for radiation oncologists showed that 60% of 309 responders preferred a QD regimen, and 76% acknowledged that QD regimen was more common in routine practice [3]. BID regimen, which is not widely used, has several limitations. Intergroup 0096 trial reported that BID regimen was associated with severe (grade 3 or more) esophagitis (32% with BID vs. 16% with QD) [1]. Modern RT technique such as intensity-modulated RT could reduce the rate of severe esophagitis, but patient’s inconvenience was an additional obstacle to adopt BID regimen which require at least 6-hour interval between fractional treatments.
However, the optimal QD RT dose/fractionation remains unknown. Although the CONVERT trial showed that 45 Gy/30 fractions (Fx) BID and 66 Gy/33 Fx QD achieved comparable survival outcomes, no standard QD regimen was established because the trial failed to show the superiority of the QD regimen [4]. The CALGB 30610/RTOG 0538 trial also reported similar results in the meeting abstract of the American Society of Clinical Oncology (ASCO) 2021, showing similar outcomes but failing to demonstrate the superiority of 70 Gy QD over 45 Gy BID [5]. A variety of QD RT regimens have been used in the real-world treatment for LS-SCLC. A previous study reported that the median survival for patients receiving 45 Gy QD, 46–59.4 Gy QD, and 60–61.2 Gy QD was 17.2, 18.3, and 19.2 months, respectively, indicating the absence of any clear dose-response relationship in terms of overall survival (OS) [2]. Without randomized evidence, according to the very up-to-date ASCO/ASTRO (American Society of Radiation Oncology) guideline, a QD RT dose of 60–70 Gy is ‘conditionally’ recommended as an acceptable alternative to BID RT (quality of evidence: moderate) [6].
However, frail patients, especially those with underlying lung disease, are currently recommended a 60–70 Gy thoracic RT dose that can sometimes cause severe side effects. An individual patient data pooled analysis from 11 trials of CCRT for LS-SCLC reported that elderly patients failed to complete treatment more often due to adverse events, and died more frequently during treatment [7]. The number of SCLC patients with underlying lung disease has been increasing, and a recent study showed that patients with interstitial lung abnormalities showed a higher rate of radiation pneumonitis than those without lung abnormalities after CCRT for LS-SCLC, even with a modest RT dose (45 Gy BID or 50 Gy QD) [8].
Traditionally, modest doses of RT (45–50 Gy) are often used in QD 1.8–2 Gy Fx in the absence of standard fractionation, and our institutions have gradually increased the thoracic RT dose from 44 to 66 Gy. Therefore, we conducted this study to compare the treatment outcomes and toxicities of modest doses (≤ 54 Gy) to those of a standard dose (> 54 Gy) using propensity score-matching (PSM) data and investigate the benefit of a standard dose based on patient factors.
Materials and Methods1. Study populationWe identified a cohort of 358 patients with LS-SCLC from two institutions between January 2004 and December 2017, after excluding patients without appropriate initial staging work-up (both brain magnetic resonance imaging [MRI] and whole-body fluorodeoxyglucose [FDG] positron emission tomography [PET]/computed tomography [CT]). All patients were histologically confirmed to be SCLC patients. We identified 225 patients treated with definitive CCRT after excluding those treated with sequential chemoradiotherapy, chemotherapy alone, or surgery with or without chemotherapy for early stage SCLC.
2. Treatment detailsThe 8th edition of the American Joint Committee on Cancer staging was used for TNM staging. Chemotherapy regimens consisted of etoposide and cisplatin or carboplatin administered every 3 weeks up to six cycles. Thoracic RT usually started with the 2nd or 3rd cycle of chemotherapy. The RT techniques for LS-SCLC have been described previously in detail [9]. Three-dimensional conformal RT was used for most patients (n=204, 90.7%), and intensity-modulated RT was used for 21 patients (9.3%). The median RT dose was 54 Gy (range, 43.2 to 72.0 Gy). Only one case of 72 Gy/18 Fx was irradiated for T1N0 SCLC which was not applied generally to common cases, and 43.2 Gy was also an exceptional case in which one patient did not complete the scheduled RT courses. The most commonly prescribed dose-fractionation was 54 Gy/27 Fx (n=120, 53.3%), followed by 60 Gy/30 Fx (n=70, 31.1%). Therefore, we divided the patients into two dose groups: > 54 Gy (n=84, 37.3%) versus ≤ 54 Gy (n=141, 62.7%) for subsequent analysis. Prophylactic cranial irradiation (PCI) consisting of 25 Gy/10 Fx was recommended to patients who had achieved complete or partial response after CCRT and its implementation was determined by the treating physician and the patients’ choice.
3. Follow-up and toxicity evaluationDuring the follow-up period, the patients underwent physical examination, laboratory tests, chest radiography, and chest CT every 2–3 months in the first year and every 3–6 months thereafter. Bone scan, brain MRI, and/or FDG-PET/CT were performed at any time in case of suspicion of metastasis. The median duration of follow-up was 32 months (range, 5 to 148 months; interquartile range, 18 to 60 months) for all patients. Severe pulmonary toxicities were defined as grade 3 or higher toxicity according to the Radiation Therapy Oncology Group criteria [10]. The determination of underlying interstitial lung disease (ILD) was based on the diagnosis of the pulmonologist who was following the corresponding patient. Pulmonary toxicity of grades 1 and 2 after initiation of RT were described according to related acute symptoms during RT or within 3 months. Grade 3 was classified as RT field associated pneumonitis, not ILD aggravation, which symptoms were severe enough to use steroids. Grade 4 was classified as a case of severe respiratory insufficiency that required oxygen or assisted ventilation, and grade 5 was classified as a case of death due to exacerbation.
4. Propensity score matchingBecause the patients treated with RT > 54 Gy were not assigned randomly, PSM was performed to adjust important baseline characteristics (years of treatment, age, sex, performance status, smoking history, T category, N category, underlying lung disease, chemotherapy cycles, and the use of PCI). The propensity score (PS) was calculated to predict the likelihood that RT > 54 Gy was administered to each patient. Based on the PS, the patients were matched at a 1:1 ratio (RT ≤ 54 Gy vs. RT > 54 Gy) using the nearest-neighbor method.
5. Statistical analysisThe chi-square test and t test were used to evaluate the distribution of characteristics between both groups. OS was defined as the time from the date of diagnosis to the date of the last follow-up or death due to any cause. Progression-free survival (PFS) was defined as the time from the date of diagnosis to the date of any progression or death during the follow-up period. Local relapse-free survival (LRFS), regional relapse-free survival (RRFS), and distant metastasis free survival (DMFS) were defined as the time from the date of diagnosis to the date of local recurrence, regional recurrence, or distant metastasis, respectively. The Kaplan-Meier method was used to estimate the survival curves, and log-rank tests were used to compare the difference in survival rates in the univariate analysis. The factors proven to have a significant impact on OS were included in the multivariate analysis using the Cox proportional hazard model. A p-value less than 0.05 was considered statistically significant, and all statistical analyses were performed using R ver. 3.5.2 (http://www.r-project.org).
Results1. Patient characteristicsOverall, there were 187 men (83.1%) and 38 women (16.9%) with a median age of 64 years (range, 39 to 84 years). The Eastern Cooperative Oncology Group performance status was mainly 0–1 in 212 patients (94.2%). The proportion of patients treated with > 54 Gy increased over time (p=0.014). More patients in the standard dose group had underlying chronic obstructive pulmonary disease (COPD; 20.6% vs. 36.9%, p=0.020). The distribution of other characteristics, such as age, sex, T category, N category, pulmonary function test, tumor marker, chemotherapy cycles, follow-up duration, and the use of PCI, was not significantly different between the two groups (Table 1). After PSM, characteristic differences were further reduced and especially the proportion of underlying lung disease was not significantly different between the two groups (S1 Table, S2 Fig.). The proportion of intensity-modulated radiotherapy technique application (6.7% in ≤ 54 Gy group vs. 10.7% in > 54 Gy group, p=0.562) and 4-dimensional RT simulation (5.3% in ≤ 54 Gy group vs. 12.0% in > 54 Gy group, p=0.246) were also not significantly different between matched groups.
2. Impact of RT dose on survival outcomesThe results of the univariate and multivariate analyses for PFS and OS in all study patients are shown in Table 2. In the univariate analysis, stage III was significantly associated with poor PFS (p=0.031) and OS (p=0.026). The RT dose of > 54 Gy showed significantly improved PFS (3-year rate, 41.5% vs. 33.0%; p=0.014) and OS (3-year rate, 53.6% vs. 46.3%; p=0.049). After the multivariate analysis, all of the above factors preserved statistical significance, and the survival benefit of the higher RT dose was also retained: hazard ratio (HR) for PFS was 0.55 (95% confidence interval [CI], 0.38 to 0.81; p=0.002) and HR for OS was 0.65 (95% CI, 0.44 to 0.96; p=0.029).
After PSM, it was confirmed that the thoracic RT dose of > 54 Gy showed significantly improved PFS (3-year rate, 42.7% vs. 24.0%; p < 0.001) (Fig. 1A) and OS (3-year rate, 56.2% vs. 38.5%; p=0.003) (Fig. 1B). To analyze whether the difference in locoregional control according to the RT dose escalation resulted in OS improvement, the differences in patterns of failure were additionally analyzed in matched cohort. The probability of locoregional recurrences decreased to 46.7% vs. 36.0% (p=0.246) by dose escalation, although it was not statistically significant, but LRFS (mean, 30.7 months vs. 47.3 months; p=0.002) and RRFS (mean, 33.6 months vs. 48.4 months; p=0.007) showed a significant difference (S3 Table). Additionally, better DMFS was observed in the RT > 54 Gy group (mean, 30.6 months vs. 45.8 months; p=0.006) (S3 Table).
We added the sensitivity analysis about the comparison results for 120 patients of 54 Gy vs. 70 patients of 60 Gy both of which were the representative groups in modest dose (≤ m54 Gy) and standard dose (> m54 Gy). Overall, the results were not different from those of the previous PSM comparison, and the 60 Gy group showed a significant difference in PFS compared to the 54 Gy group (p=0.026) (S4 Fig.).
3. Investigation of benefit of RT > 54 Gy according to each characteristicSubgroup analysis was performed in the PS-matched group to further identify patients who could derive benefit from RT > 54 Gy compared to ≤ m54 Gy. Fig. 2 shows the results of the exploratory analysis plotting HR and 95% CI and comparing OS by the administration of RT > 54 Gy for each subgroup of patients. The benefits of RT > 54 Gy were observed in most subgroups but not significantly in patients with underlying lung disease (ILD or COPD). Specifically, an RT dose > 54 Gy significantly improved OS in patients without lung disease (3-year rate, 64.4% vs. 40.3%; p=0.002) (Fig. 3A) but not in those with COPD (3-year rate, 39.8% vs. 36.4%; p=0.510) (Fig. 3B) or ILD (3-year rate, 25.0% vs. 25.0%; p=0.470) (Fig. 3C). In the sensitivity analysis consisting of 120 patients of 54 Gy and 70 patients of 60 Gy, similar subgroup analysis according to the underlying lung disease was also performed and showed the same results as the previous analysis. There was a significant OS difference in the ‘without lung disease’ group (3-year rate, 67.5% vs. 45.4%) (p=0.014), but there was no difference in the COPD (3-year rate, 36.0% vs. 41.2%; p=0.780) and ILD (3-year rate, 25.0% vs. 20.0%; p=0.510) groups (S5 Fig.).
The observed rates of severe (grade 3 or more) pulmonary toxicities were significantly different based on underlying lung disease which showed the highest rates in ILD group (p=0.001) (Table 3), but not significantly different for RT doses in each lung disease group (Table 3). Other mild (grade 1–2) pulmonary toxicities, baseline pulmonary function, and related RT planning parameters (planning target volume, irradiated lung volume, and mean lung dose) according to RT dose were all not significantly different in each underlying lung disease group (Table 3).
DiscussionOur results clearly show improved outcomes with the standard RT dose over the traditional modest dose in the course of QD CCRT for LS-SCLC. However, it remains unclear whether this is applicable to patients with underlying lung disease because subgroup analysis did not show any benefit from RT > 54 Gy. Although no increase was observed in the rate of severe pulmonary toxicities in the RT > 54 Gy group, doses of 60 Gy may not be feasible for these frail patients. Although consensus guidelines recommend ≥ 60 Gy like for other types of gross solid tumors, SCLC is traditionally regarded as a radiosensitive tumor, and frail patients may not benefit from RT dose escalation. Because these patients showed poor OS probably due to the high risk of underlying disease progression and mortality from non-cancer causes, this risk could outweigh slight improvements in OS due to dose escalation. Kobayashi et al. [8] also reported that patients with ILD showed lower OS than those without such abnormalities (median OS, 19 months vs. 68 months; p=0.034) after CCRT for LS-SCLC.
Due to the low incidence of LS-SCLC with underlying lung disease, thoracic RT dose studies have not been conducted for these patients. In fact, there are many cases where standard CCRT is not recommended in the presence of ILD, even if it is recommended, radiation oncologists could be reluctant to treat with high doses. Therefore, it is difficult to gather adequate data related to RT dose escalation in these patients. Although few previous studies reported the unfavorable impact of underlying lung disease on OS in patients with LS-SCLC, heterogeneity with regard to RT dose/fractionation, chemotherapy, and types/severity of underlying lung disease (from just interstitial radiologic abnormality to severe idiopathic pulmonary fibrosis) made it difficult to interpret [8,11]. Song et al. [12] reported that GAP (gender, age, and physiology) stage was the only predictor for acute exacerbation of idiopathic pulmonary fibrosis in 59 patients with SCLC suggesting physicians to keep this in mind. Including our study, time-dependent PFT changes after CCRT and its impact on quality of life and OS in SCLC patients with underlying lung disease had been rarely known due to its rarity as well as irregular follow-up tests although similar topics had been dealt with in patients with non-small cell lung cancer. Hence, multicenter prospective studies for LS-SCLC with underlying lung disease will be needed to validate our findings and to further analyze detailed toxicity profiles related to severity and progression of underlying disease, using such as ILD-GAP index with regular PFT follow-up.
Many previous studies retrospectively compared regimens of approximately 60 Gy QD and 45 Gy/30 Fx BID and demonstrated similar survival outcomes and mixed toxicity profiles (generally more pneumonitis in the QD group and more esophagitis in the BID group) [13–16]. However, even retrospective data for QD RT dose comparisons are scarce since the publication of the Intergroup 0096 trial [1]. Tomita et al. [17] compared 45 Gy BID, < 54 Gy QD, and ≥ 54 Gy QD in 127 LS-SCLC patients. Similar to our finding, the median OS times were significantly different: 14 months in the < 54 Gy group and 41 months in ≥ 54 Gy group. However, they did not adjust for potential differences in patient/tumor characteristics in the different dose groups and did not perform subgroup analysis based on the presence of underlying lung disease. Patients were randomized to 45 Gy BID or 70 Gy QD in the CALGB 30610/RTOG 0538 phase III trial; however, the 61.2 Gy concomitant boost arm was terminated early after the interim analysis due to unfavorable toxicity compared with 70 Gy [18]. Subsequently, no direct comparison was made between the doses of 61.2 Gy and 70 Gy [5,19]. Therefore, our study is an important academic data as one of the few studies to compare QD doses.
Recently, a randomized phase 2 trial comparing 60 Gy BID versus 45 Gy BID suggested better OS in the 60 Gy dose escalation [20], indicating that QD dose escalation will continue to be attempted. However, the results of the RTOG 0617 dose escalation study in non–small cell lung cancer failed to show any benefit from 74 Gy escalation, which could be attributed to toxicity [21]. Moreover, the previous RTOG 9712 study examining the maximum tolerated dose of thoracic CCRT showed that 60% of patients in the 64.8 Gy arm developed grade 3 acute esophagitis, resulting in 61.2 Gy as maximum tolerated dose [22]. Another study analyzed 1,228 patients in the National Cancer Database who had been treated with CCRT for non-metastatic SCLC and reported similar median OS times of 21.5 and 20.2 months for 70 Gy and 61.2 Gy, respectively [23]. Some researchers also suggested hypofractionated RT in LS-SCLC. Zayed et al. [24] showed that 40–45 Gy/15–20 Fx, corresponding to 40–50 Gy EQD2 (equivalent dose in a 2-Gy fraction), demonstrated similar survival outcomes to conventional fractionation with a total dose of ≥ 58 Gy. They also reported similar effects of the hypofractionated RT and conventional fractionation on tumor and normal tissues despite important differences in the biological effective dose values. These studies raised the possibility of applying ≤ 60 Gy in some patients. Taken together, standard QD thoracic RT dose remains controversial in the treatment of LS-SCLC. However, considering that most clinicians prefer QD over BID fractionation [3], it is necessary to determine the standard dose for QD regimens despite insufficient evidence for the benefits of QD over BID fractionation regimens.
However, there are several limitations to our study. First, its retrospective nature is a major weakness as the patients were not randomized, and sources of bias were not fully controlled, although the use of PSM reduced some of the bias. Second, the small number of patients with underlying lung disease makes it difficult to clearly show the statistical differences according to RT dose escalation as well as to assess the impact of the severity of underlying COPD or ILD on subsequent pulmonary toxicities. Moreover, pulmonary function test after CCRT had not been routinely performed and the limitations of subgroup analysis necessitate cautious interpretation. Further external validation is warranted to support the results of the current analysis. Lastly, radiation-related toxicities, especially those of less than grade 3, may have been underestimated. However, this may not be a severe limitation as grade 3 or higher lung toxicity generally warrants steroid treatment, which would have been well documented in our dataset.
In conclusion, our analysis supports that the 60 Gy RT dose should be considered in the QD regimen of CCRT for LS-SCLC without lung disease, but RT dose > 54 Gy did not seem to benefit for patients with COPD or ILD. Further prospective study for these patients is needed to establish standard QD RT regimens for LS-SCLC.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This work was approved by the Institutional Review Board of Seoul National University Hospital (No. 1906-154-1044) and Seoul Metropolitan Government Seoul National University Boramae Medical Center (No. 10-2021-86) and carried out according to the principles of the Declaration of Helsinki. Informed consent was waived by the committee due to the retrospective and anonymized nature of the study. Author Contributions Conceived and designed the analysis: Kim BH, Kim HJ. Collected the data: Kim BH, Chung JH, Son J. Contributed data or analysis tools: Kim BH, Chung JH, Son J, Kim S, Wu HG, Kim HJ. Performed the analysis: Kim BH, Chung JH, Son J, Kim S, Wu HG, Kim HJ. Wrote the paper: Kim BH, Kim HJ. Fig. 1Kaplan-Meier graph by thoracic radiotherapy dose in propensity score matched patients: (A) progression-free survival (PFS) and (B) overall survival (OS). 
Fig. 2Exploratory subgroup analysis on overall survival evaluating the benefit of 60 Gy or higher thoracic radiotherapy dose during once-daily concurrent chemoradiotherapy. BMI, body mass index; COPD, chronic obstructive pulmonary disease; CTx, chemotherapy; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ILD, interstitial lung disease; PCI, prophylactic cranial irradiation. 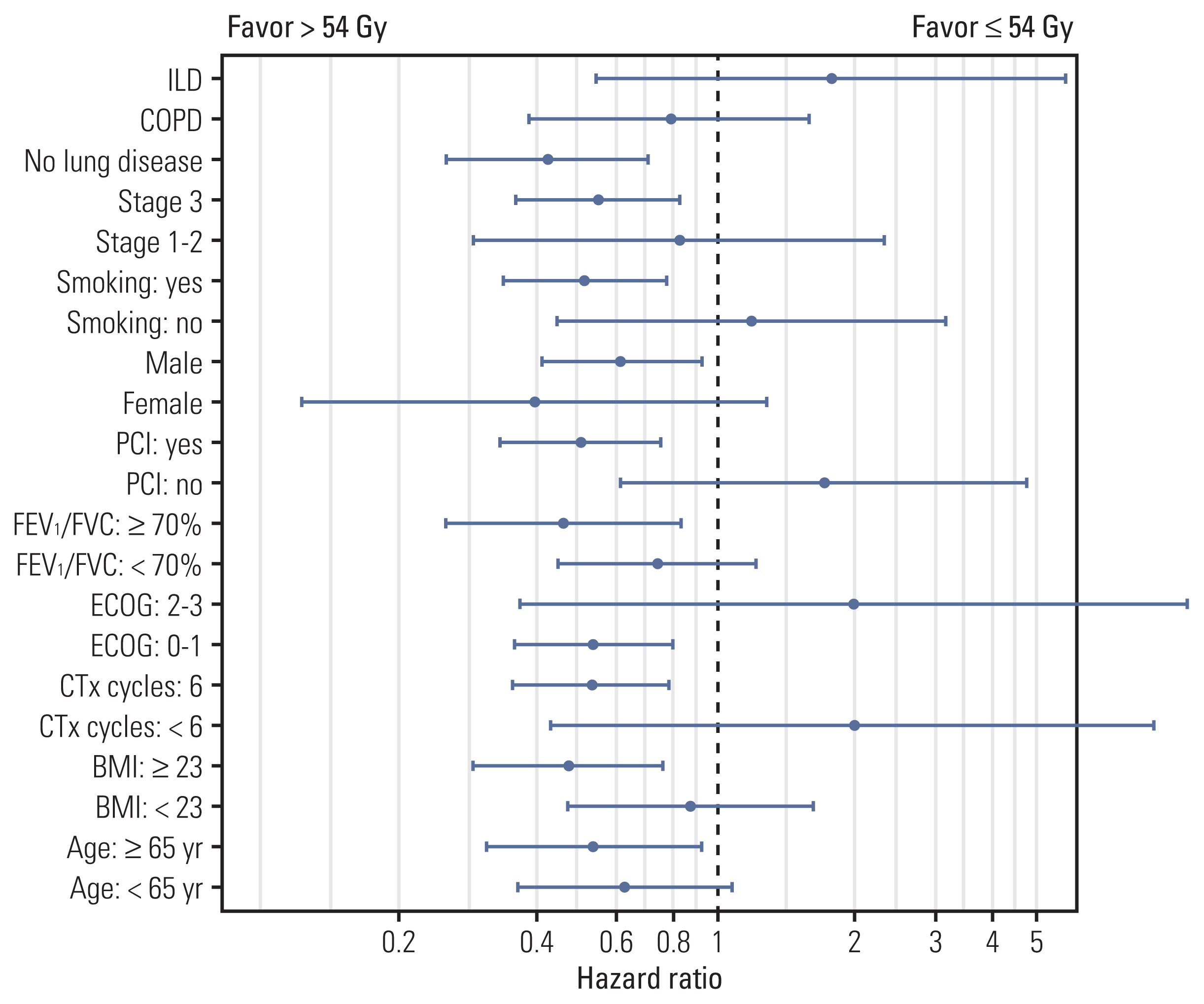
Fig. 3Overall survival (OS) graph in each lung disease group according to the thoracic radiotherapy dose in matched cohort (≤ 54 Gy and > 54 Gy): (A) without lung disease, (B) chronic obstructive pulmonary disease (COPD), and (C) interstitial lung disease (ILD). 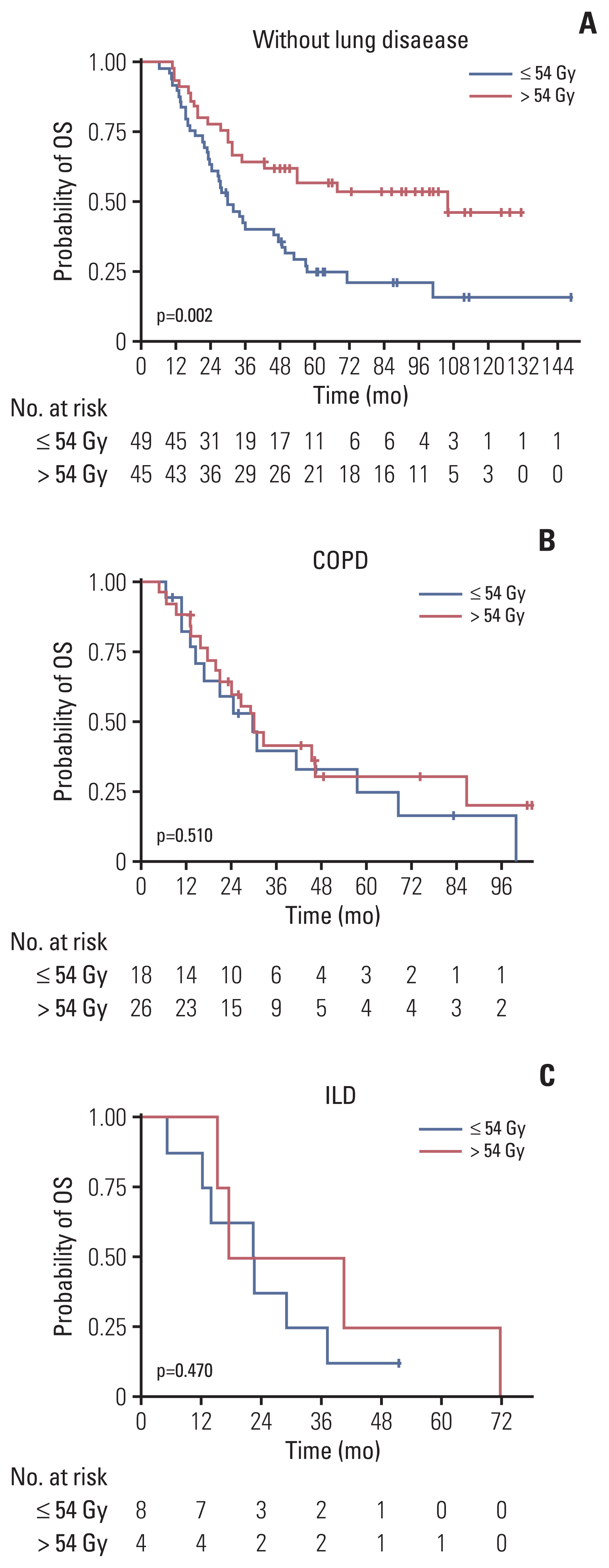
Table 1Comparison of patient characteristics according to the thoracic radiotherapy dose (≤ 54 Gy vs. > 54 Gy)
Values are presented as median (IQR), number (%), or mean±SD. 3D-CRT, three-dimensional conformal radiotherapy; COPD, chronic obstructive pulmonary disease; DLCO, carbon monoxide diffusion capacity; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ILD, interstitial lung disease; IMRT, intensity-modulated radiotherapy; IQR, interquartile range; NSE, neuron specific enolase; PCI, prophylactic cranial irradiation; RT, radiotherapy; SD, standard deviation. Table 2Univariate and multivariate analysis for PFS and OS in all patients (n=225) BMI, body mass index; CI, confidence interval; CTx, chemotherapy; DLCO, carbon monoxide diffusion capacity; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HR, hazard ratio; OS, overall survival; PCI, prophylactic cranial irradiation; PFS, progression-free survival; RT, radiotherapy. Table 3Severe pulmonary toxicities, baseline pulmonary function, and related planning parameters according to RT dose and underlying lung disease in all matched patients (n=150)
Values are presented as number (%) or mean±SD. COPD, chronic obstructive pulmonary disease; DLCO, carbon monoxide diffusion capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ILD, interstitial lung disease; RT, radiotherapy; RTOG, Radiation Therapy Oncology Group; SD, standard deviation. References1. Turrisi AT 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–71.
2. Schreiber D, Wong AT, Schwartz D, Rineer J. Utilization of hyperfractionated radiation in small-cell lung cancer and its impact on survival. J Thorac Oncol. 2015;10:1770–5.
3. Farrell MJ, Yahya JB, Degnin C, Chen Y, Holland JM, Henderson MA, et al. Radiation dose and fractionation for limited-stage small-cell lung cancer: survey of US radiation oncologists on practice patterns. Clin Lung Cancer. 2019;20:13–9.
4. Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–25.
5. Bogart JA, Wang XF, Masters GA, Gao J, Komaki R, Kuzma CS, et al. Phase 3 comparison of high-dose once-daily (QD) thoracic radiotherapy (TRT) with standard twice-daily (BID) TRT in limited stage small cell lung cancer (LSCLC): CALGB 30610 (Alliance)/RTOG 0538. J Clin Oncol. 2021;39(15 Suppl):8505.
6. Daly ME, Ismaila N, Decker RH, Higgins K, Owen D, Saxena A, et al. Radiation therapy for small-cell lung cancer: ASCO guideline endorsement of an ASTRO guideline. J Clin Oncol. 2021;39:931–9.
7. Stinchcombe TE, Fan W, Schild SE, Vokes EE, Bogart J, Le QT, et al. A pooled analysis of individual patient data from National Clinical Trials Network clinical trials of concurrent chemoradiotherapy for limited-stage small cell lung cancer in elderly patients versus younger patients. Cancer. 2019;125:382–90.
8. Kobayashi H, Wakuda K, Naito T, Mamesaya N, Omori S, Ono A, et al. Chemoradiotherapy for limited-stage small-cell lung cancer and interstitial lung abnormalities. Radiat Oncol. 2021;16:52.
9. Han TJ, Kim HJ, Wu HG, Heo DS, Kim YW, Lee SH. Comparison of treatment outcomes between involved-field and elective nodal irradiation in limited-stage small cell lung cancer. Jpn J Clin Oncol. 2012;42:948–54.
10. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–6.
11. Li F, Zhou Z, Wu A, Cai Y, Wu H, Chen M, et al. Preexisting radiological interstitial lung abnormalities are a risk factor for severe radiation pneumonitis in patients with small-cell lung cancer after thoracic radiation therapy. Radiat Oncol. 2018;13:82.
12. Song MJ, Lim SY, Park JS, Yoon HI, Lee JH, Kim SY, et al. Prognosis of small cell lung cancer with idiopathic pulmonary fibrosis: assessment according to GAP stage. J Oncol. 2019;2019:5437390.
13. Kim K, Moon S, Kim Y, Kim T, Cho K, Han J, et al. Treatment outcomes of limited-stage small cell lung cancer patients treated with concurrent chemoradiation therapy: a comparative analysis of different radiation dose fractionation schedules in a single institution. Int J Radiat Oncol. 2014;90(1 Suppl):S632.
14. Han D, Hao S, Tao C, Zhao Q, Wei Y, Song Z, et al. Comparison of once daily radiotherapy to 60 Gy and twice daily radiotherapy to 45 Gy for limited stage small-cell lung cancer. Thorac Cancer. 2015;6:643–8.
15. Watkins JM, Fortney JA, Wahlquist AE, Shirai K, Garrett-Mayer E, Aguero EG, et al. Once-daily radiotherapy to > or =59.4 Gy versus twice-daily radiotherapy to > or =45.0 Gy with concurrent chemotherapy for limited-stage small-cell lung cancer: a comparative analysis of toxicities and outcomes. Jpn J Radiol. 2010;28:340–8.
16. Gazula A, Baldini EH, Chen A, Kozono D. Comparison of once and twice daily radiotherapy for limited stage small-cell lung cancer. Lung. 2014;192:151–8.
17. Tomita N, Kodaira T, Hida T, Tachibana H, Nakamura T, Nakahara R, et al. The impact of radiation dose and fractionation on outcomes for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2010;76:1121–6.
18. Bogart J, Wang X, Masters G, Zhu H, Komaki R, Gaspar L, et al. Interim toxicity analysis for patients with limited stage small cell lung cancer (LSCLC) treated on the experimental thoracic radiotherapy (TRT) arms of CALGB 30610 (Alliance)/RTOG 0538. Ann Oncol. 2019;30:v711.
19. Bogart JA, Wang X, Masters GA, Gao J, Komaki R, Gaspar LE, et al. Short communication: interim toxicity analysis for patients with limited stage small cell lung cancer (LSCLC) treated on CALGB 30610 (Alliance)/RTOG 0538. Lung Cancer. 2021;156:68–71.
20. Gronberg BH, Killingberg KT, Flotten O, Brustugun OT, Hornslien K, Madebo T, et al. High-dose versus standard-dose twice-daily thoracic radiotherapy for patients with limited stage small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet Oncol. 2021;22:321–31.
21. Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-term results of NRG oncology RTOG 0617: standard-versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38:706–14.
22. Komaki R, Swann RS, Ettinger DS, Glisson BS, Sandler AB, Movsas B, et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer: report of Radiation Therapy Oncology Group (RTOG) protocol 97–12. Int J Radiat Oncol Biol Phys. 2005;62:342–50.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||