AbstractPurposeOsimertinib is a third-generation, irreversible, oral epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) that potently and selectively inhibits both EGFR sensitizing mutation and EGFR T790M and has demonstrated efficacy in non-small cell lung cancer (NSCLC) central nervous system (CNS) metastases. We present results of a subgroup analysis of Korean patients from the pooled data of two global phase II trials: AURA extension (NCT01802632) and AURA2 (NCT02094261).
Materials and MethodsEnrolled patients had EGFR T790M-positive NSCLC and disease progression during or after EGFR-TKI therapy. Patients received osimertinib 80 mg orally once daily until disease progression. The primary endpoint was objective response rate (ORR).
ResultsIn total, 66 Korean patients received osimertinib treatment with a median treatment duration of 19 months. In the evaluable-for-response population (n=62), ORR was 74% (95% confidence interval [CI], 61.5 to 84.5) and median duration of response was 9.8 months (95% CI, 7.1 to 16.8). In the full analysis set (n=66), median progression-free survival was 10.9 months (95% CI, 8.3 to 15.0; data cutoff November 1, 2016), and median overall survival was 29.2 months (95% CI, 24.8 to 35.7; data cutoff May 1, 2018). Eight patients with CNS metastases were evaluable for response, none of whom showed CNS progression. The most common adverse events were rash (53%), cough (33%), paronychia, diarrhea, and decreased appetite (each 32%).
IntroductionEpidermal growth factor receptor (EGFR) tyrosine kinase
inhibitors (TKIs) are the standard first-line treatment for patients with advanced non-small cell lung cancer (NSCLC) harboring an EGFR-TKI sensitizing mutation (EGFRm) [1-3].
The majority of patients who initially respond to EGFR-TKIs
resistance to a first- or second-generation EGFR-TKI, T790M is observed in approximately 50% [7].
Osimertinib is a third-generation, irreversible, oral EGFR-TKI that potently and selectively inhibits both EGFRm and EGFR T790M and has demonstrated efficacy in NSCLC central nervous system (CNS) metastases [8-12]. In a pooled analysis of two phase II studies, AURA extension (NCT0180-2632) and AURA2 (NCT02094261) [13-15], in patients with T790M positive advanced NSCLC and disease progression after prior EGFR-TKI therapy, high objective response rates (ORR; 66%; 95% confidence interval [CI], 61 to 70), and prolonged median progression-free survival (PFS; 9.9 months; 95% CI, 9.5 to 12.3) were observed with osimertinib. The pooled phase II results were consistent with that observed in the AURA3 phase III trial (NCT02151981), in which osimertinib therapy was compared with platinum-based chemotherapy plus pemetrexed in patients with T790M-positive NSCLC following disease progression on first-line EGFR-TKI. Median PFS was consistently longer with osimertinib: 10.1 versus 4.4 months (hazard ratio, 0.30; 95% CI, 0.23 to 0.41; p < 0.001) [12]. Osimertinib also provided a higher and more durable CNS response rate, and longer CNS PFS compared with platinum-pemetrexed [16].
Here we present results from a post-hoc analysis of the Korean subgroup from the two global phase II studies, AURA extension and AURA2, which investigated the efficacy and safety of osimertinib in patients with pre‑treated EGFRm advanced NSCLC with centrally determined T790M positive status. This subgroup was selected because Korean patients made up a significant proportion of the global population in these studies and, as in the overall East Asian population, the rate of EGFR mutation among patients with NSCLC is much higher in the Korean than in the Caucasian population (30%- 34% vs. 10%-17%) [17-20].
Materials and Methods1. Study designAURA extension and AURA2 were phase II, single-arm, open-label, multicenter studies. Full methodology for each study has been previously reported [13-15].
The study protocols were designed by the sponsor (Astra-Zeneca) and the study investigators.
2. Eligibility criteriaBoth studies enrolled patients who were 18 years or older with a histologically or cytologically confirmed diagnosis of NSCLC and T790M positive status with radiological disease progression on their most recent treatment regimen. Eligible patients had a World Health Organization (WHO) performance status of 0 or 1 and disease progression following previous EGFR-TKI therapy, either with or without additional anti-cancer regimens. Patients with spinal cord compression or brain metastases could be included if the disease was stable, asymptomatic and did not require steroids for 4 weeks prior to the first dose of study drug.
3. Study treatmentAll patients received osimertinib 80 mg orally once daily until disease progression (as defined by Response Evaluation Criteria in Solid Tumors [RECIST] ver. 1.1) or until a discontinuation criterion was met. Patients could continue to receive osimertinib after disease progression provided they showed clinical benefit (investigator assessed). If study treatment was discontinued for any reason other than disease progression, RECIST ver. 1.1 assessments continued every 6 weeks until disease progression, regardless of further treatment regimens.
4. Study endpointsThe primary endpoint of AURA extension and AURA2 was ORR by blinded independent central review (BICR). Additional efficacy endpoints included duration of response (DoR), PFS, and tumor shrinkage. Safety and tolerability, and overall survival (OS), were evaluated in all patients receiving at least one dose of osimertinib (full analysis set). Investigators recorded and graded adverse events (AEs) according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events ver. 4.0.
5. Study assessmentsTumor response was evaluated by BICR. Computed tomography or magnetic resonance imaging scans were taken at baseline, then every 6 weeks from the first dose of osimertinib until disease progression. RECIST ver. 1.1 was used to assess ORR, PFS, DoR, disease control rate and tumor shrinkage, and a sensitivity analysis was performed using investigator assessment of RECIST ver. 1.1.
6. Statistical analysisThe Korean subgroup comprised all patients from each study who were enrolled at Korean sites and treated with osimertinib. The full analysis set (FAS) was defined as all patients enrolled who received at least one dose of study treatment. The evaluable-for-response (EFR) analysis set was defined as all patients who received at least one dose of study treatment and had measurable disease at baseline according to BICR. Response endpoints including ORR, DoR and change in tumor size were analyzed in the EFR set, with a data cutoff of November 1, 2016. PFS, defined as the time from date of first dose until the date of objective disease progression or death (regardless of whether the patient withdrew from study treatment or received another anti-cancer therapy prior to progression), was analyzed in the FAS at data cutoff November 1, 2016. CNS methodology has been previously published [21]. Briefly, CNS efficacy was assessed in an evaluable-for-CNS-response analysis set, which included patients with at least one measurable CNS lesion on baseline brain scan (RECIST ver. 1.1) by BICR (assessed by neuroradiologists), with an earlier data cutoff of November 1, 2015. OS data were immature at the time of data cutoff for the primary efficacy analyses (November 1, 2016); therefore, OS was analyzed in the FAS at a later cutoff date of May 1, 2018. Safety and tolerability analyses are also presented for the later cutoff date of May 1, 2018, to maximize available safety data for this patient population.
Statistical analyses were performed by PPD (Wilmington, NC). All calculations were performed with SAS software ver. 9.2 (SAS Institute Inc., Cary, NC), unless otherwise stated.
7. Ethical statementAll participating sites in both studies obtained approval from their independent institutional review boards or independent ethics committees. The AURA extension and AURA2 studies were performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with International Conference on Harmonisation/Good Clinical Practice, applicable regulatory requirements, and AstraZeneca’s policy on bioethics. All patients provided written informed consent prior to their participation in the studies.
Results1. PatientsOf those patients included in the AURA2 and AURA extension studies, 66 of 411 (16%) were Korean. These patients were enrolled from seven sites and received osimertinib across the two phase II trials; 41 of 201 patients in the AURA extension study and 25 of 210 patients in the AURA2 study. Patient demographics and baseline characteristics are summarized in Table 1. The majority of patients were female (70%), never smokers (73%), with a median age of 60.5 years. The most recent therapy for 70% of patients was an EGFR-TKI and 56% had previously received platinum chemotherapy. 94% of patients had metastatic disease and 30% had CNS metastases.
2. EfficacyAt data cutoff for the primary analysis (November 1, 2016), the median duration of exposure to osimertinib was 19.3 months (range, 0.5 to 28.1 months; mean, 17.4 months); 19 patients (29%) were still receiving treatment.
Four patients’ results were not considered evaluable: one had an unconfirmed partial response, two had a complete response but had no measurable disease and one had no evaluable follow-up assessments. In the EFR Korean population (n=62), the ORR was 74% (46/62; 95% CI, 61.5 to 84.5). In the EFR, 2 of 62 patients (3%) had a best objective response of complete response, 44 of 62 patients (71%) had a partial response and 13 (21%) had stable disease ≥ 6 weeks (Table 2). Disease control rate in the EFR was 95% (59/62; 95% CI, 86.5 to 99.0). The mean best percentage change in target lesion size was –52% (Fig. 1). Among patients with an objective response (n=46), median time to response from first dose was 5.6 weeks and median DoR was 9.8 months (95% CI, 7.1 to 16.8) (Fig. 2A).
At the primary data cutoff (November 1, 2016), the median duration of follow-up was 9.6 months. In total, 45 of 66 patients (68%) had reported a progression event. Among the 21 patients with no disease progression at data cutoff according to investigator assessment, 13 patients were progression-free, four patients withdrew consent, and four patients had RECIST progression or death censored (RECIST-defined disease progression or death occurred 19 weeks after the last evaluable RESIST assessment). None of these patients were lost to follow up or discontinued treatment. The median PFS in the FAS was 10.9 months (95% CI, 8.3 to 15.0), and 80% of patients were alive and progression-free at 6 months, 46% at 12 months and 20% at 24 months (Fig. 2B).
In the FAS, 20 patients had CNS metastases at study entry, of whom eight were evaluable for CNS response after receiving osimertinib once daily. These patients’ demographics were closely aligned with the overall subset population: median age of 62 years, 75% female and all were Asian. The median lesion size in these evaluable patients was 20 mm (range, 10 to 41 mm), with a total of four (50%) having received any previous local treatment for brain metastases (excluding whole brain radiotherapy), assessed by medical review. Of the patients evaluable for response, three (38%) showed confirmed partial CNS response while the remaining five patients (63%) showed stable CNS disease of ≥ 6 weeks. None of the eight patients showed CNS disease progression at the time of data cutoff for the CNS efficacy analysis (November 1, 2015); median PFS for these eight patients was 4 months (95% CI, 2.7 to not calculable).
At the later data cutoff of May 1, 2018, the median duration of exposure to osimertinib was 19.3 months (range, 0.5 to 46.0 months; mean, 20.3 months); 7 patients (11%) remained on treatment. The median duration of follow-up was 27.9 months. A total of 44 patients (67%) had died. Median OS was 29.2 months (95% CI, 24.8 to 35.7; FAS). OS at 12, 24, and 36 months was 83%, 64%, and 37%, respectively.
3. SafetyAt least one AE was reported in all patients (100%, 66/66) (Table 3). Fifty-eight patients (88%) reported AEs that were possibly causally related to osimertinib (assessed by the investigator). AEs grade ≥ 3 were reported in 23 of 66 patients (35%), Grade ≥ 3 AEs possibly casually-related to osimertinib were reported in six patients (9%). There was one report of a fatal AE (cerebral infarction); however, this was not considered to be causally related to osimertinib.
The most commonly reported AEs were rash (grouped term including rashes and acnes; 53% of patients, 0% grade ≥ 3), pruritus (32%; 0% grade ≥ 3), cough (33%; 0% grade ≥ 3), paronychia (grouped term including general nail/nail bed conditions; 32%; 0% grade ≥ 3), diarrhea (32%; 2% grade ≥ 3) and decreased appetite (32%; 0% grade ≥ 3) (Table 4). One patient developed interstitial lung disease and one patient developed pneumonitis, both of which were grade 1 in severity and were considered to be possibly causally related to osimertinib by the investigator. Both events resolved on discontinuation of osimertinib. No patients had AEs of QT prolongation recorded.
DiscussionIn this subgroup analysis of Korean patients from two global phase II studies in pre-treated patients with T790M positive advanced NSCLC (AURA extension and AURA2), osimertinib therapy resulted in an ORR of 74% with a median DoR of 9.8 months and a median PFS of 10.9 months (as of data cutoff November 1, 2016). Longer-term follow-up (data cutoff May 1, 2018; median follow-up, 27.9 months) showed a median OS of 29.2 months. The safety profile of osimertinib in Korean patients was consistent with the overall safety profile of osimertinib [12-14], with a low rate of grade ≥ 3 possibly causally related AEs.
In the global phase II pooled analysis from AURA extension and AURA2 studies, the ORR was 66%, with a median DoR of 12.3 months and median PFS of 9.9 months, and median OS 26.8 months on prolonged follow-up [15]. In the phase III AURA3 trial, ORR and median DoR by investigator assessment with osimertinib were 71% and 9.7 months respectively, while median PFS by BICR and investigator assessment was 11.0 and 10.1 months, respectively, showing concordance between the two [12]. The efficacy results of the current analysis in a Korean population are consistent with the global population, with ORR slightly higher. This confirms that the efficacy of osimertinib is not different in patients of Korean ethnicity, and supports the recommendation for use of osimertinib in Korean patients with T790M positive advanced NSCLC following disease progression after EGFR-TKI therapy [2,17].
The AEs reported in this Korean subgroup were consistent with the global phase II and III studies [12-14]. Rash, diarrhea, paronychia, and dry skin have consistently been reported as the most common AEs with osimertinib treatment. For each of these AEs, the majority of cases reported were grade 1 or 2 in severity. Potential differences in reported AEs in the Korean subgroup compared with the global pooled population [15] were the overall frequencies of diarrhea (Korean subgroup 32% vs. global population 49%), dry skin (15% vs. 36%), pruritus (32% vs. 18%), platelet count decreased (27% vs. 14%), and anemia (27% vs. 18%). However, comparisons should be viewed with caution because of the small number of patients in this Korean subgroup.
Brain metastases have been reported to occur in 25%-40% of patients with EGFR mutation–positive NSCLC [22]. Phase II and III studies with osimertinib report CNS activity in patients with T790M positive NSCLC [13,16,21]. The CNS activity in this Korean subgroup appears to be consistent with previous reports, supporting the use of osimertinib as treatment of choice for patients with CNS metastases and EGFRm NSCLC.
A limitation of this analysis is the small number of Korean patients that were enrolled and received osimertinib. However, the efficacy, safety, and tolerability profiles of osimertinib in this Korean subgroup were consistent with the global phase II studies, which is indicative of the broadly beneficial effects of osimertinib in the wider Korean population.
In conclusion, osimertinib provides a high response rate and prolonged PFS and OS, with a low incidence of grade ≥ 3 AEs in Korean patients with T790M-positive advanced NSCLC.
Conflicts of InterestDong-Wan Kim reports personal expenses from Novartis. Jin-Hyoung Kang reports research funding from AstraZeneca, during the conduct of the study; research funding from CKD, Ono Pharmaceutical, and Johnson & Johnson; advisory board participation for AstraZeneca, Eli Lilly, Merck Sharp & Dohme, and Genexin; honoraria from Roche, Novartis, Merck Sharp & Dohme, Ono Pharmaceutical, and Bristol-Myers Squibb, outside the submitted work. James Chih-Hsin Yang reports personal fees from Boehringer Ingelheim, Eli Lilly, Bayer, Roche/Genentech, Chugai, Astellas, Merck Sharp & Dohme, Merck Serono, Pfizer, Novartis, Clovis Oncology, Celgene, Merrimack, Yuhan Pharmaceuticals, Bristol-Myers Squibb, Ono Pharmaceuticals, Daiichi Sankyo, Astrazeneca, and Hansoh Pharmaceuticals, outside the submitted work. Tetsuya Mitsudomi Reports Personal Fees From Astrazeneca, During The Conduct Of The Study; Grants And Personal Fees From Boehringer Ingelheim, Grants And Personal Fees From Chugai, Personal Fees From Roche, Personal Fees From Pfizer, Grants And Personal Fees From Merck Sharp & Dohme, Grants And Personal Fees From Ono Pharmaceutical, Personal Fees From Bristol-myers Squibb, And Grants And Personal Fees From Taiho, Outside The Submitted Work. The other authors report no conflicts of interest. AcknowledgmentsThe authors would like to thank the patients and their families. The studies NCT01802632 and NCT02094261 were funded by Astra-Zeneca, Cambridge, UK, the manufacturer of osimertinib. The authors would like to acknowledge Bernadette Tynan, MSc, of iMed Comms, Macclesfield, UK, an Ashfield Company, part of UDG Healthcare plc, for medical writing support that was funded by AstraZeneca, Cambridge, UK, in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Fig. 1.Waterfall plot of target lesion size, best percentage change from baseline by blinded independent central review (evaluable-for-response set). Best percentage change in target lesion size is the maximum reduction from baseline or the minimum increase from baseline in the absence of a reduction. Data cutoff November 1, 2016. 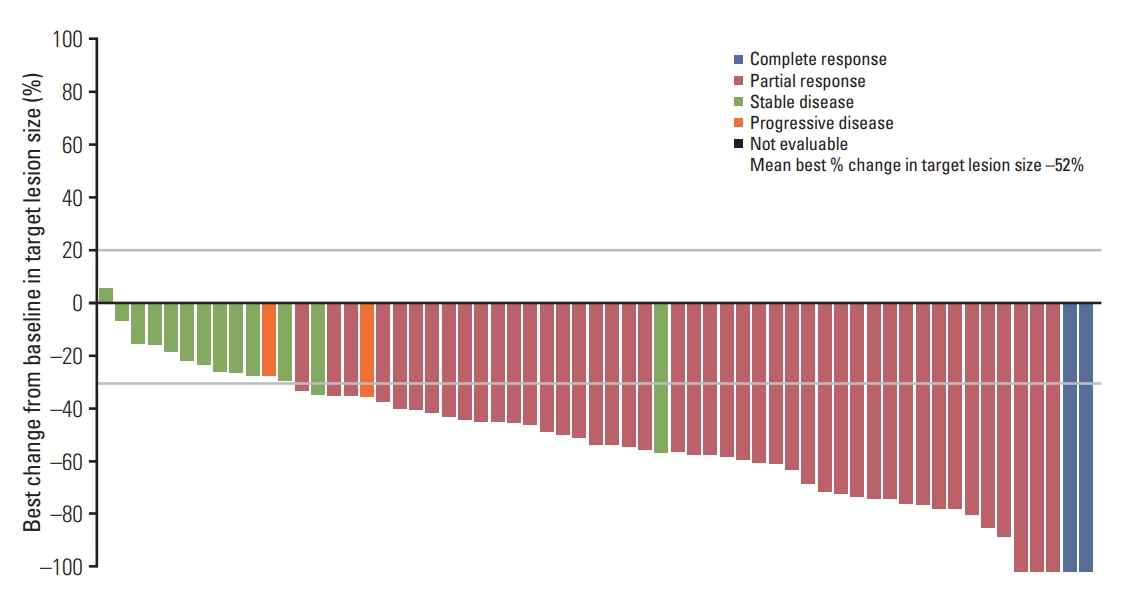
Fig. 2.Kaplan-Meier analysis of osimertinib 80 mg once daily treatment in Korean patients from AURA extension and AURA 2 studies. (A) Duration of response (DoR) by blinded independent central review (evaluable-for-response set) by study and total. (B) Progression-free survival (PFS) by blinded independent central review (full analysis set) by study and total. Tick marks indicate censored observations. Data cutoff November 1, 2016. CI, confidence interval. 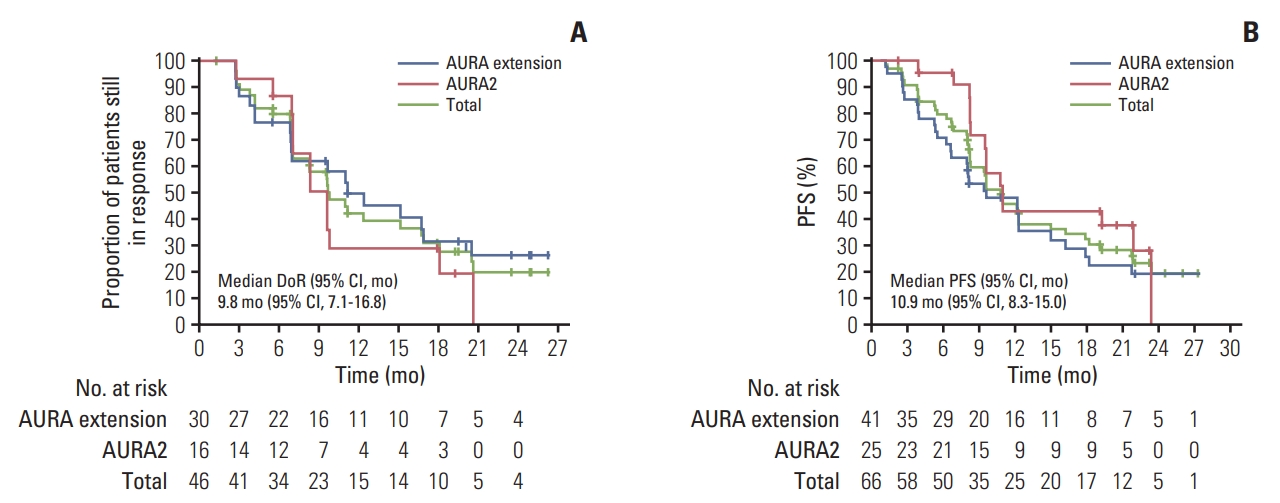
Table 1.Baseline demographics and disease characteristics, full analysis set
Values are presented as number (%) unless otherwise indicated. WHO, World Health Organization; NOS, not otherwise specified; EGFR, epidermal growth factor receptor; CNS, central nervous system; TKI, tyrosine kinase inhibitor. Table 2.Best objective response in the evaluable-for-response analysis set
Table 3.Summary of adverse events (full analysis set)
Table 4.All causality adverse events occurring in ≥ 10% of patients (full analysis set)
References1. Tan DS, Yom SS, Tsao MS, Pass HI, Kelly K, Peled N, et al. The International Association for the Study of Lung Cancer Consensus Statement on optimizing management of EGFR mutation-positive non-small cell lung cancer: status in 2016. J Thorac Oncol. 2016;11:946–63.
2. Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2017;35:3484–515.
3. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–237.
4. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as firstline treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46.
5. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57.
6. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34.
7. Wang ZF, Ren SX, Li W, Gao GH. Frequency of the acquired resistant mutation T790 M in non-small cell lung cancer patients with active exon 19Del and exon 21 L858R: a systematic review and meta-analysis. BMC Cancer. 2018;18:148.
8. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–61.
9. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFRmutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
10. Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS efficacy of osimertinib in patients with T790Mpositive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36:2702–9.
11. Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:3290–7.
12. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40.
13. Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017;35:1288–96.
14. Goss G, Tsai CM, Shepherd FA, Bazhenova L, Lee JS, Chang GC, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–52.
15. Ahn MJ, Tsai CM, Shepherd FA, Bazhenova L, Sequist LV, Hida T, et al. Osimertinib in patients with T790M mutationpositive, advanced non-small cell lung cancer: long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer. 2019;125:892–901.
16. Mok T, Ahn MJ, Han JY, Kang JH, Katakami N, Kim H, et al. CNS response to osimertinib in patients (pts) with T790M-positive advanced NSCLC: data from a randomized phase III trial (AURA3). J Clin Oncol. 2017;35(15 Suppl):9005.
17. Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v1–27.
18. Douillard JY, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, singlearm study. Br J Cancer. 2014;110:55–62.
19. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46.
20. Lee SH, Kim WS, Choi YD, Seo JW, Han JH, Kim MJ, et al. Analysis of mutations in epidermal growth factor receptor gene in Korean patients with non-small cell lung cancer: summary of a nationwide survey. J Pathol Transl Med. 2015;49:481–8.
|
|