AbstractPurposeThis study aimed to report the final analysis of time-on-treatment (TOT) and overall survival (OS) in patients with advanced-stage epidermal growth factor receptor (EGFR)+ non–small cell lung cancer (NSCLC) who received sequential afatinib and osimertinib and to compare the outcomes with other second-line regimens (comparator group).
Materials and MethodsIn this updated report, the existing medical records were reviewed and rechecked. TOT and OS were updated and analyzed according to clinical features using the Kaplan-Meier method and log-rank test. TOT and OS were compared with those of the comparator group, in which most patients received pemetrexed-based treatments. A multivariable Cox proportional hazard model was used to evaluate features that could affect survival outcomes.
ResultsThe median observation time was 31.0 months. The follow-up period was extended to 20 months. A total of 401 patients who received first-line afatinib were analyzed (166 with T790M+ and second-line osimertinib, and 235 with unproven T790M and other second-line agents). Median TOTs on afatinib and osimertinib were 15.0 months (95% confidence interval [CI], 14.0 to 16.1) and 11.9 months (95% CI, 8.9 to 14.6), respectively. The median OS in the osimertinib group was 54.3 months (95% CI, 46.7 to 61.9), much longer than that in the comparator group. In patients who received osimertinib, the OS was longest with Del19+ (median, 59.1; 95% CI, 48.7 to 69.5).
IntroductionLung cancer is the leading cause of death worldwide, accounting for almost 22% of all cancer–related deaths in males and 14% in females [1]. There is an increasing trend in lung cancer incidence in Eastern Asia, especially in the female population [2]. Although the most common histological type of lung cancer may vary between countries, adenocarcinoma is currently more prevalent than squamous cell carcinoma [3]. The incidence of adenocarcinoma in females continues to rise in several countries, while it remains stable in males [4]. A recent study in South Korea reported an increasing rate of adenocarcinoma and a decreasing trend of squamous cell carcinoma [5].
The selection of treatment strategy is an integral part of cancer management because it can lead to a significant improvement in survival outcomes. Tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib, and afatinib, have been the mainstay for the management of advanced non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations. The FLAURA phase 3 trial recommended single-agent osimertinib as the preferred first-line treatment for advanced EGFR+ NSCLC [6]. However, the question remains whether osimertinib should be administered as the first- or second-line treatment following first- or second-generation TKIs.
The T790M mutation is the most common resistance mechanism to first-generation (erlotinib and gefitinib) and second-generation (afatinib) TKIs during first-line treatment [7]. In the AURA3 trial, osimertinib as a second-line treatment demonstrated a striking effect against the T790M mutation [8]. Given that post-osimertinib treatment is challenging and the drug is not reimbursable as a first-line treatment in some countries such as South Korea, many clinicians would reserve osimertinib for T790M positive progression.
At present, few studies, including randomized controlled trials and real-world reports, are available on the activity of sequential TKI treatments in EGFR+ advanced NSCLC. For example, a post-hoc analysis of the LUX-Lung 7 study reported a 3-year survival rate of greater than 90% in patients who received sequential afatinib and osimertinib [9]. GioTag and UpSwinG are other studies showing the effectiveness of sequential afatinib and osimertinib therapies in real-world practice [10,11]. However, these studies might be limited by the small number of Asian patients and lack of a comparator group. Given the ethnic differences in the clinical effects of EGFR-TKIs, there is still a paucity of data on sequential afatinib and osimertinib treatment in Asian populations.
The real-world multicenter RESET study from South Korea might have provided an insight into the optimal sequence of EGFR-TKIs in Asian populations by investigating more than 700 advanced EGFR+ NSCLC patients [12]. The preliminary results were promising with time-on-treatment (TOT) on first-line afatinib of 15.7 months and TOT on second-line osimertinib treatment for 11.9 months. Overall survival (OS) was not achieved in patients receiving afatinib and subsequent osimertinib treatment. Many patients were still on the afatinib and osimertinib treatment. Therefore, it would be worthwhile to report updated patient data in the RESET study.
Materials and Methods1. Datasets and patient selectionThe design of the RESET study has been described previously [12]. In brief, RESET was a retrospective observational study conducted across 16 medical centers in South Korea. This study was designed to evaluate the real-world effectiveness of sequential afatinib and osimertinib treatments in patients with advanced EGFR+ NSCLC. Currently, osimertinib is the only approved EGFR-TKI after the failure of first-line TKIs in patients in South Korea. In a previous report, 164 patients were still receiving treatment, and 56 patients continued osimertinib at the final follow-up. Therefore, we expanded the final follow-up date from October 2020 to 30 June 2022 and collected the updated survival outcome for those patients. In addition, we rechecked the information on the clinical, molecular, and histologic features as well as the data regarding treatment outcomes, such as the date of treatment initiation and discontinuation and the occurrence of treatment-related events.
The data-processing flow is illustrated in Fig. 1. The original cohort dataset comprised 735 patients. In the first selection process, 289 patients were excluded for the following reasons: 54 continued afatinib, 12 had unavailable updated data, 77 were transferred or lost to follow-up, and 148 had no data for second-line treatment. In the next step, in which patients had information on both first- and second-line treatment, 45 patients were excluded: 26 received osimertinib without evidence of T790M and 19 were administered drugs other than osimertinib despite having T790M+. Consistent with a previous report, we classified the patients according to whether they received osimertinib as a second-line treatment. All patients in the osimertinib group 100% presented with the T790M mutation after afatinib failure. All patients in the comparator group were negative or unproven for T790M and received other therapies.
2. VariablesIn our previous report, we investigated features, including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking status (never, former, and current), tissue type of NSCLC at the initial diagnosis (adenocarcinoma or others), type and presence of EGFR mutation (deletion 19 [Del19], L858R, and others), which was detected using the peptic nucleic acid-mediated real-time polymerase chain reaction clamping method (Panagene, Daejeon, Korea) or the Roche Cobas EGFR mutation test (Roche Molecular Systems, Pleasanton, CA), tumor stage assessed by the eighth edition of the American Joint Committee on Cancer (AJCC) stating manual, number of metastatic organs, presence of metastasis in specific organs, type of brain metastasis at the initial work-up, change in brain metastasis during afatinib, and dose adjustment for afatinib. In addition, we also collected information on anthropometric indices, such as height and weight, longest tumor diameter, and TNM stage at the initial diagnosis.
3. OutcomesThe analysis of treatment-related outcomes was exploratory. The primary purpose of this updated report was to expand the final follow-up period to 20 months, as mentioned above. OS was defined as the length of time from the initiation of first-line afatinib therapy to death from any cause. TOT was also updated; the period was estimated for first-line afatinib and second-line osimertinib or other therapies, separately. TOT was defined as the period between the start of treatment and discontinuation of the drug for any reason, including tumor progression, drug toxicity, or death.
4. StatisticsClinical characteristics were summarized as numbers with percentages for categorical variables and means with standard deviations for continuous variables. Clinical features were compared using chi-square test or Fisher’s exact test for categorical features and Student’s t test for continuous variables.
TOT and OS were estimated and visualized using the Kaplan-Meier method. The log-rank test was used to compare the differences between survival outcomes within the categorical variables. The median period (months) and 95% confidence interval (CI) were also measured. TOT and OS were updated in both the afatinib→osimertinib and comparator groups.
Additionally, a subgroup analysis was performed by stratifying the types of second-line treatments other than osimertinib. At the time of primary data collection, we observed that most of the patients received pemetrexed alone or in combination with platinum-based agents as second-line agents. OS was compared between osimertinib vs. pemetrexed-containing regimens, and osimertinib vs. pemetrexed-platinum doublet vs. pemetrexed monotherapy.
The Cox proportional hazard model was used to identify the features that could affect TOT and OS. Multivariable analyses were performed using factors with p < 0.1 in the univariable model.
All statistical analyses were performed using R software ver. 4.2.2 for Windows (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS Statistics for Windows ver. 25.0 (IBM Corp., Armonk, NY).
ResultsA total of 401 patients across 16 medical centers were included in the analysis. The median observation time was 31.0 months (interquartile range, 19.8 to 45.9). The male and female patients were equally distributed (Table 1). Patients who received osimertinib as second-line treatment had a higher AJCC stage than those who received other therapies. The rate of Del19+ cells was higher in the osimertinib-treated group. Patients who received osimertinib had a higher rate of recurrence or new detection of brain metastasis more frequently than those in the comparator group.
The median TOT during afatinib was 15.0 months (95% CI, 14.0 to 16.1) (Table 2, S1 Fig.). Median TOT during afatinib was 16.6 (95% CI, 15.2 to 18.0) in the osimertinib-treated group and 13.9 (95% CI, 12.4 to 15.3) in the comparator group with p of 0.043 (data not shown). The TOT during osimertinib was 11.9 months (95% CI, 8.9 to 14.6) (Table 2, S2 Fig.), which was significantly longer than that in patients who received other treatments (5.1 months; 95% CI, 4.2 to 5.9) with a p-value of < 0.001. TOT during afatinib treatment was longer in patients with a Del19 mutation (15.7 months; 95% CI, 14.1 to 17.3) than in patient with a L858R mutation or other mutations (p=0.037). However, the period did not differ significantly between the types of mutations during osimertinib treatment.
Updated median OS in all 401 patients were estimated as 49.1 months (95% CI, 43.6 to 54.6). The median OS in patients who received sequential afatinib and osimertinib was 54.3 months (95% CI, 46.7 to 61.9) (Table 3, Fig. 2). The OS was longer in patients who received osimertinib as second-line treatment than in patients who received other regiments (41.3 months; 95% CI, 32.9 to 49.8; p=0.019). OS was the longest in patients with a Del19 mutation (59.1 months; 95% CI, 48.7 to 69.5).
The multivariable Cox proportional hazard model showed that poor ECOG PS, histologic types other than adenocarcinoma, EGFR mutations other than Del19 and L858R, higher numbers of metastatic organs, and no dose adjustment during afatinib treatment were related to an increased risk of poor TOT (Table 4). Meanwhile, in terms of OS, the hazard ratio was higher in patients with poor PS and the presence of liver metastasis during afatinib and osimertinib treatments (Table 5). In patients receiving afatinib followed by other regimens, other types of EGFR mutations, liver metastasis, and no dose adjustment during first-line afatinib were associated with a decrease in OS.
The types of second-line agents other than osimertinib in the comparator groups are summarized in S3 Table. As noted above, most patients (n=146, 66.7%) were treated with pemetrexed-based treatments: 74 (33.3%) received pemetrexed-platinum doublet and 74 (33.3%) received pemetrexed monotherapy. Patients who received sequential afatinib and osimertinib showed longer OS (median, 54.3 months; 95% CI, 48.5 to not available [NA]) than those who received pemetrexed-containing regimens (median, 41.7 months; 95% CI, 36.7 to 67.3; p=0.039) for 12.6 months numerically (Fig. 3, S4 Table). In the comparator group, pemetrexed-platinum doublet therapy showed longer OS than pemetrexed monotherapy, although both regimens conferred shorter OS than the osimertinib group (p=0.005) (Fig. 4, S4 Table). However, when comparing patients administered osimertinib with pemetrexed-platinum doublet therapy, a statistical significance was not reached (p=0.6), although median OS was numerically longer in osimertinib group of 54.3 months than in doublet group (50.0 months; 95% CI, 37.6 to NA).
DiscussionThe RESET study is the first multicenter study in South Korea to report survival outcomes in patients with advanced EGFR+ NSCLC who received sequential afatinib and osimertinib treatment. All patients who received osimertinib as a second-line treatment were all T790M positive. This study has several strengths. First, this final analysis of RESET is one of the largest studies in Asian populations analyzing the real-world effectiveness of osimertinib after afatinib treatment. Second, the RESET study brought the comparator group into the survival outcome analysis, which was absent in other real-world studies. Sequential afatinib and osimertinib were superior to other agents, mostly pemetrexed-based treatments, although the presence of the T790M mutation is the key to deciding the second-line treatment. Third, the survival outcomes were subject to comprehensive analyses based on various clinical factors. The encouraging activity of sequential afatinib and osimertinib in real-world data supports the feasibility of applying this treatment sequence in clinical practice by reserving osimertinib as a second-line regimen. Further scrutiny of prospective clinical trials is required to apply our results to real-world clinical practice. For example, a randomized open-label phase 4 trial in Germany, AFAMOSI, is going to evaluate the efficacy of afatinib followed by osimertinib in treatment-naïve patients with EGFR+ and T790M non-squamous NSCLC (NCT04413201).
The primary objective of RESET is to report the updated OS. The median OS was not reached in our previous study, and the updated median OS herein was 54.3 months. In terms of sequential afatinib and osimertinib treatment, only a few prospective studies have reported OS in patients with EGFR + NSCLC who received TKIs in that sequence. For example, median OS was ‘not evaluable’ with afatinib versus 46.0 months with gefitinib in patients who received the following osimertinib in a sub-analysis of the LUX-Lung 7 trial [9]. However, only 20 patients who were treated with afatinib received osimertinib as second-line treatment, and 23 patients treated with gefitinib received osimertinib.
In this final report, the estimated median TOT was 15.0 months on afatinib and 11.9 months on osimertinib. The results from previous randomized controlled trials substantiate the findings of RESET. The median duration of afatinib was 13.7 months in the post-hoc analysis of the LUX-Lung 7 trial [9]. First-line afatinib in the Asian population demonstrated a median progression-free survival of 11.0 months in the LUX-Lung 6 trial [13]. For osimertinib, the results from the phase 3 AURA trial showed that the median period of progression-free survival was 10.1 months in patients who received the drug after disease progression with first-line TKIs [8]. However, only 20 patients (7%) were treated with afatinib before osimertinib treatment. A subgroup analysis of the AURA 3 study in 63 Japanese patients showed slightly longer period of progression-free survival of 12.5 months [14].
Interestingly, patients who received pemetrexed-platinum doublet therapy as a second-line treatment had a longer OS period than those who received pemetrexed monotherapy. This observation does not necessarily imply that combination therapy is superior to monotherapy. Patients in the former were 3.8 years younger than those in the latter, and the proportion of patients with ECOG PS ≥ 2 was 12.3% in the combination group versus 6.7% in the monotherapy group. A phase 2 randomized clinical trial comparing the efficacy of pemetrexed-carboplatin doublet versus pemetrexed monotherapy as a second-line treatment in patients with advanced NSCLC yielded similar findings [15]. Patients who received combination therapy had a significantly longer progression-free survival.
Given the lack of data from prospective trials, evidence from real-world practice may provide additional insights into the optimization of treatment sequences within TKIs. Currently, except for RESET, two global multinational observational studies are available. The UpSwinG study enrolled 191 patients across nine countries with advanced EGFR+ NSCLC who were treated with first-line afatinib, following the detection of T790M, and second-line osimertinib [11]. The study analyzed 118 Asians, whereas our study included 166 South Koreans. In the UpSwinG study, the median OS in Asian patients was 42.3 months (95% CI, 33.2 to 63.5), which is slightly shorter than the OS in our study. Another global, multinational non-interventional study was GioTag [10]. However, GioTag only involved 50 Asian patients with a median OS of 44.8 months (95% CI, 37.0 to 57.8).
These real-world experiences show that the survival benefit is particularly promising in Asian patients with Del19+ disease. In GioTag study, the median OS in Asians with Del19+ versus all patients with Del19+ was 44.8 months (90% CI, 37.0 to 57.8) versus 41.6 months (90% CI, 36.9 to 45.0) [10]. A combined analysis of GioTag and UpSwinG studies showed that the median OS in Asian patients were significantly different between Del19 (n=109) and L858R (n=59) mutations; 63.5 months (95% CI, 42.3 to 71.1) and 39.1 (95% CI, 29.3 to 48.5), respectively [16]. These findings are comparable with the results from the updated RESET report, where 98 patients tested positive for Del19 and 55 were positive for L858R (Table 6). In a group received sequential afatinib and osimertinib therapy, the median OS was 59.1 months for the Del19+ and 46.5 months for L858R+ mutation. Consequently, our data support the notion that sequential afatinib treatment followed by osimertinib is an effective therapeutic option in Asian patients with advanced EGFR+ NSCLC, especially those with Del19+.
In addition, the authors of the above two real-world studies noted that they were largely limited by the lack of comparator arms. In this regard, our study additionally investigated specific regimens that were used as second-line treatments. Numerically, patients treated with osimertinib showed a 13.0-month extension of OS compared to the comparator group and 12.6-month extension to the pemetrexed-containing treatments. However, it should be noted that the T790M mutation was not detected or unproved in the comparator group. Several studies have reported similar results for the RESET. Progression-free survival was longer in patients with T790M+ NSCLC after initial TKI failure than in patients with T790M negativity [17]. In addition to second-line treatment, T790M mutation expression is associated with indolent progression during first-line treatment with both TKIs and chemotherapy agents [18]. These clinical results were further supported by experimental models. Cells harboring the T790M mutation showed a slower rate of growth in a preclinical study [19]. Mice expressing T790M showed a longer latency to tumorigenesis than those expressing other EGFR mutations [20].
Currently, there are no approved targeted treatments for patients who experience disease progression after osimertinib treatment. Platinum-based doublet chemotherapy may be the next step for these patients. A phase 1/1b Chrysalis-2 trial is an effort to examine post-osimertinib treatments using lazertinib as monotherapy or in combination with amivantamab (NCT04077463). A substantial proportion of patients receiving osimertinib develop resistance, despite a durable response. Especially, C797S point mutation in exon 20 is particularly important for osimertinib resistance [21], accounting for 10%–26% of cases of resistance after second-line osimertinib [8]. However, TKI resistance mechanisms may differ in the presence of osimertinib or afatinib. In an in vitro examination, mutations developed differently between cancer cells exposed to either osimertinib or afatinib [22]. Another preclinical examination support that a combination of osimertinib and afatinib rather than either drug alone was more effective in an appearance of drug-resistant cells [22].
The controversy regarding the optimal sequence of osimertinib may be intensified by the results of the FLAURA study: an updated OS in patients received first-line osimertinib was 38.6 months in the first-line osimertinib group [23], which was much shorter than the RESET. There could be several explanations around this difference. First, while 68% of patients who received osimertinib as a first-line treatment in the FLAURA study administered cytotoxic chemotherapy as a second-line treatment [23], patients in RESET study received two subsequent EGFR-TKIs, afatinib followed by osimertinib. Second, the medical environment and a health-care system in South Korea is generally considered to be advanced and of a high standard. According to a study of cancer statistics published in South Korea, cancer survival rates were generally higher than those in other countries [24]. Third, as we have shown in Table 6, survival data of RESET study was comparable to the previous real-world studies. Although our results are based on a retrospective design, relatively enough patients were analyzed, and survival periods such as TOT and OS were comprehensively estimated according to various clinical features. Our findings suggest that sequential therapy with afatinib followed by osimertinib is effective and could potentially become an option for patients with advanced EGFR+ NSCLC.
Despite the strengths of our study, because of the inherent nature of the retrospective study, it has several limitations, as noted in a previous report [12]. Selection bias could exist, since this study was restricted to South Korea, where osimertinib is reimbursable only for patients in whom first-line EGFR-TKI failed and T790M upon re-biopsy subsequently tested positive, and this issue could not be corrected. Further studies investigating the survival outcomes after first- and second-line osimertinib treatment would be valuable, given that osimertinib is the preferred first-line treatment option for advanced EGFR+ NSCLC based on the FLAURA study findings [7]. Misclassification may also occur. We attempted to minimize this problem by reviewing and rechecking the collected data. Survival data were not mature in our previous report, but we expanded the observation period up to 20 months. Another study limitation was the lack of data on adverse events, which might have affected the accuracy and completeness of our findings regarding drug safety and tolerability.
In this study, a final analysis of the RESET was conducted. Patients receiving osimertinib rather than other agents, such as pemetrexed-platinum doublet, as second-line treatments, had longer survival outcomes. Reserving osimertinib for second-line use after failure of first-line afatinib could be a feasible strategy in Asian patients with EGFR+ advanced NSCLC, particularly for those with Del19+. This report suggests that using first- or second-generation TKIs followed by osimertinib could potentially provide a survival benefit in advanced NSCLC patients. However, further prospective trials are required to confirm this strategy and determine the best approach to improving survival outcomes, quality of life, and tolerability in NSCLC patients receiving TKIs.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The study for the updated analysis was approved by the Institutional Review Board of the Kosin University Gospel Hospital (KUGH no. 2022-06-038). The study was conducted following the Declaration of Helsinki. All procedures were performed in accordance with relevant guidelines and regulations. The need for informed consent was waived as this study was in retrospective nature. Author Contributions Conceived and designed the analysis: Kim T, Jang TW. Collected the data: Jang TW, Choi CM, Kim MH, Lee SY (Sung Yong Lee), Chang YS, Lee KY, Kim SJ, Yang SH, Ryu JS, Lee JE, Lee SY (Shin Yup Lee), Park CK, Lee SH, Jang SH, Yoon SH, Oh HJ. Contributed data or analysis tools: Kim T. Performed the analysis: Kim T. Wrote the paper: Kim T. Fig. 1Patient selection process. AJCC, American Joint Committee on Cancer; EGFR, epidermal growth factor receptor; NSCLC, non–small cell lung cancer. 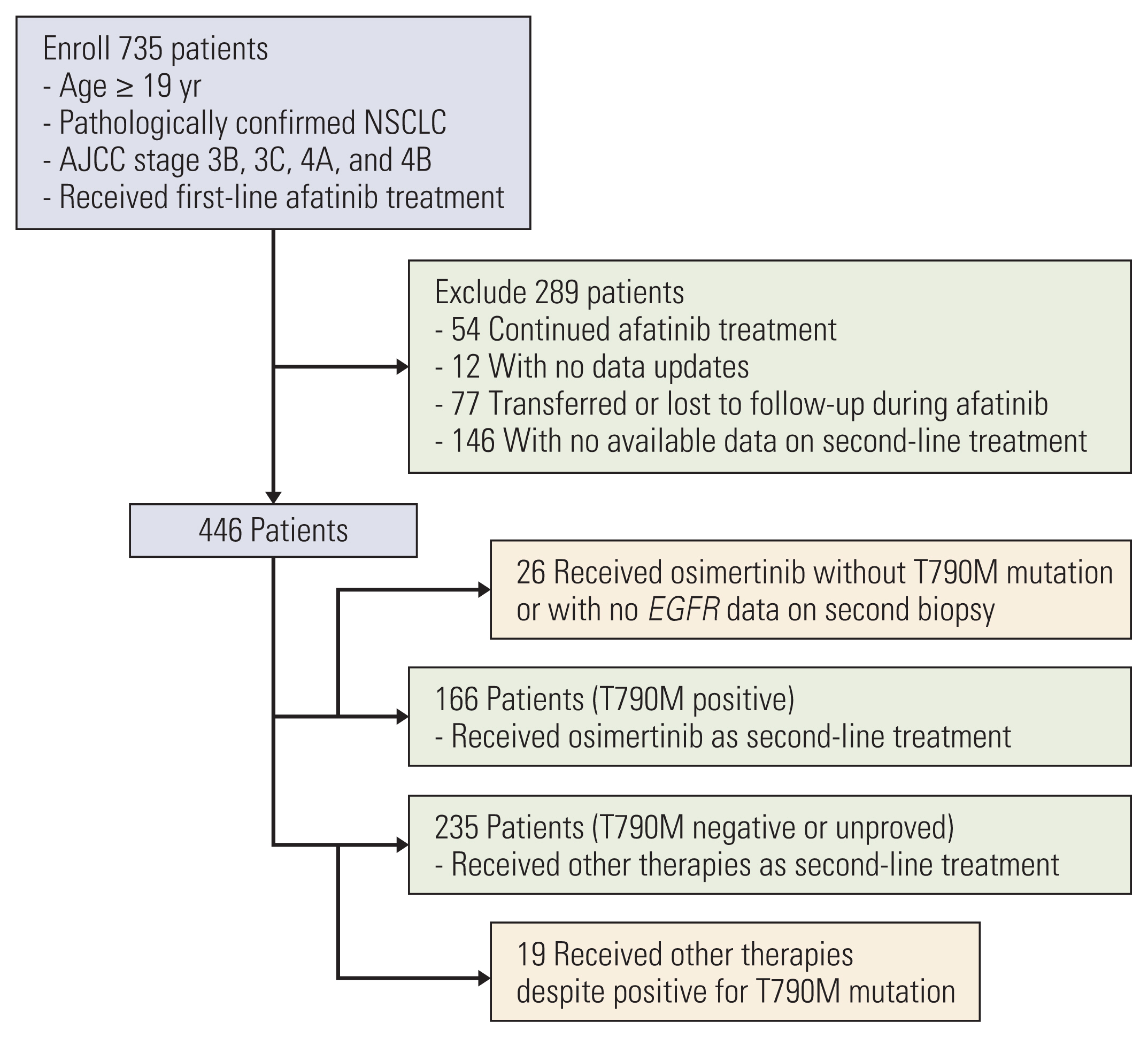
Fig. 4Overall survival comparing osimertinib, pemetrexed-platinum doublet, and pemetrexed monotherapy. 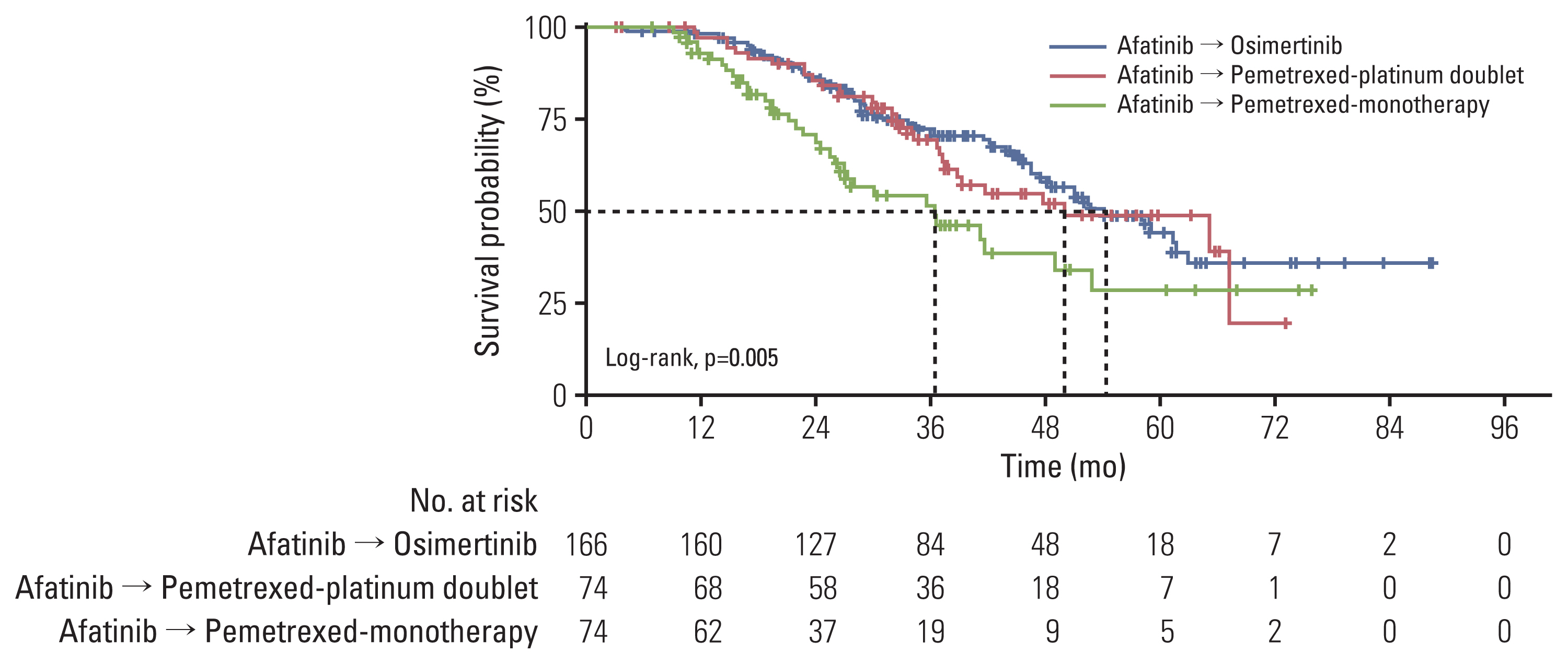
Table 1Baseline characteristics
Table 2Time-on-treatment (months) according to the treatments
Table 3Overall survival (months) according to the treatments
Table 4Factors affecting time-on-treatment during first-line afatinib
Table 5Factors affecting overall survival during first- and second-line treatments
Table 6Comparison of RESET with other previous real-world studies
References1. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–89.
2. Zhang Y, Luo G, Etxeberria J, Hao Y. Global patterns and trends in lung cancer incidence: a population-based study. J Thorac Oncol. 2021;16:933–44.
3. Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The international epidemiology of lung cancer: latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11:1653–71.
4. Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84:13–22.
5. Lee JG, Kim HC, Choi CM. Recent trends of lung cancer in Korea. Tuberc Respir Dis. 2021;84:89–95.
6. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
7. Ke EE, Zhou Q, Zhang QY, Su J, Chen ZH, Zhang XC, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol. 2017;12:1368–75.
8. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40.
9. Paz-Ares L, Tan EH, O’Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28:270–7.
10. Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang JC, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol. 2020;16:2799–808.
11. Popat S, Jung HA, Lee SY, Hochmair MJ, Lee SH, Escriu C, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: a global non-interventional study (UpSwinG). Lung Cancer. 2021;162:9–15.
12. Kim T, Jang TW, Choi CM, Kim MH, Lee SY, Park CK, et al. Sequential treatment of afatinib and osimertinib or other regimens in patients with advanced non-small-cell lung cancer harboring EGFR mutations: results from a real-world study in South Korea. Cancer Med. 2021;10:5809–22.
13. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22.
14. Akamatsu H, Katakami N, Okamoto I, Kato T, Kim YH, Imamura F, et al. Osimertinib in Japanese patients with EGFR T790M mutation-positive advanced non-small-cell lung cancer: AURA3 trial. Cancer Sci. 2018;109:1930–8.
15. Smit EF, Burgers SA, Biesma B, Smit HJ, Eppinga P, Dingemans AM, et al. Randomized phase II and pharmacogenetic study of pemetrexed compared with pemetrexed plus carboplatin in pretreated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:2038–45.
16. Miura S, Jung HA, Lee SY, Lee SH, Lee MK, Lee YC, et al. Sequential afatinib and osimertinib in Asian patients with EGFR mutation-positive non-dmall vell lung cancer and acquired T790M: combined analysis of two global non-interventional studies. Onco Targets Ther. 2022;15:873–82.
17. Kogure Y, Shigematsu F, Oki M, Saka H. T790M correlates with longer progression-free survival in non-small cell lung carcinomas harboring EGFR mutations. In Vivo. 2018;32:1199–204.
18. Gaut D, Sim MS, Yue Y, Wolf BR, Abarca PA, Carroll JM, et al. Clinical implications of the T790M mutation in disease characteristics and treatment response in patients with epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2018;19:e19–28.
19. Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3:90ra59.
20. Regales L, Balak MN, Gong Y, Politi K, Sawai A, Le C, et al. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PLoS One. 2007;2:e810.
21. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–37.
22. Yonesaka K, Kobayashi Y, Hayashi H, Chiba Y, Mitsudomi T, Nakagawa K. Dual blockade of EGFR tyrosine kinase using osimertinib and afatinib eradicates EGFR-mutant Ba/F3 cells. Oncol Rep. 2019;41:1059–66.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||