AbstractPurposeThe purpose of this study was to retrospectively investigate the contribution of 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG-PET/CT) to detection of metastatic bladder cancer.
Materials and MethodsThe present study included 79 patients (69 men and 10 women) undergoing 18F-FDG-PET/CT upon suspicion of metastatic bladder cancer between July 2007 and April 2013. The mean age was 66.1 years with a standard deviation of 10.7 years (range, 21 to 85 years). Patients were required to fast for 6 hours prior to scanning, and whole-body PET scanning from the skull base to the upper thighs was performed approximately 1 hour after intravenous injection of 555 MBq of 18F-FDG. Whole body CT scanning was performed in the cranio-caudal direction. FDG-PET images were reconstructed using CT data for attenuation correction. Suspicious recurrent or metastatic lesions were confirmed by histopathology or clinical follow-up.
IntroductionBladder cancer is the ninth most common cancer worldwide, with 380,000 new cases reported annually. The ratio of male:female bladder cancer patients is 3.8:1 [1]. According to data from the United States, the prevalence of bladder cancer has been increasing substantially [2]. Despite recent advances, 15,250 deaths from bladder cancer are expected to occur in 2013 [3]. Moreover, according to the Surveillance, Epidemiology and End Results database, there has been no significant change in bladder cancer deaths over the last 30 years [4]. Bladder cancer is a heterogeneous disease, with 70% of patients presenting with superficial tumors that tend to recur, but are generally not life threatening, and 30% presenting as muscle-invasive disease associated with a high risk of death from distant metastases [5]. Transitional cell carcinoma accounts for more than 90% of all bladder cancers, followed by squamous cell carcinomas (5%), adenocarcinomas (2%), and undifferentiated cancers (< 1%) [5]. The risk of metastases is very low when the disease is superficial. Therapy for superficial tumors includes complete endoscopic resection with or without additional intravesical chemotherapy. The standard treatment for patients with muscle-invasive bladder cancer is radical cystectomy. However, this only provides a 5-year survival in about 50% of patients. Although radical cystectomy is the preferred treatment for muscle invasive disease, metastases develop in about 25% of cases with tumors only invading the muscular layer, and in about 50% with tumors extending into the perivesical tissue. The main cause of death in this malignancy is the metastases. Despite radical treatments, metastasis occurs in 2 years in 50% of the patients with local disease [6]. However, it is not clear if the reason for this is the progressive or unpredictable biological behavior of the disease or micrometastases remaining undiagnosed at the time of diagnosis. Although answer to this question is not clear, the relationship between survival and stage of the disease in cases of radical cystectomy indicates that metastases undiagnosed at the time of diagnosis significantly influence survival. Indeed, the survival rate for stage T2 was 60% to 70% in cases of radical cystectomy investigated by Stein et al. [6], whereas it was 15%-20% for stage T4. Thus, failure of clinical staging is the biggest barrier to predicting the survival and planning additional treatment protocols. Clinical staging using bimanual palpation, computed tomography (CT) or magnetic resonance imaging (MRI) may result in over- and understaging, as indicated by a staging accuracy of only 70% [7]. Pelvic nodes > 8 mm and abdominal nodes > 10 mm in maximum short-axis diameter that are detected by CT or MRI should be regarded as pathologically enlarged. CT and MRI have low sensitivity (48%-87%) for lymph node metastasis because they evaluate the lymph node based on its size [8], and it is well known that metastasis may exist in normal-sized lymph nodes. Accordingly, positron emission tomography/computed tomography (PET/CT) is added upon detection of abnormal nodes because the anatomic views provide more accurate data.
Currently, diagnosis of patients with metastatic bladder cancer is still a great challenge. Furthermore, tests with high sensitivity and specificity are needed in these patients to predict residual disease and monitor treatment response. In this context, 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (18F-FDG-PET/CT), in which particularly functional and anatomic images are processed, is the most important diagnostic tool. Therefore, the present study was conducted to retrospectively review the contribution of 18F-FDG-PET/CT to detection of metastatic bladder cancer. The histology of the lesions (if available), or all of the clinical and radiological investigations (CT, MRI) were used as references.
Materials and MethodsA total of 7,938 patients were evaluated and 10,553 18F-FDG-PET/CT scans were performed in the Department of Nuclear Medicine, Sifa University, Izmir, Turkey between July 2007 and April 2013. Of this group, 79 patients underwent 18F-FDG-PET/CT because of suspicion of metastatic bladder cancer. Sixty-nine of these patients (87.3%) were male and ten (12.7%) were female. The mean age was 66.1 years and the standard deviation was 10.7 years (range, 21 to 85 years). All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients prior to inclusion in the study. Additional informed consent was obtained from all patients for which identifying information is included in this article.
Data on histological sub-types of bladder cancer based on pathological and immunohistochemical investigations were available for 75 patients (94.9%). Overall, 69 patients (87.3%) had high-grade transitional cell carcinoma (TCCa), three (3.8%) had low-grade TCCa, two (2.5%) had undifferentiated carcinoma, one (1.2%) had squamous cell carcinoma, and data on histological sub-type were unavailable for four patients (5%). All patients had 18F-FDG-PET/CT upon suspicion of metastasis. These patients were retrospectively evaluated and their pathological and 18F-FDG-PET/CT findings were recorded. The baseline characteristics of the patients are summarized in Table 1.
1. Imaging and interpretation of data
18F-FDG was synthesized using an in-house cyclotron (RDS 111 Cyclotron, Siemens Healthcare, Erlangen, Germany) and an automated synthesis system according to a previously authorized procedure. After five hours of fasting, the blood glucose level of each patient was measured and the patient was then intravenously injected with 370 MBq of 18F-FDG. One hour after 18F-FDG injection, a CT scan of the area from the vertex to the proximal thigh was performed without contrast agent, and these images were then used for attenuation correction and image fusion. This was followed by whole-body three-dimensional PET acquisition with eight bed positions that each consisted of 3 minutes of emission scan time using a dedicated PET/CT scanner (HI-REZ Biograph 6, Siemens Healthcare). This scanner provides an in-plane spatial resolution of 4.8 mm and an axial field view of 16.2 cm. The PET data were then reconstructed using a Gaussian filter with an ordered-subset expectation maximization algorithm (3 iterations, 8 subsets), reoriented in transverse, coronal and sagittal planes, and assessed by comparison with corresponding CT images.
PET scans were analyzed visually and semi-quantitatively using maximum standardized uptake value (SUVmax) measurement. SUV was expressed in terms of body weight (SUVbw, g/mL). Parameters such as patient's weight (kg), height (cm), radioactivity during injection (MBq), residual radioactivity (MBq) after the injection, starting time of injection, and half life of the radioisotope (taken to be 109.8 minutes for 18F-FDG) were calculated automatically by the software.
Two physicians experienced in nuclear medicine blindly and independently reviewed the hybrid 18F-FDG-PET/CT scans to determine if they were positive or negative for primary tumor sites. Every focal tracer uptake that deviated from physiological distribution was considered to favor disease spread. The background deviation and difference in activity between suspected lesions and the surrounding tissues were used to differentiate benign from malignant lesions. An SUVmax > 2.5 threshold was employed.
Results
18F-FDG-PET/CT imaging was negative in 24 patients (30.4%) and positive in 55 (69.6%). There were widespread metastases with high SUV values (mean, 8.3; range, 3.3 to 20.9) involving at least three organs (i.e., lungs or liver, bones, lymph nodes) in ten patients (12.7%), lymph node metastases in 24 (30.3%; mean SUVmax, 8.1), lung metastases in 18 (22.8%; mean SUVmax, 7.5), bone metastases in 15 (19%; mean SUVmax, 7.8), soft-tissue metastases in four (5%; mean SUVmax, 11.4), liver metastases in three (3.8%; mean SUVmax, 8.3), peritonitis carcinomatosa in two (2.5%; mean SUVmax, 6.6), cerebral metastasis in one (1.2%; mean SUVmax, 11) and concomitant upper tract urothelial carcinoma (UTUC) in one (1.2%; mean SUVmax, 14). The distribution of metastases is given in Fig. 1.
Grade and histological sub-types of the bladder cancer and PET/CT results were assessed. According to the grading system of the International Society of Urological Pathology (ISUP) and European Association of Urology (EAU), PET/CT was negative in three patients (100%) with low-grade disease, while it was negative in 18 patients (26%) and positive in 51 patients (74%) in the group of high-grade disease, and negative in all patients in the group of squamous cell carcinoma, positive in all patients in the group of undifferentiated carcinoma, and negative in three patients (75%) and positive in one patient (25%) in the group of carcinoma of unknown histological sub-type (Table 2). The percentage of positive PET findings increased with increasing grade. A significant correlation was observed between 18F-FDG-PET/CT results and histological degree (p < 0.05).
Suspicious recurrent or metastatic lesions were confirmed by histopathology or clinical follow-up. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of 18F-FDG-PET/CT were 89%, 78%, 90%, 75%, and 86%, respectively.
DiscussionMetastatic bladder carcinoma has a poor prognosis, and survival decreases parallel to the stage of disease, with a dramatic decrease occurring in the presence of metastatic disease. Overall, 10% to 15% of patients with metastatic cancer are metastatic at the time of diagnosis. Additionally, the rate of local recurrence is about 30% after radical cystectomy, and development of metastatic disease is more common in muscular invasive bladder carcinomas. Even though the disease does not begin with metastases, metastasis occurs in 24 months in almost half of the bladder cancers treated with radical methods. The main cause of death in the bladder carcinoma is metastases [6]. Accordingly, making accurate diagnoses and monitoring responses to treatment are of vital importance. Currently, there is no diagnostic tool capable of detecting occult metastases with high accuracy. Among all metastases, those in the visceral organs are markers of very poor prognosis in terms of survival. Systematic chemotherapy using multidrug regimens is the standard therapy for metastatic disease. However, accurately staging bladder cancer through conventional diagnostic methods is still currently challenging. The success rate of clinical staging (bimanual exam, CT, MRI) has been reported as 70% in the literature [7]. In patient-based analyses, it has been shown that 18F-FDG-PET/CT leads to changes in the therapeutic plan in 68% of patients because it can detect 40% more cases compared to conventional investigations based on CT and MRI. Moreover, 18F-FDG-PET/CT has been found to provide additional diagnostic data for clinical management of the disease owing to the high sensitivity and specificity of metastatic muscle-invasive bladder carcinoma (MIBC) [9] (Figs. 2-4).
Jadvar et al. [10] evaluated the diagnostic capacity of PET/CT retrospectively in patients with MIBC and found that clinical management had changed in 17% of the patients. Lu et al. [11] conducted a meta-analysis that revealed that diagnostic accuracy was sufficient in staging and restaging with 18F-FDG-PET/CT in patients with MIBC and metastatic bladder cancer in their meta-analysis, while no diagnostic capacity of 18F-FDG-PET/CT was observed for detecting bladder wall lesions with primary malignancy. Because it is excreted with urine, FDG may not play an important role in evaluation of bladder and detrusor lesions; however, it was shown to be useful for staging and determining metastatic disease [11]. Overall, tests with high sensitivity and specificity are needed to predict residual disease and monitor treatment response in such patients. In this context, 18F-FDG-PET/CT in which particularly functional and anatomic images are processed is a very important diagnostic tool. In the present study, patients undergoing PET/CT screening upon suspicion of metastatic disease were evaluated retrospectively, and the capacity of PET/CT to detect metastatic disease was investigated.
The most distinctive feature of cancer tissue is that it shows higher glucose metabolism than normal tissues (Warburg effect). High uptake of 18F-FDG in cancerous lesions of transitional carcinoma was first demonstrated by Harney et al. [12] in rats. Drieskens et al. [13] found that metabolism-based anatomical information gathered by the addition of 18F-FDG-PET to CT provided high diagnostic accuracy in preoperative staging of invasive transitional cancers, particularly invasive bladder carcinoma. 18F-FDG-PET/CT is now an established standard for preoperative staging and detection of metastatic lesions of bladder cancer [11,14,15]. During systemic circulation, 18F-FDG undergoes glomerular filtration and is not absorbed, but largely excreted in the urine. This poses a problem to identification of kidney, ureter, bladder, and prostate tumors. Another limitation is the faint 18F-FDG uptake by some malignant neoplasms, such as renal, prostate, and hepatocellular carcinomas. This has been attributed to high glucose-6-phosphatase activity. An important reason for decreased sensitivity is that primary tumors can express low levels of glucose transporters such as GLUT-1, which are responsible for the accumulation of 18F-FDG [16].
Use of urinary activity of 18F-FDG in diagnosis of primary tumors is currently still challenging; however, oral hyperhydration, forced diuresis with furosemide, evacuating the bladder with a catheter, and taking late pelvic images partially eliminates such problems. Our case series took into consideration the metastatic activity. Thus, urinary activity of 18F-FDG was only a limitation of our study as it relates to UTUCs. 18F-FDG activity of the bladder in metastases of pelvic lymph nodes was recorded as a feature partially limiting the evaluations owing to superposition. Diuresis has been shown to effectively decrease background radioactivity in the urinary tract and hence facilitate the identification of hypermetabolic lesions on 18F-FDG-PET.
Anjos et al. [17] found a sensitivity of 54% when 18F-FDG-PET/CT was applied to detect malignant areas on the bladder wall in 11 patients with MIBC. In a similar study, Harkirat et al. [18] found sensitivity and specificity of 86.7% and 100%, respectively, for detection of primary lesions with 18F-FDG-PET/CT in 22 patients with MIBC. Sensitivity and specificity rates given in both studies were found using late pelvic images taken along with forced diuresis.
In a study of 51 patients, Swinnen et al. [19] compared the 18F-FDG-PET/CT results of patients with MIBC for whom radical cystectomy was scheduled with pathological results obtained by extended pelvic lymph node dissection. The sensitivity, specificity, and accuracy of 18F-FDG-PET/CT were found to be 46%, 97%, and 84%, respectively. In a similar study, Lodde et al. [20] found a PPV of 100% for 18F-FDG-PET/CT applied to detect lymph node metastasis, and a higher specificity rate compared to CT (33% vs. 57%). Taken together, these previous studies indicate the superiority of 18F-FDG-PET/CT on CT for detection of lymph node metastasis (Figs. 5, 6).
Kibel et al. [15] found a sensitivity, specificity, PPV, and NPV of 70%, 94%, 78%, and 91%, respectively in a 18F-FDG-PET/CT investigation of 43 patients with stage T2/3N0M0 urothelial cancer. Specifically, they found occult metastatic disease in seven of 42 patients and that preoperative 18F-FDG-PET/CT might be influential in the decision of whether or not to conduct treatment prior to radical cystectomy. The relationship between PET findings and survival was also assessed in that study, and the results showed a 24-month recurrence-free survival of 24% among PET-positive patients, compared to a survival of 55% among PET-negative patients [15]. Jensen et al. [21] compared 18F-FDG-PET/CT and MRI for N-staging prior to radical cystectomy in 18 patients and found specificity rates of 93% and 80%, respectively, which were significantly different. Additionally, the NPVs were found to be 87.5% and 80%, respectively, although these values were not statistically significant.
Drieskens et al. [13] found sensitivity, specificity, and accuracy rates of 60%, 88%, and 78%, respectively, for the detection of metastatic disease by 18F-FDG-PET/CT in 55 patients with MIBC. In that series, 40 patients had undergone pathological correlation. Apolo et al. [14] evaluated 47 patients for diagnosis of metastatic disease and 135 individual metastatic lesions and found a sensitivity of 88% and specificity of 87% upon organ-based analyses. In that study, 18F-FDG-PET/CT was found to lead to changes in the therapeutic plan in 68% of the patients because it could detect 40% more cases than conventional investigations using CT and MRI. The authors concluded that 18F-FDG-PET/CT has excellent sensitivity and specificity for detection of metastatic bladder cancer and provides additional diagnostic information that enhances clinical management relative to CT/MRI alone. In the present study, specificity, sensitivity, accuracy rates, PPV, and NPV were found to be 89%, 78%, 90% (50/55), 75% (18/24), and 86%, respectively, which was in accordance with the results of previous studies. Liu et al. [22] found a sensitivity of 77% and specificity of 97% for 18F-FDG-PET/CT when detecting metastatic disease in 46 chemotherapy-negative patients. In a recent systemic review and meta-analysis by Lu et al. [11], a sensitivity of 89% and specificity of 82% were found for detection of metastatic lesions in bladder cancer. In that meta-analysis, the authors found that diagnostic accuracy was sufficient in staging and restaging with 18F-FDG-PET/CT for patients with MIBC and metastatic bladder cancer, while no diagnostic capacity of 18F-FDG-PET/CT was observed during detection of bladder wall lesions with primary malignancy. Because of its urinary excretion, 18F-FDG may not play an important role in evaluation of bladder and detrusor lesions; however, the authors found that it might be useful for staging and in determining metastatic disease [11].
In a study conducted in 2014, Mertens et al. [23] evaluated the relationship of 18F-FDG-PET/CT results with mortality in patients with MIBC (n=211; median follow-up, 18 months). In that study, disease-specific survival was 50 months in PET-negative patients, while it fell to 16 months in PET-positive patients. The presence of extravesical disease was found to be an independent prognostic factor for mortality in PET-positive patients.
Studies have also been conducted to investigate the diagnostic performance of alternative diagnostic tools to 18F-FDG-PET/CT. In a study using choline, Golan et al. [24] evaluated 20 patients for a total of 51 lesions with abnormal activity. For all lesions, the PPV was 84.7% for 11choline-PET/CT, while it was 90.7% for 18F-FDG-PET/CT. Upon evaluation of extravesical lesions, the PPV was 79.4% and 88.2%, respectively. That study indicated that 11choline-PET/CT was not superior to 18F-FDG-PET/CT for detecting metastatic bladder cancer, despite its histopathological correlation disadvantage. The diagnostic performance of 18F-FDG-PET/CT applied to metastatic bladder cancer reported in previous studies is summarized in Table 3.
The metabolic rate of low grade transitional cell carcinomas is close to that of normal tissues. The increased glucose metabolism in patients with high grade MIBC allows visualization of the lesions on the detector due to increased FDG uptake.
False-positive or false-negative findings of FDG uptake cannot be explained solely by glucose metabolism of tumor tissue. Previous studies have demonstrated that 18F-FDG-PET/CT scans can only provide information in the presence of a number of increased tumor cells with abnormal glucose metabolism (104-107). Such diagnostic failures are particularly important in solid organ metastases, such as those found in the lungs and liver. In general, 18F-FDG-PET/CT cannot accurately evaluate metastases measuring less than 5 mm in size. It is unknown why lung lesions below this threshold do not produce high SUVs; however, it may be caused by motion artifacts and low metabolic activity of the metastatic lesion. The reduction of motion artifacts using certain techniques, achieving a better spatial resolution and finding a higher cutoff SUV values for such lesions can increase diagnostic accuracy [25].
The findings of PET/CT scans must be verified by histopathological work-up to confirm disease recurrence. Theoretically, this remains the gold standard. Unfortunately, this is seldom possible in daily practice owing to clinical reasons, feasibility of the procedure and effective advantages of this approach in the absence of a radical surgical intent. In our study, histological confirmation was available in 14 patients, while all others were compared based on clinical and radiological findings.
It should be noted that this study was limited by its retrospective nature. Additionally, there may have been some selection bias since it is likely that only metastatic bladder carcinoma patients suspected to have recurrence were referred for PET/CT.
ConclusionThe present study retrospectively reviewed the contribution of 18F-FDG-PET/CT to detection of metastatic bladder cancer and found it to be consistent with the results of previous studies. The most important parameters limiting the current study and similar ones in the literature were a low correlation of histopathology, inability to prevent respiratory artifacts when detecting lung carcinomas smaller than 5 mm and thus inability to diagnose microscopic disease with high accuracy. It is believed that 18F-FDG-PET/CT may be useful in the diagnosis of metastatic bladder cancer owing to its high sensitivity and PPVs. Additionally, it may be useful to evaluation of the response to systemic chemotherapy due to its high performance.
AcknowledgmentsI would like to thank Inanç Karapolat, the head of Department of Nuclear Medicine, Sifa University and director of Cyclotron Unit.
Fig. 2.(A) Coronal 18F-FDG-PET/CT scans (white arrow: right lung metastasis; SUVmax, 14.6; red arrow: false-positive FDG accumulation in the left lung, pneumonia; SUVmax, 5.0). (B) Coronal 18F-FDG-PET/CT scans (arrow: liver metastasis; SUVmax, 6.4). (C) Coronal 18F-FDG-PET/CT scans (arrow: metastatic mass filling the neural foramina at the level of L2 vertebra; SUVmax, 12.0). 18F-FDG-PET/CT, fluorodeoxyglucose-positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value. 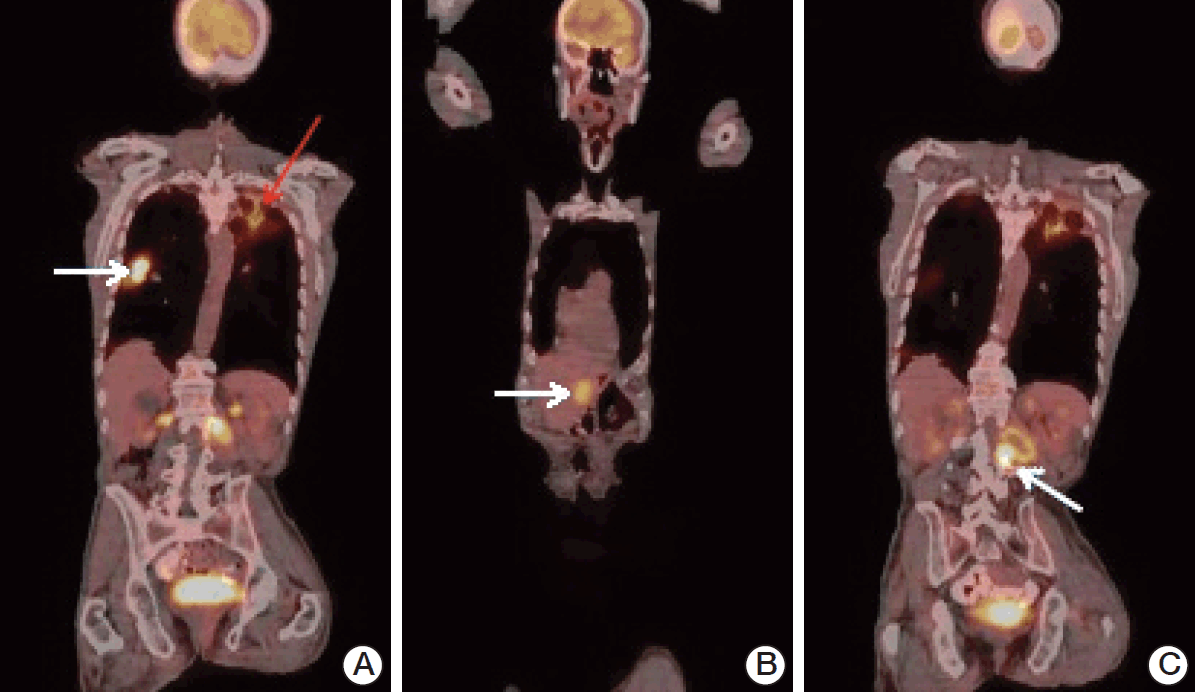
Fig. 3.(A) Axial 18F-FDG-PET/CT scans (arrow: false-positive FDG accumulation in the left lung, pneumonia; SUVmax, 5.0). (B) Axial 18F-FDG-PET/CT scans (arrow: right lung metastasis; SUVmax, 14.6). (C) Axial 18F-FDG-PET/CT scans (arrow: liver metastasis; SUVmax, 6.4). (D) Axial 18F-FDG-PET/CT scans (arrow: metastatic mass filling the neural foramina at the level of L2 vertebra; SUVmax, 12.0). 18F-FDG-PET/CT, fluorodeoxyglucose-positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value. 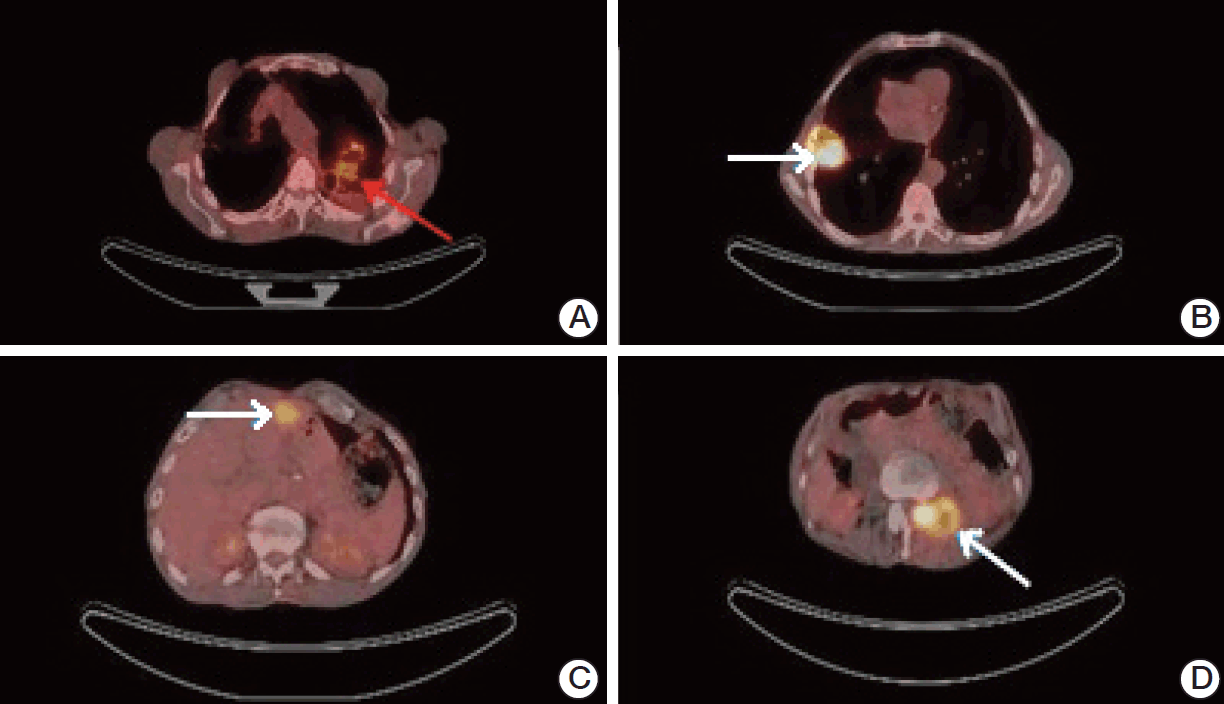
Fig. 4.(A) Maximum intensity projection (MIP) images of a patient (blue arrow: right lung metastasis; red arrow: false-positive fluorodeoxyglucose accumulation in the left lung, pneumonia). (B) MIP images of a patient (arrow: L2 vertebral metastasis). 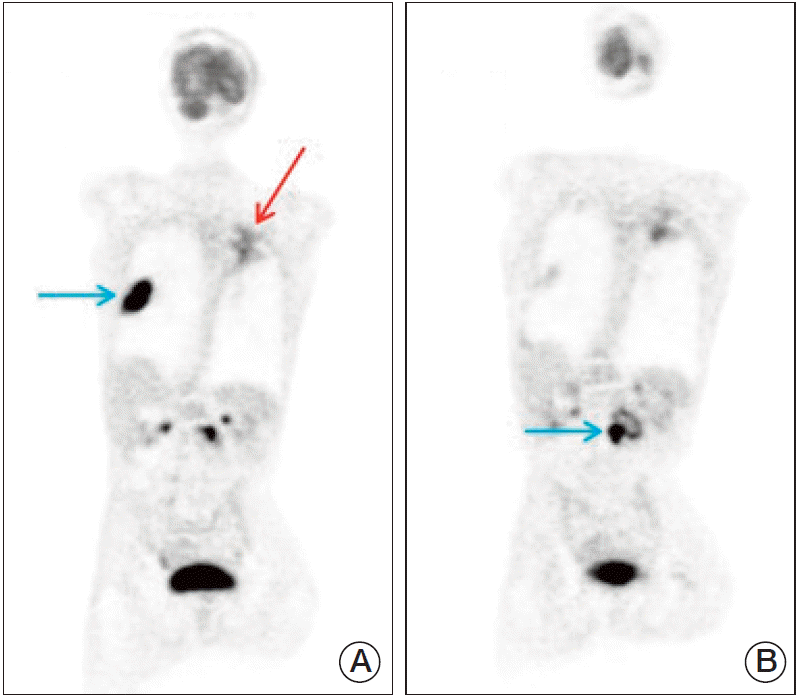
Fig. 5.(A) Coronal 18F-FDG-PET/CT scans (arrow: widespread retroperitoneal lymph node metastasis; SUVmax, 20.3). (B) MIP images of a patient (arrow: widespread retroperitoneal lymph node metastasis). (C) Coronal 18F-FDG-PET/CT scans (arrow: soft tissue metastasis; SUVmax, 18.2). (D) MIP images of a patient (arrow: soft tissue metastasis). 18F-FDG-PET/CT, fluorodeoxyglucose-positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value; MIP, maximum intensity projection. 
Fig. 6.(A) Coronal 18F-FDG-PET/CT scans (arrow: widespread conglomerate retroperitoneal lymph node metastasis; SUVmax, 10.8). (B) MIP images of a patient (arrow: widespread conglomerate retroperitoneal lymph node metastasis). (C) Coronal 18F-FDG-PET/CT scans (arrows: widespread bone metastasis; SUVmax, 13.5). (D) MIP images of a patient (arrows: widespread bone metastasis). 18F-FDG-PET/CT, fluorodeoxyglucose-positron emission tomography/computed tomography; SUVmax, maximum standardized uptake value; MIP, maximum intensity projection. 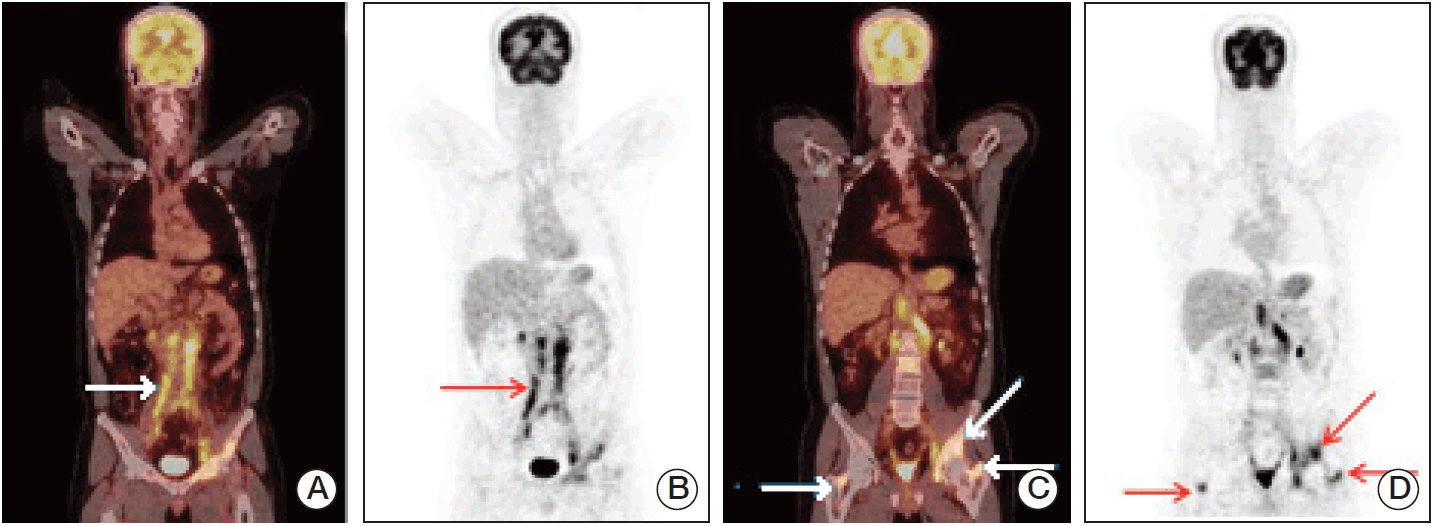
Table 1.Baseline characteristics of patients (n=79) Table 2.PET/CT results according TCCa grade and histology of primary tumor Table 3.Diagnostic performance of 18F-FDG-PET/CT studies in the literature
References1. Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93.
2. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212.
4. Abdollah F, Gandaglia G, Thuret R, Schmitges J, Tian Z, Jeldres C, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol. 2013;37:219–25.
6. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75.
7. Sternberg CN, Pansadoro V, Calabro F, Schnetzer S, Giannarelli D, Emiliozzi P, et al. Can patient selection for bladder preservation be based on response to chemotherapy? Cancer. 2003;97:1644–52.
8. Barentsz JO, Engelbrecht MR, Witjes JA, de la Rosette JJ, van der Graaf M. MR imaging of the male pelvis. Eur Radiol. 1999;9:1722–36.
9. Bouchelouche K, Turkbey B, Choyke PL. PET/CT and MRI in bladder cancer. J Cancer Sci Ther. 2012;S14:7692.
10. Jadvar H, Quan V, Henderson RW, Conti PS. [F-18]-Fluorodeoxyglucose PET and PET-CT in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int J Clin Oncol. 2008;13:42–7.
11. Lu YY, Chen JH, Liang JA, Wang HY, Lin CC, Lin WY, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: a systemic review and meta-analysis. Eur J Radiol. 2012;81:2411–6.
12. Harney JV, Wahl RL, Liebert M, Kuhl DE, Hutchins GD, Wedemeyer G, et al. Uptake of 2-deoxy, 2-(18F) fluoro-D-glucose in bladder cancer: animal localization and initial patient positron emission tomography. J Urol. 1991;145:279.
13. Drieskens O, Oyen R, Van Poppel H, Vankan Y, Flamen P, Mortelmans L. FDG-PET for preoperative staging of bladder cancer. Eur J Nucl Med Mol Imaging. 2005;32:1412–7.
14. Apolo AB, Riches J, Schoder H, Akin O, Trout A, Milowsky MI, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol. 2010;28:3973–8.
15. Kibel AS, Dehdashti F, Katz MD, Klim AP, Grubb RL, Humphrey PA, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol. 2009;27:4314–20.
16. Lin EC, Alavi A. Urologic tumorsIn : Lin EC, Alavi A, editors. PET and PET/CT: a clinical guide. New York: Thieme; 2009. p. 204–11.
17. Anjos DA, Etchebehere EC, Ramos CD, Santos AO, Albertotti C, Camargo EE. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med. 2007;48:764–70.
18. Harkirat S, Anand S, Jacob M. Forced diuresis and dual-phase F-fluorodeoxyglucose-PET/CT scan for restaging of urinary bladder cancers. Indian J Radiol Imaging. 2010;20:13–9.
19. Swinnen G, Maes A, Pottel H, Vanneste A, Billiet I, Lesage K, et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol. 2010;57:641–7.
20. Lodde M, Lacombe L, Friede J, Morin F, Saourine A, Fradet Y. Evaluation of fluorodeoxyglucose positron-emission tomography with computed tomography for staging of urothelial carcinoma. BJU Int. 2010;106:658–63.
21. Jensen TK, Holt P, Gerke O, Riehmann M, Svolgaard B, Marcussen N, et al. Preoperative lymph-node staging of invasive urothelial bladder cancer with 18F-fluorodeoxyglucose positron emission tomography/computed axial tomography and magnetic resonance imaging: correlation with histopathology. Scand J Urol Nephrol. 2011;45:122–8.
22. Liu IJ, Lai YH, Espiritu JI, Segall GM, Srinivas S, Nino-Murcia M, et al. Evaluation of fluorodeoxyglucose positron emission tomography imaging in metastatic transitional cell carcinoma with and without prior chemotherapy. Urol Int. 2006;77:69–75.
23. Mertens LS, Mir MC, Scott AM, Lee ST, Fioole-Bruining A, Vegt E, et al. 18F-fluorodeoxyglucose: positron emission tomography/computed tomography aids staging and predicts mortality in patients with muscle-invasive bladder cancer. Urology. 2014;83:393–8.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||