INTRODUCTION
Advances in diagnostic techniques and treatment methods for gastric cancer have improved overall treatment outcomes. The early diagnosis of Borrmann type IV gastric cancer is difficult, as no ulceration or elevation appears on the mucosal surface in the early phases of the tumor. These tumors are; therefore, often diagnosed at an advanced stage, making the prognosis very poor (1,2). Peritoneal dissemination is a major problem and the leading cause of death in patients with Borrmann type IV gastric cancer (1). The recurrence rate of a peritoneal dissemination after a resection is very high, making its prevention necessary for improved survival (2,3) Although many surgeons have made efforts to prevent peritoneal recurrence, these have not been successful. To date, there is no effective treatment for a peritoneal dissemination after a resection for Borrmann type IV gastric cancer. To improve the treatment results for Borrmann type IV gastric cancer, detailed clarification of the prognostic factors is important. Therefore, we retrospectively analyzed the clinicopathological characteristics and prognostic factors affecting the survival rate of patients with Borrmann type IV gastric cancer.
MATERIALS AND METHODS
The medical records of 370 patients with Borrmann type IV gastric cancer, who underwent exploratory surgery at the Department of Surgery, Asan Medical Center, between 1989 and 1997, were retrospectively analyzed. These patients constituted 9% of the 4,063 patients with gastric cancers who underwent exploratory surgery during the same period. The age, gender, histological findings, and the location and depth of tumor invasion, lymph node metastasis, tumor stage and the clinical course for recurrence and prognosis were retrospectively examined from the medical chart and data of the National Statistical Office. No patient was lost during the median 27 months follow up period (ranging from 1 to 109 months). One hundred and eighty two patients underwent resection for the primary tumor, and when the tumor involved an adjacent organ, that organ was also resected. The histological type, and location and depth of tumor invasion, the extent of lymph node metastasis and the stage of the primary tumors were determined according to the classification of gastric carcinomas of the Japanese Gastric Cancer Association (4).
Survival curves were determined using the Kaplan-Meier method. An analysis of the difference between the survival rates was performed using the log-rank test. The multivariate analysis was performed using Cox's proportional hazard model. Differences between groups were considered statistically significant at p<0.05. The SPSS statistical software was used for the above analysis.
RESULTS
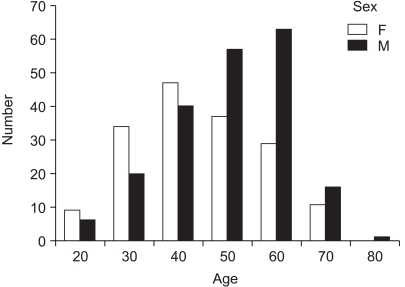
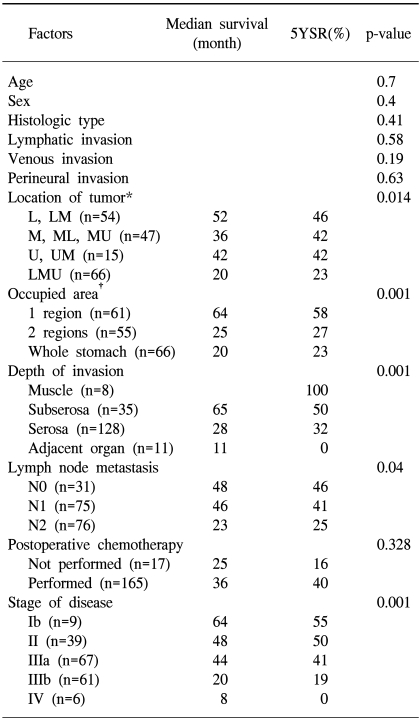
The average age of the 370 patients (203 males, 167 females) who underwent a resection for Borrmann type IV gastric cancer was 53 years, ranging from 21 to 81 years. Although the overall male to female ratio was 1.23 to 1, this was reversed in younger patients (Fig. 1). Tumors were located with similar frequency in the antral and body regions, with 156 tumors (42.2%) involving the whole stomach. One hundred and thirteen tumors (30.5%) were localized to only one region, while the remainder occupied two regions, either the antral and body regions or the body and cardiac regions. Serosa-exposure was observed in 322 patients (87%), while lymph node metastasis was confirmed in 223 (60.3%). Although peritoneal dissemination was detected in 141 patients (38.1%) at the time of surgery, hepatic metastasis was observed in only 14 (3.9%, Table 1). A curative resection was performed on 182 patients (49.1%). The overall 5 year survival rate of patients with Borrmann type IV gastric cancer was 24.3%; whereas, for patients either receiving a curative resection or not resected were 38.4 and 4.5%, respectively (Fig. 2). The univariate analysis showed the prognostic factors affecting the survival rate after a curative resection were the location (p=0.014), occupied region (p<0.001) and depth (p<0.001) of the primary tumor, as well as the presence of lymph node metastasis (p=0.04) and the tumor stage (p<0.001, Table 2). The multivariate analysis indicated that the tumor location and stage were significant independent prognostic factors after a curative resection for Borrmann type IV gastric cancer (Table 3). Of the 121 patients who experienced a recurrence after a curative resection, peritoneal dissemination was the main site of the recurrence in 90 (74.3%). The most frequent site of hematogenous metastasis was the bone marrow (Table 4). Of the patients in whom peritoneal dissemination was observed at the time of surgery, the survival rate for those who were resected was longer than for those who were not (p<0.05, Fig. 3). Among the resected patients with peritoneal dissemination, the prognosis was the same for those with both positive and negative resection margins (p=0.84, Fig. 4).
DISCUSSION
The clinical characteristics of Borrmann type IV gastric cancer include a high rate of incidence in young females (1,2,5), delayed diagnosis and detection at an advanced stage (1,2,5~7), peritoneal dissemination at the first operation (1), many peritoneal recurrences after a curative resection (1~3), low hepatic metastasis (1,2) and a low curative resection rate (2). Difficulties in treating this type of tumor may be due to both these specific characteristics and the late diagnosis. Thus, patients with Borrmann type IV gastric cancer have a poorer prognosis than those with other types of gastric cancer.
We found that 9% of patients with gastric cancers had Borrmann type IV gastric cancer, an incidence rate similar to that observed in other studies (1,5,8,9). We also found that the mean age of patients with Borrmann type IV gastric cancer was similar to that of all gastric cancer patients in Korea (8). In contrast, we observed a lower male to female ratio in Borrmann type IV gastric cancer patients than previously reported of all gastric cancer patients (8).
It has been hypothesized that patients with gastric linitis plastica who have liver metastasis, peritoneal dissemination and serosal involvement, or local extension of the tumor, would not benefit from a total gastrectomy, making only 20% of these patients candidates for palliative surgery (10). For the remainder, it has been suggested that alternative forms of treatment, other than surgery, including chemotherapy and/or radiation therapy, should be selected (10). It has been reported; however, that very extensive surgery, consisting of left upper abdominal exenteration plus the Appleby's method, improved the survival of patients with scirrhous cancer (11). The prognosis of patients with a scirrhous carcinoma of the stomach is mainly determined by the depth of penetration and curability, making early detection crucial for better survival (9,10,12). As the stage of tumor was found to be an independent prognostic factor in our multivariate analysis, the diagnosis at an early stage is very important in lengthening the survival time. However, only 6.7% of the patients with Borrmann type IV gastric cancer initially present with a stage II tumor (13). In our study, 49.1% of patients were treated with a curative resection, with the 5 year survival rate in this group being 38.4%, which was longer than that observed in other studies (2,14). This may have been due to the higher incidence of earlier stage patients in our study, providing further evidence that early diagnosis and treatment are essential to improve the survival of patients with Borrmann type IV gastric cancer.
It has been reported that a palliative gastrectomy may improve the prognosis of selected patients with peritoneal dissemination, but may be ineffective in patients with synchronous liver metastasis (15). It has been suggested that surgery should not be confined to a gastrectomy, but should also include a lymphadenectomy (16). In our patients with peritoneal dissemination, the median survival time of the resected group (11 months) was found to be significantly longer than that of the non-resected group (7 months). Although our study was not a randomized, controlled trial, the results suggest that a non- curative resection may lengthen the survival time in selected patients with peritoneal disseminations. Since patients with negative and positive resection margins of the gastrectomy of the peritoneal disseminations had similar survival rates, it is not necessary to obtain a negative safety margin at the time of a palliative non-curative resection. However, to determine the effect of a noncurative resection, a randomized trial may be necessary. Finally, in our study, 90 (64%) patients with peritoneal disseminations could not undergo a curative or palliative resection. Considering this undesirable result, it is very important to advance the diagnostic tools for peritoneal disseminations, such as laparoscopic staging work-up.
CONCLUSION
Borrmann type IV gastric cancer typically presents as an advanced stage tumor, with serosa-exposure and metastasis to the lymph nodes, as well as peritoneal dissemination. The early diagnosis and treatment are essential for better survival of these patients. Although these tumors are usually at an advanced stage, with surgery usually being palliative, a noncurative palliative resection may improve the prognosis.