AbstractPurposeIn men with metastatic castration-resistant prostate cancer (mCRPC), new bone lesions are sometimes not properly categorized through a confirmatory bone scan, and clinical significance of the test itself remains unclear. This study aimed to demonstrate the performance rate of confirmatory bone scans in a real-world setting and their prognostic impact in enzalutamide-treated mCRPC.
Materials and MethodsPatients who received oral enzalutamide for mCRPC during 2014-2017 at 14 tertiary centers in Korea were included. Patients lacking imaging assessment data or insufficient drug exposure were excluded. The primary outcome was overall survival (OS). Secondary outcomes included performance rate of confirmatory bone scans in a real-world setting. Kaplan-Meier analysis and multivariate Cox regression analysis were performed.
ResultsOverall, 520 patients with mCRPC were enrolled (240 [26.2%] chemotherapy-naïve and 280 [53.2%] after chemotherapy). Among 352 responders, 92 patients (26.1%) showed new bone lesions in their early bone scan. Confirmatory bone scan was performed in 41 patients (44.6%), and it was associated with prolonged OS in the entire population (median, 30.9 vs. 19.7 months; p < 0.001), as well as in the chemotherapy-naïve (median, 47.2 vs. 20.5 months; p=0.011) and post-chemotherapy sub-groups (median, 25.5 vs. 18.0 months; p=0.006). Multivariate Cox regression showed that confirmatory bone scan performance was an independent prognostic factor for OS (hazard ratio 0.35, 95% confidence interval, 0.18 to 0.69; p=0.002).
IntroductionMetastatic castration-resistant prostate cancer (mCRPC) rates are increasing worldwide. This cancer has a dismal prognosis, with a 5-year survival of approximately 30% [1]. After docetaxel was first approved as treatment with survival benefit for mCRPC, many drugs were actively developed, such as abiraterone, enzalutamide, cabazitaxel, and radium-223 [2,3]. Enzalutamide is one of the approved drugs that inhibit androgen-receptor signaling. The PREVAIL and AFFIRM randomized clinical trials have demonstrated the safety and efficacy of enzalutamide in men with mCRPC, both those who are chemotherapy-naïve and post-chemotherapy [4-6].
Due to the innate bone tropism of prostate cancer (PCa), approximately 90% of mCRPC patients have detectable bone metastases on imaging studies [7]. The Prostate Cancer Working Group (PCWG)-2 established specific assessment criteria for bone lesion evaluation [8]. They took into account the bone scan flare phenomenon, which is defined as unconfirmed new bone scan lesions with stable or responding disease, based on prostate-specific antigen (PSA) changes or soft-tissue imaging in PCa [6,9-11]. This indicates a favorable osteoblastic reaction, representing a healing process. However, without confirmatory bone scans performed at specific intervals after initial assessment, this can easily be mistaken for progression, and is termed pseudoprogression, which occurs in 10%-50% during various PCa treatments (androgendeprivation therapy [ADT], abiraterone, radium-223, and enzalutamide) [12]. Accordingly, pivotal randomized trials (PREVAIL and AFFIRM) clearly protocolized mandatory confirmatory bone scans when new bone lesions were encountered during enzalutamide treatment [4,5]. A post-hoc, secondary analysis of the trial data indeed supported the favorable treatment response in the pseudoprogression group [13]. Nevertheless, clinical data in a real practice setting on whether a confirmatory bone scan is appropriately performed to distinguish pseudoprogression from the true progression, or whether the performance of confirmatory bone scan itself affects survival outcome, are lacking.
In this study, we investigated the performance rate of confirmatory bone scans in real clinical practice for patients with mCRPC receiving enzalutamide, using a multicenter cohort. We hypothesized that not performing a confirmatory bone scan may be associated with worse oncological outcomes in chemotherapy-naïve or post-docetaxel settings. We also hypothesized that the pseudoprogression group would have similar oncological outcomes to those who responded to treatment as assessed by other means.
Materials and Methods1. Study design and patientsThis study included 627 patients who received oral enzalutamide between 2014 and 2017 at 14 tertiary centers in Korea. The inclusion criteria were histologically confirmed prostate adenocarcinoma, treated with enzalutamide between 2014 and 2017 due to mCRPC. Our cohort included both chemotherapy-naïve and post-chemotherapy groups. Unlike the AFFIRM or PREVAIL trials, our trial did not use particularly stringent eligibility criteria, in order to reflect a real-life clinical environment better [14,15]. The exclusion criteria were as follows: (1) metastases in the brain or active epidural disease (patients treated for epidural disease were allowed), (2) enzalutamide used in clinical trials or combined with other active drugs. Enzalutamide was administered daily at an initial dose of 160 mg and ADT was administered in a conventional manner.
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline [16].
2. Final cohort for the analysis of confirmatory bone scan efficacyOf the 627 patients who received enzalutamide, 107 were excluded due to lack of imaging assessment data or insufficient drug exposure (treatment discontinuation within less than 1 month for various causes, such as drug side effects and high drug costs) (S1 Fig.), resulting in final inclusion of 520 patients in the analysis. For the present analysis, we focused on men with stable disease or disease responding to therapy according to non-bone disease manifestations, including PSA levels and soft-tissue criteria. Overall, 260 patients (50.0%) showed stable disease without new bone lesions, while 92 patients (17.7%) showed new bone lesions. These were further divided into those who underwent confirmatory bone scan and those who did not.
3. Radiographic assessmentTc 99m-labeled methylene diphosphonate bone scans were performed at each participating center, interpreted locally at each center, and analyzed centrally for confirmation. Unconfirmed bone lesions were defined as the detection of two or more new lesions on post-baseline bone scans within 6 months of enzalutamide initiation, without subsequent new lesions detected on later scans (≥ 4-week intervals), according to the PCWG2 criteria.
The assessment of soft tissue progression was based on the Response Evaluation Criteria in Solid Tumors ver. 1.1 (RECIST v1.1). This was largely classified into complete response, partial response, stable disease, or progressive disease.
4. Study endpointsWe used modified study endpoints to reflect a real clinical situation. The primary endpoint was overall survival (OS), defined as the time from the start of enzalutamide treatment to death from any cause. Secondary endpoints included time to PSA progression, radiographic progression-free survival (rPFS), and performance rate of confirmatory bone scans in a real-world setting. Radiographic progression was defined according to RECIST 1.1, as soft tissue disease or the appearance of ≥ 2 new lesions on a bone scan.
5. Data collection and statistical analysisStudy data were collected online using the Research Electronic Data Capture (REDCap) program hosted by the Department of Urology at Seoul National University Hospital. REDCap is a secure, web-based application designed to support data capture for research studies [16]. For secondary verification, all the bone scan images from each center were also uploaded to the database.
Estimates of the median and 95% confidence intervals (CIs) for the time-to-event analyses were determined using the Kaplan-Meier method. The hazard ratio (HR) was determined using a Cox proportional hazards regression model. Statistical analyses were mainly performed in the following subgroup analyses: (1) groups without (–) and with (+) confirmatory bone scans in both the chemotherapy-naïve and post-docetaxel groups; (2) patients with stable disease vs. those confirmed to have undergone pseudoprogression.
Results1. Entire cohort of enzalutamide treatmentIn total, 520 patients with mCRPC were included in this study, of whom 240 patients (46.2%) were chemotherapynaive, and 280 patients (53.8%) received enzalutamide after chemotherapy. Patients received a mean of 10.8 cycles of enzalutamide (S2 Table). Among them, 168 patients (32.3%) showed no decrease in PSA or soft-tissue progression, demonstrating non-responsiveness. Baseline patient demographics, treatment history before enzalutamide administration, and primary and metastatic characteristic differences were recorded between chemotherapy-naïve and post-chemotherapy treatment types (Table 1).
2. Association of confirmatory bone scan with oncological outcomesAmong the 352 enzalutamide responders, 92 patients (26.1%) showed new bone lesions during the course of enzalutamide treatment. Confirmatory bone scans were performed in 44.6% (41 patients). All baseline clinical characteristics were comparable between the groups with and without confirmatory bone scans (Table 2).
Multivariate Cox regression analysis showed that confirmatory bone scan performance was an independent prognostic factor (OS) (HR, 0.35; 95% CI, 0.18 to 0.69; p=0.002) (Table 3). Confirmatory bone scan performance was associated with a significantly prolonged OS (median, 30.9 vs. 19.7 months; p < 0.001), time to PSA progression (median, 10.0 vs. 5.8 months; p < 0.001), and rPFS (median, 11.7 vs. 6.1 months; p < 0.001) (Fig. 1). In subgroup analysis, the group with confirmatory bone scans demonstrated OS benefit in both the chemotherapy naïve group (median survival, 47.2 vs. 20.5 months; p=0.011) and the post-chemotherapy group (median survival, 25.5 vs. 18.0 months; p=0.006) (Fig. 2).
To exclude situations where PSA increase occurred at the time of observing unconfirmed bone lesions, additional analysis was conducted on patients who demonstrated consistent PSA responses to enzalutamide over the permissible period defined as six months in the definition of unconfirmed bone lesions. All baseline clinical characteristics were comparable between the groups with and without confirmatory bone scans (Table 2). All baseline clinical characteristics were comparable between the groups with and without confirmatory bone scans (S3 Table). Confirmatory bone scan performance was still associated with a significantly prolonged OS (median, 33.5 vs. 21.4 months; p < 0.001) (S4 Fig.).
3. Association of bone scan pseudoprogression with efficacyAmong the 352 enzalutamide responders, 260 patients (73.9%) were classified as a “stable disease” group, whose disease was responding to treatment by both PSA and soft-tissue standards, with no new bone lesions detected on follow-up bone scans for at least the first 6 months. The number of pseudoprogression group was 17 patients, out of 41 patients who underwent confirmatory bone scan. This group was considered to show the bone flare phenomenon temporarily in response to enzalutamide. There were no differences in clinical characteristics between the two groups (stable disease group and pseudoprogression group) (S5 Table). Kaplan-Meier survival analysis revealed no difference in OS (median 52.0 months [stable disease] vs. not reached [pseudoprogression], p=0.195) (S6 Fig.).
DiscussionSince a Tc 99m–labeled methylene diphosphonate bone scan demonstrates osteoblastic activity derived from both the healing response and from cancer lesions, the PCWG (2-3) guidelines [17] recommend performing a confirmatory bone scan when new, unconfirmed bone lesions are detected on a first follow-up scan in mCRPC patients. This allows clinicians to distinguish true progression from pseudoprogression easily. This is clinically important because, without a confirmatory bone scan, patients showing a transient bone flare may be misdiagnosed as harboring resistance to an androgen receptor–targeting agent. Two global enzalutamide clinical trials, PREVAIL and AFFIRM, were thoroughly designed in accordance with these PCWG2 guidelines [18], so that only 16 men in the PREVAIL and no men in the AFFIRM study discontinued enzalutamide treatment for unconfirmed bone lesions, i.e., without first obtaining confirmatory bone scans. Such trial study results may not fully reflect real-world data. In support of this, a recent retrospective study presented by the Memorial Sloan Kettering Cancer Center group showed that 42 of 257 patients who received androgen receptor– axis–targeted drugs did not undergo a confirmatory bone scan [19]. Thus, in this study, we attempted to assess the performance rate of confirmatory bone scans for the new bone lesions and their survival outcomes in enzalutamide-treated mCRPC using multicenter real-world data. The novelty of this study is that we provided clinical evidence bolstered by a multicenter setting with regard to the clinical impact of confirmatory bone scans in enzalutamide-treated mCRPC, which is strikingly underperformed.
Essentially, we found no clinicopathological differences between patients with or without confirmatory bone scans, indicating that a confirmatory bone scan is somewhat randomly performed in a multicenter (14 tertiary centers) real-world setting. Nevertheless, performance of a confirmatory bone scan significantly extended the enzalutamide maintenance duration by approximately 8 months, which ultimately led to prolonged OS, rPFS, and time to PSA progression. On the other hand, the pseudoprogression group showed similar oncological outcomes to the stable disease group, in agreement with the PREVAIL and AFFIRM results. We next compared the incidence of unconfirmed bone lesions with the previous post-hoc analysis of the PREVAIL and AFFIRM data [13]. The new bone lesions were observed in 17.7% (92/520) of all patients in this study, which was comparable to the frequencies in previous trials. In subgroup analyses, pre-chemotherapy and post-chemotherapy settings showed a similar prevalence, i.e., 17.1% (41/240) and 17.9% (50/280), respectively. Through analysis of the multicenter-based real-world data, we revealed that only about 44.6% of patients underwent confirmatory bone scans when clinicians encountered the newly developed bone lesions. This indicates that, although the PCWG3 guidelines emphasize the clinical importance of the bone flare phenomenon and the necessity of a confirmatory bone scan, the confirmatory bone scan performance rate remains far from expected in real clinical practice.
Our patient cohort harbored different clinical characteristics from those in the AFFIRM and PREVAIL trials. First, for the pre-chemotherapy group, compared with the PREVAIL trial, the incidence of bone metastasis only group was higher (69.9% vs. 39.9%), and the ratio of the International Society of Urological Pathology (ISUP) grade group (GG) ≥ 4 was also markedly higher (76.9% vs. 50.6%). The group using two or more types of prior ADT accounted for 62.9%, which was almost triple of that (21.4%) in the PREVAIL trial, indicating that our cohort showed more aggressive mCRPC with a higher disease burden. In fact, this trend was similar to the distribution in an East Asian subpopulation shown in the post-hoc analysis results of the PREVAIL study [20]. Compared with the overall study population, a higher percentage of East Asian patients had a Gleason score ≥ 8 and a greater percentage had bone disease (East Asian vs. overall: 80% vs. 50.6%), suggesting a higher disease burden [20]. Additionally, this study was timely, given the current trend of increasingly advanced stages and an aggressive PCa, as well as increasing prevalence of bone-metastatic mCRPC [21-23]. This shift has been primarily driven by increased diagnosis of high-grade disease [24]. The same trend was observed in the post-chemotherapy group. Patients with ISUP GG 4-5 were 80.2% in our cohort, which was more than one and a half times that in the AFFIRM trial (50.4%).
In our comparison of OS between those with and those without new bone lesions, the former group showed significantly shorter survival than the pre-docetaxel (30.9 vs. 63.1 months, p < 0.001) and post-docetaxel (21.4 vs. 37.9 months, p < 0.001) values. This was in stark contrast to the results of previous studies (PREVAIL and AFFIRM), which consistently performed confirmatory bone scans. In short, the AFFIRM trial showed shortened OS, but no difference in rPFS in men with unconfirmed bone lesions, while the PREVAIL trial did not show differences in any oncological outcome, regardless of whether newly developed unconfirmed bone lesions were present. Approximately 40% of patients who underwent confirmatory bone scans revealed that the lesions represented pseudoprogression. This emphasizes that patients with new bone lesions who have not undergone confirmatory bone scans (55.4%) should be strongly recommended to undergo confirmatory bone scans to categorize their oncological risk appropriately and decide whether enzalutamide treatment should be maintained or switched to the next sequential drug. Our results may raise awareness of these new bone lesions among the community healthcare network and convey the necessity of a confirmatory bone scan when these lesions are encountered. At present, men who are likely to develop pseudoprogression rather than true progression cannot be identified prospectively, and thus, all patients with mCRPC who are receiving enzalutamide should be carefully observed for this phenomenon.
This study had some limitations. The first is the retrospective nature of the analysis, possibly because of inherent patient selection biases. A second limitation is the relatively small number of patients with pseudoprogressive lesions compared to the PREVAIL and AFFIRM trials. The third limitation is the lack of information on accompanying bone pain related to bone metastasis, which could have provided a more accurate clinical context. Fourth, this study did not suggest any useful clinical biomarkers for predicting pseudoprogression. Although the pre-enzalutamide laboratory test revealed that hemoglobin (11.7±1.6 [true progression] vs. 12.9±1.0 [pseudoprogression], p=0.008) was significantly different and lactate dehydrogenase (333±249 vs. 112±103, p=0.076) and alkaline phosphatase (305±454 vs. 129±62, p=0.072) demonstrated borderline significance, no sequential laboratory data could be obtained. Improved functional imaging, such as prostate-specific membrane antigen positron emission tomography–computed tomography [25], or automated bone scan index [19] may provide useful information for equivocal lesions that cannot be fully discriminated with confirmatory bone scans.
In our multicenter study using real-world data of enzalutamide-treated mCRPC, a confirmatory bone scan was not performed in a considerable number of patients showing new bone lesions in their early scan (< 6 months after treatment), contrary to the recommended guidelines. The performance of a confirmatory bone scan itself may be associated with prolonged OS in chemotherapy-naïve or post-chemotherapy subpopulations. Pseudoprogression occurred in approximately 40% of patients who underwent a confirmatory bone scan, and their OS was not different from that of men responding to treatment without new bone lesions. Based on these findings, we suggest that premature discontinuation of enzalutamide without a confirmatory bone scan should be discouraged to improve oncological outcomes.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 2004-070-1117). Informed consent was waived owing to the retrospective study design. The study was performed in accordance with applicable laws and regulations, good clinical practices, and ethical principles as described in the Declaration of Helsinki. Author Contributions Conceived and designed the analysis: Jeong CW, Kwak C. Collected the data: Jeong CW, Han JH, Kwon DD, Joung JY, Kim CS, Ahn H, Hong JH, Kim TH, Chung BH, Jeon SS, Kang M, Hong SK, Jung TY, Park SW, Yun SJ, Lee JY, Lee SH, Kang SH, Kwak C. Contributed data or analysis tools: Jeong CW, Han JH, Kwon DD, Joung JY, Kim CS, Ahn H, Hong JH, Kim TH, Chung BH, Jeon SS, Kang M, Hong SK, Jung TY, Park SW, Yun SJ, Lee JY, Lee SH, Kang SH, Kwak C. Performed the analysis: Jeong CW, Han JH, Kwon DD, Joung JY, Kim CS, Ahn H, Hong JH, Kim TH, Chung BH, Jeon SS, Kang M, Hong SK, Jung TY, Park SW, Yun SJ, Lee JY, Lee SH, Kang SH, Kwak C. Wrote the paper: Jeong CW, Han JH, Kwak C. Administrative, technical, and material support: Jeong CW, Han JH, Kwon DD, Joung JY, Kim CS, Ahn H, Hong JH, Kim TH, Chung BH, Jeon SS, Kang M, Hong SK, Jung TY, Park SW, Yun SJ, Lee JY, Lee SH, Kang SH, Kwak C. Fig. 1.Kaplan-Meier curve of groups with (red) and without (blue) confirmatory bone scan (BS) for overall survival (A), radiographic progression-free survival (B), and prostate-specific antigen (PSA) progression-free survival (C) in cases with enzalutamide-treated metastatic castration-resistant prostate cancer. 
Fig. 2.Kaplan-Meier curve of groups with (red) and without (blue) confirmatory bone scan (BS) for overall survival in cases with predocetaxel (A), and post-docetaxel (B) enzalutamide-treated metastatic castration-resistant prostate cancer. 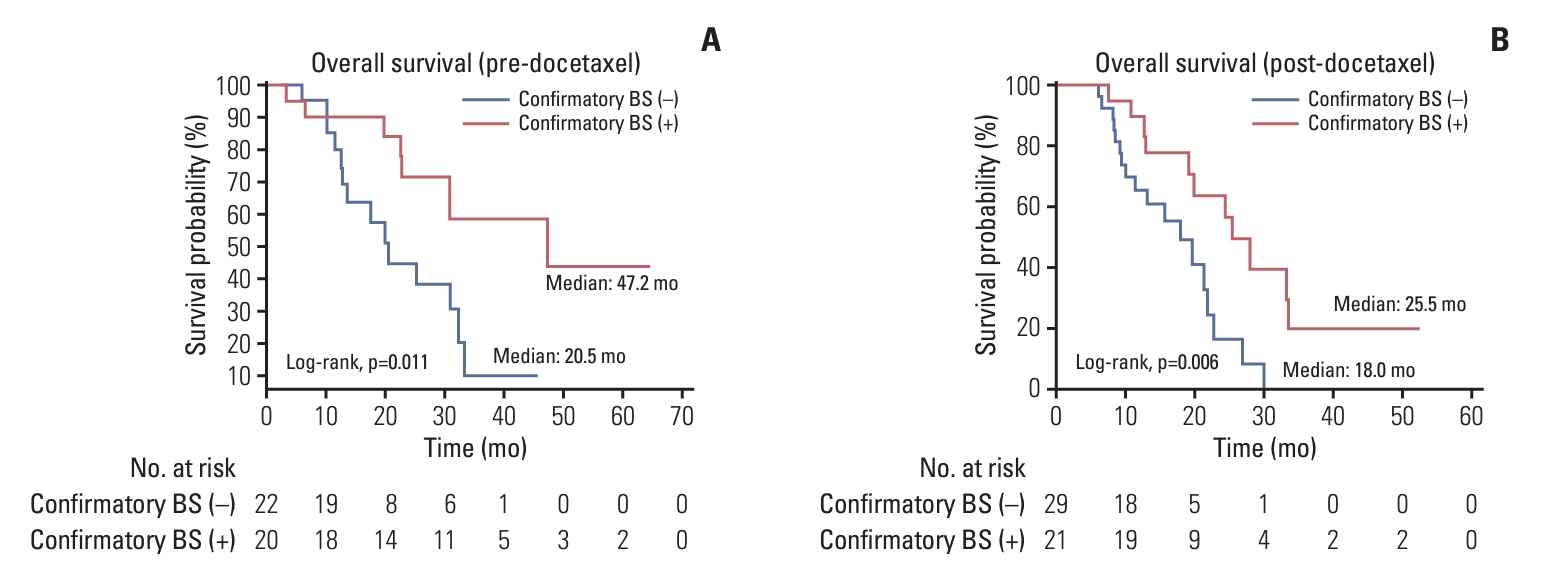
Table 1.Characteristics of pre- and post-docetaxel enzalutamide Values are presented as mean±SD, median (IQR), or number (%). BMI, body mass index; DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; ENZA, enzalutamide; HTN, hypertension; ISUP GG, International Society of Urological Pathology Grade Group; IQR, interquartile range; PSA, prostate-specific antigen; SD, standard deviation. Table 2.Characteristics of patients with/without confirmatory bone scan Values are presented as mean±SD, median (IQR), or number (%). BMI, body mass index; DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; HTN, hypertension; ISUP GG, International Society of Urological Pathology Grade Group; IQR, interquartile range; PSA, prostate-specific antigen; SD, standard deviation. References1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
2. Lorente D, Fizazi K, Sweeney C, de Bono JS. Optimal treatment sequence for metastatic castration-resistant prostate cancer. Eur Urol Focus. 2016;2:488–98.
4. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
5. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
6. Kim CS, Theeuwes A, Kwon DD, Choi YD, Chung BH, Lee HM, et al. The PREVAIL trial of enzalutamide in men with chemotherapy-naive, metastatic castration-resistant prostate cancer: post hoc analysis of Korean patients. Investig Clin Urol. 2016;57:174–83.
7. Isensee G, Peporte A, Muller J, Schmid S, Gillessen S, Omlin A. Is there a flare phenomenon on bone scintigraphy in men with advanced prostate cancer treated with radium-223? Clin Genitourin Cancer. 2018;16:349–54.
8. Sonpavde G, Pond GR, Armstrong AJ, Galsky MD, Leopold L, Wood BA, et al. Radiographic progression by Prostate Cancer Working Group (PCWG)-2 criteria as an intermediate endpoint for drug development in metastatic castration-resistant prostate cancer. BJU Int. 2014;114:E25–31.
9. Pollen JJ, Shlaer WJ. Osteoblastic response to successful treatment of metastatic cancer of the prostate. AJR Am J Roentgenol. 1979;132:927–31.
10. Coleman RE, Mashiter G, Whitaker KB, Moss DW, Rubens RD, Fogelman I. Bone scan flare predicts successful systemic therapy for bone metastases. J Nucl Med. 1988;29:1354–9.
11. Ryan CJ, Shah S, Efstathiou E, Smith MR, Taplin ME, Bubley GJ, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–61.
12. Conteduca V, Poti G, Caroli P, Russi S, Brighi N, Lolli C, et al. Flare phenomenon in prostate cancer: recent evidence on new drugs and next generation imaging. Ther Adv Med Oncol. 2021;13:1758835920987654.
13. Armstrong AJ, Al-Adhami M, Lin P, Parli T, Sugg J, Steinberg J, et al. Association between new unconfirmed bone lesions and outcomes in men with metastatic castration-resistant prostate cancer treated with enzalutamide: secondary analysis of the PREVAIL and AFFIRM randomized clinical trials. JAMA Oncol. 2020;6:217–25.
14. Jung SI, Kim MS, Jeong CW, Kwak C, Hong SK, Kang SH, et al. Enzalutamide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer: a retrospective Korean multicenter study in a real-world setting. Investig Clin Urol. 2020;61:19–27.
15. Jeong CW, Kang M, Jung SI, Kim TH, Park SW, Joung JY, et al. Importance of androgen-deprivation therapy during enzalutamide treatment in men with metastatic castration-resistant prostate cancer following chemotherapy: results from retrospective, multicenter data. Prostate Cancer Prostatic Dis. 2019;22:150–8.
16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadatadriven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
17. Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
18. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59.
19. Anand A, Heller G, Fox J, Danila DC, Bjartell A, Edenbrandt L, et al. Automated bone scan index to optimize Prostate Cancer Working Group radiographic progression criteria for men with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2022;20:270–7.
20. Kim CS, Choi YD, Lee SE, Lee HM, Ueda T, Yonese J, et al. Post hoc analyses of East Asian patients from the randomized placebo-controlled PREVAIL trial of enzalutamide in patients with chemotherapy-naive, metastatic castration-resistant prostate cancer. Medicine (Baltimore). 2017;96:e7223
21. Fletcher SA, von Landenberg N, Cole AP, Gild P, Choueiri TK, Lipsitz SR, et al. Contemporary national trends in prostate cancer risk profile at diagnosis. Prostate Cancer Prostatic Dis. 2020;23:81–7.
22. Wallace KL, Landsteiner A, Bunner SH, Engel-Nitz NM, Luckenbaugh AN. Increasing prevalence of metastatic castration-resistant prostate cancer in a managed care population in the United States. Cancer Causes Control. 2021;32:1365–74.
23. Ryan C, Stoltzfus KC, Horn S, Chen H, Louie AV, Lehrer EJ, et al. Epidemiology of bone metastases. Bone. 2022;158:115783.
|
|
|||||||||||||||||||||||||||||||||||||||||