AbstractPurposeThis study aims to evaluate the efficacy and safety of a new combination treatment of vinorelbine and pyrotinib in human epidermal growth factor receptor 2 (HER2)–positive metastatic breast cancer (MBC) and provide higher level evidence for clinical practice.
Materials and MethodsThis was a prospective, single-arm, phase 2 trial conducted at three institutions in China. Patients with HER2-positive MBC, who had previously been treated with trastuzumab plus a taxane or trastuzumab plus pertuzumab combined with a chemotherapeutic agent, were enrolled between March 2020 and December 2021. All patients received pyrotinib 400 mg orally once daily plus vinorelbine 25 mg/m2 intravenously or 60-80 mg/m2 orally on day 1 and day 8 of 21-day cycle. The primary endpoint was progression-free survival (PFS), and the secondary endpoints included the objective response rate (ORR), disease control rate (DCR), overall survival, and safety.
ResultsA total of 39 patients were enrolled. All patients had been pretreated with trastuzumab and 23.1% (n=9) of them had accepted trastuzumab plus pertuzumab. The median follow-up time was 16.3 months (95% confidence interval [CI], 5.3 to 27.2), and the median PFS was 6.4 months (95% CI, 4.0 to 8.8). The ORR was 43.6% (95% CI, 27.8% to 60.4%) and the DCR was 84.6% (95% CI, 69.5% to 94.1%). The median PFS of patients with versus without prior pertuzumab treatment was 4.6 and 8.3 months (p=0.017). The most common grade 3/4 adverse events were diarrhea (28.2%), neutrophil count decreased (15.4%), white blood cell count decreased (7.7%), vomiting (5.1%), and anemia (2.6%).
IntroductionBreast cancer has risen to the top spot for the most commonly diagnosed tumor type, with an estimated 2.3 million new cases (11.7%) [1]. Approximately 15%-20% of patients with breast cancer have amplification of the human epidermal growth factor receptor 2 (HER2) expression. HER2-positive breast cancer exhibits aggressive behavior and poor prognosis [2,3]. The booming development of anti-HER2 agents including trastuzumab, pertuzumab, ado-trastuzumab emtansine (T-DM1), fam-trastuzumab deruxtecan (DS-8201), lapatinib, and neratinib, have significantly improved the outcome of patients with HER2-positive breast cancer [4-9].
For second-line treatment of HER2-positive metastatic breast cancer (MBC) patients, the preferred regimen is trastuzumab deruxtecan [7], which is recommended by international guidelines. However, trastuzumab deruxtecan is not approved for use in some countries including China. Therefore, pyrotinib plus capecitabine, T-DM1, neratinib plus capecitabine, lapatinib plus capecitabine, and other regimens are recommended in the second-line setting.
Pyrotinib is an irreversible tyrosine kinase inhibitor (TKI) targeting epidermal growth factor receptor (EGFR), HER2, and HER4, which has shown clinically meaningful efficacy and acceptable tolerability when combined with capecitabine in previous trials [10-14]. However, alternative chemotherapies as combination partners are always required in clinical practice. Vinca alkaloid vinorelbine is an important chemotherapy agent for patients with MBC, reflecting a synergistic effect with trastuzumab in breast cancer cells and non-inferior efficacy compared to taxanes when in combination with trastuzumab in clinical trials [15-17]. Previous retrospective studies have exhibited promising effects of pyrotinib plus vinorelbine with a median progression-free survival (PFS) of 7.8 months and an objective response rate (ORR) of 34.3% and the superior efficacy of pyrotinib plus vinorelbine over lapatinib plus capecitabine in HER2-positive MBC patients pretreated with trastuzumab and taxane [18,19]. A retrospective, multicenter real-world study, which enrolled 172 HER2-positive MBC patients treated with pyrotinib-based therapy, demonstrated that vinorelbine plus pyrotinib provided similar median PFS as capecitabine plus pyrotinib [20]. Besides, vinorelbine has both oral and intravenous administration patterns and different toxicity profiles from capecitabine. Therefore, vinorelbine might be a good alternative combination option with pyrotinib. However, the efficacy and safety of vinorelbine in combination with pyrotinib are not investigated so far in prospective trials. Herein, we conducted this multicenter, prospective study to evaluate the efficacy and safety of vinorelbine plus pyrotinib and provide higher level evidence for clinical practice.
Materials and Methods1. Study design and patientsThis was a prospective, multicenter, single-arm study (ClinicalTrials.gov ID: NCT04605575) recruiting patients from Sun Yat-sen University Cancer Center, Meizhou People’s Hospital, and Shantou Central Hospital from March 2020 to December 2021. This study strictly followed the Declaration of Helsinki and Good Clinical Practice guidelines. All the patients provided written informed consent. The study was approved by the relevant institutional review board or ethics committee of each study center.
Major inclusion criteria were as follows: (1) age 18-70 years; (2) histologically confirmed HER2-positive (immunohistochemistry 3+ or fluorescence in situ hybridization showing HER2 gene amplification, according to the 2018 American Society of Clinical Oncology–College of American Pathologists guidelines [21]) relapsed or metastatic breast cancer; (3) progression during a taxane and trastuzumab treatment in adjuvant or neoadjuvant setting or within 6 months after treatment of early-stage disease or progression with the first-line trastuzumab-containing therapy. Patients who had previously used chemotherapy plus trastuzumab and pertuzumab in the first-line setting with no progression by the first response evaluation (end of 2-3 cycles) will be allowed; (4) at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 or bone-only metastasis; (5) Baseline laboratory tests required to assess eligibility were absolute neutrophil count, platelet count, hemoglobin, total bilirubin, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, serum creatinine, creatinine clearance, left ventricular ejection fraction, and Fridericia-corrected QT interval; (6) Eastern Cooperative Oncology Group (ECOG) performance status of 0 (asymptomatic) or 1 (restricted in strenuous activity but ambulatory and able to do light work).
Key exclusion criteria were as follows: (1) prior treatment with any TKI targeting HER2; (2) previous treatment with pertuzumab in the neoadjuvant or adjuvant setting; (3) symptomatic central nervous system metastases; (4) current severe, uncontrolled systemic disease (for example, clinically significant cardiovascular, pulmonary, or metabolic disease); (5) presence of conditions that could affect gastrointestinal absorption.
2. ProceduresContinuous oral pyrotinib 400 mg once daily plus oral vinorelbine 80 mg/m2 (following a first cycle at 60 mg/m2) or intravenous vinorelbine 25 mg/m2 on day 1 and day 8 of the 21-day cycle were administered until disease progression, unacceptable toxicity, death, consent withdrawal or investigator decision. Dose reductions and interruptions owing to adverse events were defined in the protocol. For pyrotinib, dose reductions were permitted stepwise from 400 mg to 320 mg to 240 mg. The dose of orally taken vinorelbine was permitted to be reduced stepwise by 25%. Vinorelbine injection dosage was allowed to be decreased at the first time to 50% and the second time to 25% of the original daily dose. Guidelines for primary prophylaxis and treatment of diarrhea and vomiting are available in the protocol.
3. AssessmentsTumor assessments were performed at baseline and every two cycles thereafter until disease progression by high-resolution contrast-enhanced computed tomography or magnetic resonance imaging; an additional assessment was required after progression. Complete or partial responses were confirmed by repeated assessment at least 4 weeks later (at the next tumor assessment). Blood routine examination was done at baseline, every week for the first two cycles, and subsequently every cycle. Other laboratory assessments, 12-lead electrocardiograms, and vital signs were performed at baseline and every cycle thereafter. The left ventricular ejection fraction was measured by means of echocardiography every 12 weeks. Adverse events (AEs) were monitored continuously and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), ver. 5.0.
4. OutcomesThe primary endpoint was progression-free survival, which was defined as the time from treatment initiation to first documented radiographic progression or death from any cause. Secondary endpoints were ORR (the proportion of patients with a best overall response of complete or partial response), disease control rate (the proportion of patients with a best overall response of complete response, partial response, or stable disease), overall survival (the time from treatment initiation to death from any cause), and safety.
5. Statistical analysisThis trial is an exploratory study, no formal hypothesis testing was performed and results regarding all endpoints were presented descriptively. The full analysis set and safety analysis set were defined as all enrolled participants who received at least one dose of study drugs. The primary and secondary endpoints were assessed in the full analysis set. A safety analysis was performed in the safety analysis set. PFS was analyzed with the Kaplan-Meier method. We calculated the proportion of patients who achieved an objective response and estimated a 95% confidence interval (CI) using the Clopper-Pearson method. PFS according to pertuzumab exposure was estimated using the Kaplan-Meier method and compared by the log-rank test. AEs by the proportion of the total number of the safety set were summarized. All tests were two-sided, and p < 0.05 was considered statistically significant. All statistical tests were performed using the SPSS ver. 24.0 (IBM Corp., Armonk, NY).
Results1. Patient CharacteristicsBetween March 2020 and December 2021, a total of 39 HER2-positive MBC patients who had previously received trastuzumab or trastuzumab combined with pertuzumab in three institutions across China were enrolled in this prospective, single-arm study (Fig. 1). The median follow-up was 16.3 months (95% CI, 5.3 to 27.2). The median patient age was 52 years (range, 30 to 70 years). ECOG performance status was 0 in 13 patients (33.3%) and 1 in 26 patients (66.7%). Twenty-two patients (56.4%) had hormone receptor-positive disease, 23 (59.0%) had visceral metastasis, and 33 participants (84.6%) had measurable lesions. Prior HER2-targeted therapy included trastuzumab (n=39), pertuzumab (n=9), and T-DM1 (n=1). Fifteen patients (38.5%) were trastuzumab-resistant which was defined as relapse during or within 6 months after adjuvant trastuzumab or progression within 3 months of trastuzumab treatment for metastatic disease. Prior systemic therapies included anthracycline (n=23), taxanes (n=18), other chemotherapy (n=17), and endocrine therapy (n=15). Sixteen participants (41.0%) never received chemotherapy and 23 (59.0%) had received one chemotherapy regimen for locally advanced or metastatic disease. The characteristics of patients are summarized in Table 1.
2. EfficacyThe median PFS was 6.4 months (95% CI, 4.0 to 8.8) (Fig. 2). The proportion of patients who achieved complete response or partial response was 17 of 39, hence the ORR was 43.6% (95% CI, 27.8% to 60.4%). Sixteen patients achieved stable disease, thus the disease control rate (DCR) was 84.6% (95% CI, 69.5% to 94.1%) (Table 2). As shown in Fig. 3, 27 of the 33 participants (81.8%) who had an evaluable tumor assessment obtained some degree of tumor shrinkage. At 12 months of follow-up, the PFS rate was 28.3% (95% CI, 15.1% to 52.7%). The PFS of patients with versus without prior pertuzumab exposure was 4.6 vs. 8.3 months (p=0.017) (Fig. 4). Only one death (2.6%) had occurred by the data cutoff on December 31, 2021, due to tumor progression and the OS data was immature. In this study, 30 patients were treated with oral vinorelbine combined with pyrotinib, and nine patients received intravenous vinorelbine combined with pyrotinib. The ORR, DCR, and median PFS of patients in these two groups are shown in Table 3. It’s indicated that the data of efficacy in oral or intravenous vinorelbine groups was similar, and there is no statistically significant difference in PFS between the two groups (p=0.326) (Fig. 5).
3. SafetyAEs that occurred in all 39 participants are listed in Table 4. Diarrhea (89.7%) occurred most frequently, followed by vomiting (48.7%), anemia (38.5%), white blood cell count decreased (30.8%), neutrophil count decreased (28.2%), alanine aminotransferase increased (28.2%), and aspartate aminotransferase increased (28.2%). The most common grade 3 or 4 AEs were diarrhea (28.2%), neutrophil count decreased (15.4%), white blood cell count decreased (7.7%), vomiting (5.1%), and anemia (2.6%). Two patients (5.1%) developed grade 4 neutrophil count decreased, but no febrile neutropenia was reported. There was no treatment-related death during the trial. Dose interruption occurred in seven patients (17.9%) and seven patients (17.9%) had dosage reduction because of AEs. Two patients (5.1%) withdrew from the study due to grade 3 diarrhea. As shown in Table 5, diarrhea was the most common side effect in both oral and intravenous vinorelbine groups (86.7% vs. 88.9%). The incidence of bone marrow suppression with oral vinorelbine plus pyrotinib was lower than that of the intravenous vinorelbine group, including white blood cell count decreased (23.3% vs. 77.8%), neutrophil count decreased (13.3% vs. 77.8%), and anemia (33.3% vs. 55.6%). The gastrointestinal reactions were more common in the oral vinorelbine group compared with the intravenous vinorelbine group, such as vomiting (70.0% vs. 11.1%) and nausea (23.3% vs. 11.1%).
DiscussionIn the past several decades, there has been a lack of effective treatment for pretreated HER2-positive MBC. In the EMILIA phase III study, T-DM1 beat lapatinib plus capecitabine with a prolonged PFS (median, 9.6 months vs. 6.4 months) and an increased ORR (43.6% vs. 30.8%) [6]. Therefore, T-DM1 was once the recommended therapy for patients whose disease progresses after pertuzumab and trastuzumab in combination with a taxane. Afterwards, the DESTINY-Breast03 trial enrolled HER2-positive MBC patients previously treated with trastuzumab and a taxane to receive DS-8201 or T-DM1 randomly. At 12 months, the percentage of patients who still live without disease progression was 75.8% in the DS-8201 group, which significantly improved in comparison to 34.1% in the T-DM1 group (p < 0.001). An overall response occurred in 79.7% of the patients who accepted DS-8201 and in 34.2% of those who received T-DM1 [7]. Based on the result of the above study, DS-8201 is recommended as the standard treatment in the second-line setting by National Comprehensive Cancer Network (NCCN) guidelines, but unfortunately, DS-8201 is not yet on the market in China. According to retrospective studies, pyrotinib combined with vinorelbine showed promising effect with a median PFS of 7.8 months with an ORR of 34.3%, which was similar to that of pyrotinib plus capecitabine and longer than that of lapatinib plus capecitabine [18-20]. Thus, pyrotinib combined with chemotherapeutic agents are also recommended in the second-line setting by the Chinese Society of Clinical Oncology (CSCO). In our study, which is the first prospective study to evaluate the efficacy and safety of pyrotinib plus vinorelbine in second-line setting to the best of our knowledge, the median PFS for patients with HER2-positive MBC was observed to be 6.4 months, the ORR was 43.6% and the DCR was 84.6% for second-line pyrotinib plus vinorelbine treatment. Approximately 82% of the participants with an evaluable tumor assessment presented tumor shrinkage. Therefore, pyrotinib combined with vinorelbine could offer an alternative treatment as a second-line treatment, to some extent. However, the efficacy of pyrotinib plus vinorelbine in this study was not as impressive as that of pyrotinib plus capecitabine reported in the PHOEBE and PHENIX phase III trials with median PFSs of 12.5 months and 11.1 months and ORRs of 67.2% and 68.6% [14,22]. There were some causes attributed to these unsatisfactory results. First of all, all patients had accepted previous trastuzumab therapy and a portion of patients had received prior pertuzumab or T-DM1 in this study. In contrary, the PHOEBE trial and the PHENIX study didn’t recruit any patient who was previously treated with pertuzumab and T-DM1. Hence, we have recruited a relative treatment refractory population. In China, pertuzumab and T-DM1 have been approved these years and they are more commonly prescribed as front-line therapy for HER2-positive breast cancer patients. As such, the characteristics of our study population may be more closer to those of real-world second-line patients and the results might provide available data for the treatment of general HER2-positive MBC patients in settings outside clinical trials. In addition, the chemotherapeutic agent in combination with pyrotinib was vinorelbine instead of capecitabine in our study, which may lead to the difference of the efficacy data.
As mentioned above, the PHOEBE study and the PHENIX trial both excluded patients with previous pertuzumab treatment. In the current study, we recruited nine patients (23.1%) who had received pertuzumab and found that the PFS of patients without prior pertuzumab exposure was significantly longer than that of patients who have received pertuzumab (8.3 vs. 4.6 months, p=0.017). This study not only provided data of second-line therapeutic effect of pyrotinib-containing regimens after pertuzumab treatment, but also indicated that previous pertuzumab exposure may affect the effectiveness of pyrotinib. The potential mechanisms of resistance to anti-HER2 antibodies including HER2 splicing, alternative elevations of other receptor tyrosine kinases, and intracellular alterations such as phosphoinositide 3-kinase (PI3K) pathway–associated mutations, Fc-gamma receptor polymorphisms, and so on [23-25]. In addition, pyrotinib resistance may be associated with activation of PI3K/AKT and mitogen-activated protein kinase signaling according to previous study [26]. It’s speculated that PI3K pathway, which was the common pathway associated with pertuzumab and pyrotinib resistance, may play a role in the shorter PFS of pertuzumab-treated patients than that of pertuzumab-naive patients. Experiments exploring the potential mechanism and larger trials recruiting more pertuzumab-treated patients are required to verify this interesting finding.
The safety profile of pyrotinib plus vinorelbine in our study was consistent with that reported in previous studies of pyrotinib-containing regimens [14,18,19,22]. Diarrhea (89.7%) and vomiting (48.7%) were found to be the most common AEs in the present trial. The primary prophylaxis for diarrhea and vomiting were permitted and diarrhea could be generally reversed with anti-diarrhea treatment, dose interruption, or dose reduction. The incidences of grade 3 diarrhea and vomiting were present in 28.2% and 5.1% of participants with no grade 4 diarrhea or vomiting in the current study. Moreover, 10.3% and 7.7% of patients experienced grade 3 or 4 neutropenia and leukopenia, respectively. The incidence was higher than that reported in previous retrospective studies of pyrotinib and vinorelbine [18,19], where the AEs were underrated by cause of missing data. Two patients (5.1%) presented grade 4 neutrophil count decreased, but no febrile neutropenia were reported. All AEs were effectively controlled and there were no treatment-related death during the trial.
Interestingly, patients in our study used vinorelbine either orally or intravenously. There was no significant difference in the efficacy between the oral and intravenous vinorelbine groups. Although the absolute value of PFS in the oral group was higher than that in the intravenous group, there was no significant difference. In terms of side effects, patients received oral vinorelbine experienced more obvious gastrointestinal reactions, but had lower incidence of myelosuppression. It’s suggested that doctors should pay more attention to the management of gastrointestinal reactions when using double oral regimen. In addition, one of the most important toxicities that clinicians are concerned about is bone marrow suppression, as there is a risk of febrile neutropenia, infection, and even septic shock. From this point of view, the dual oral regimen with lower incidence of myelosuppression has a safety advantage compared with intravenous vinorelbine plus pyrotinib. In addition, given that pyrotinib is an oral agent, oral vinorelbine can be a better combination partner for patients because the oral regimen avoid phlebitis and the implantation of venous access, which reduces the risk of venous access-related thrombosis or infection. According to previous studies, pharmacokinetic behavior and efficacy were similar for oral and intravenous vinorelbine at therapeutic dosage levels [27-32]. Besides, a survey on the acceptance of oral chemotherapy in breast cancer patients showed that 89% of patients who had previously received oral chemotherapy tended to choose oral chemotherapy, but among the patients who were treated with intravenous chemotherapy, most patients (67%) would choose oral chemotherapy [33]. It’s demonstrated that most breast cancer patients were more inclined to choose oral chemotherapy when the treatment effect is equivalent. In addition, compared with intravenous chemotherapy, oral chemotherapy improve the quality of life of breast cancer patients, as the physiological and psychological function of patients who received oral chemotherapy tend to be normal [34].
Our study has several limitations. Firstly, due to the single-arm design of the study, selection bias couldn’t be ruled out without a comparator treatment arm. Secondly, the length of follow-up was not long enough to obtain the overall survival data. Furthermore, the sample size was too small to draw the definitive efficacy and the safety profile. Nevertheless, the present study had certain strengths. To our knowledge, this is the first prospective clinical trial that provides evidence to support the use of pyrotinib plus vinorelbine in HER2-positive MBC patients. Besides, we recruited patients with prior pertuzumab and T-DM1 exposure, who were more closer to real-world patients, providing a theoretical guidance for clinicians.
Promising effects and tolerable toxicity of the combination therapy of pyrotinib and vinorelbine in the second-line treatment of HER2-positive MBC patients with prior trastuzumab or trastuzumab plus pertuzumab were observed in this multicenter, single-arm, prospective phase II trial. Since this is a single-arm study with small sample size, additional large-sample randomized controlled trials are required to further exploit the potential of pyrotinib plus vinorelbine.
NotesEthical Statement The study was approved by the relevant institutional review board or ethics committee of Sun Yat-sen University Cancer Center (IRB No. B2020-111-01), Meizhou People’s Hospital (IRB No. 2020-C-35), and Shantou Central Hospital (IRB No.[2020]001). All the patients provided written informed consent. Author Contributions Conceived and designed the analysis: Jiang K, Hong R, Xia W, Xu F, Wang S. Collected the data: Jiang K, Hong R, Xia W, Lu Q, Li L, Huang J, Zhang J. Contributed data or analysis tools: Li L, Huang J, Shi Y, Yuan Z, Zheng Q, An X, Xue C, Huang J, Bi X, Chen M, Xu F, Wang S. Performed the analysis: Jiang K, Hong R, Xia W, Lu Q. Wrote the paper: Jiang K, Hong R, Xia W, Lu Q, Li L, Huang J, Shi Y, Yuan Z, Zheng Q, An X, Xue C, Huang J, Bi X, Chen M, Zhang J, Xu F, Wang S. AcknowledgmentsThis work was partially supported by the Sun Yat-sen University Clinical Research 5010 Program (2017011).
We would like to thank all the patients participating in this study. We are grateful to all medical staff, staff nurses, and research nurses at the three medical centers.
Fig. 4.Kaplan-Meier plot for progression-free survival of patients with or without prior pertuzumab exposure. 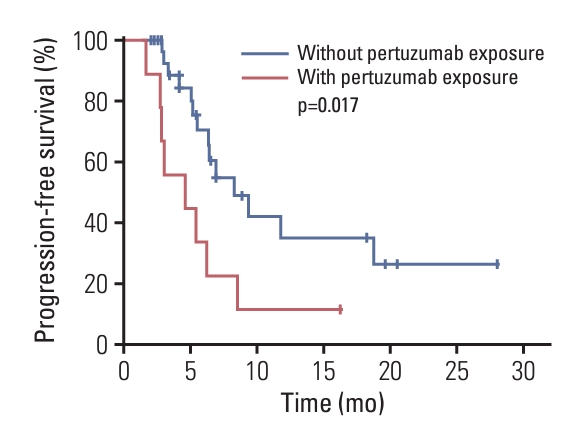
Fig. 5.Kaplan-Meier plot for progression-free survival of patients treated with oral or intravenous vinorelbine 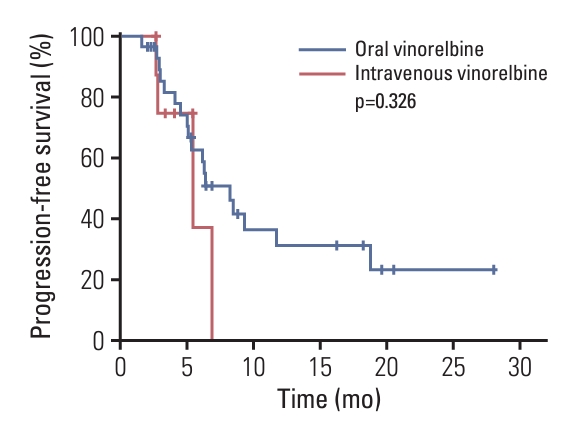
Table 1.Baseline demographic and clinical characteristics of the patients
Table 2.Efficacy outcomes Table 3.Efficacy outcomes of patients treated with oral or intravenous vinorelbine Table 4.Adverse events in the safety population Table 5.Adverse events in patients treated with oral or intravenous vinorelbine References1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
3. Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–9.
4. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
5. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34.
6. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
7. Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–54.
8. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43.
9. Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2:1557–64.
10. Li X, Yang C, Wan H, Zhang G, Feng J, Zhang L, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51–61.
12. Li Q, Guan X, Chen S, Yi Z, Lan B, Xing P, et al. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase I clinical trial. Clin Cancer Res. 2019;25:5212–20.
13. Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Oncol. 2019;37:2610–9.
14. Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:351–60.
15. Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–49.
16. Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007;110:965–72.
17. Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol. 2011;29:264–71.
18. Li Y, Qiu Y, Li H, Luo T, Li W, Wang H, et al. Pyrotinib combined with vinorelbine in HER2-positive metastatic breast cancer: a multicenter retrospective study. Front Oncol. 2021;11:664429.
19. Xie Y, Li Y, Ting L, Sang D, Yuan P, Li W, et al. Pyrotinib plus vinorelbine versus lapatinib plus capecitabine in patients with previously treated HER2-positive metastatic breast cancer: a multicenter, retrospective study. Front Oncol. 2021;11:699333.
20. Yin S, Chi Y, Du Y, Wang J, Shan C, Yi W, et al. Efficacy and safety of pyrotinib-containing regimen in the patients with HER2-positive metastatic breast cancer: a multicenter real-world study. Cancer Med. 2023;12:2333–44.
21. Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22.
22. Yan M, Bian L, Hu X, Zhang Q, Ouyang Q, Feng J, et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res. 2020;1:1–13.
23. Singla H, Kalra S, Kheterpal P, Kumar V, Munshi A. Role of genomic alterations in HER2 positive breast carcinoma: focus on susceptibility and trastuzumab-therapy. Curr Cancer Drug Targets. 2017;17:344–56.
24. Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62.
25. Singla H, Munshi A. HER2 tyrosine kinase inhibitors in the sensitization to cancers resistant to HER2 antibodies. Crit Rev Oncog. 2020;25:241–50.
26. Su B, Huang T, Jin Y, Yin H, Qiu H, Yuan X. Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways. Gastric Cancer. 2021;24:352–67.
27. Marty M, Fumoleau P, Adenis A, Rousseau Y, Merrouche Y, Robinet G, et al. Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors. Ann Oncol. 2001;12:1643–9.
28. Bonneterre J, Chevalier B, Focan C, Mauriac L, Piccart M. Phase I and pharmacokinetic study of weekly oral therapy with vinorelbine in patients with advanced breast cancer (ABC). Ann Oncol. 2001;12:1683–91.
29. Petain A, Zhong D, Chen X, Li Z, Zhimin S, Zefei J, et al. Effect of ethnicity on vinorelbine pharmacokinetics: a population pharmacokinetics analysis. Cancer Chemother Pharmacol. 2019;84:373–82.
30. Jassem J, Ramlau R, Karnicka-Mlodkowska H, Krawczyk K, Krzakowski M, Zatloukal P, et al. A multicenter randomized phase II study of oral vs. intravenous vinorelbine in advanced non-small-cell lung cancer patients. Ann Oncol. 2001;12:1375–81.
31. Yang Y, Chang J, Huang C, Zhang Y, Wang J, Shu Y, et al. A randomised, multicentre open-label phase II study to evaluate the efficacy, tolerability and pharmacokinetics of oral vinorelbine plus cisplatin versus intravenous vinorelbine plus cisplatin in Chinese patients with chemotherapy-naive unresectable or metastatic non-small cell lung cancer. J Thorac Dis. 2019;11:3347–59.
32. Huang L, Wang X, Zhou L, Di L, Zheng H, Jiang Z, et al. Oral vinorelbine versus intravenous vinorelbine, in combination with epirubicin as first-line chemotherapy in Chinese patients with metastatic breast cancer. Cancer Chemother Pharmacol. 2020;85:205–15.
|
|