AbstractPurposeThis study aimed to determine the role of local ablative radiotherapy (LART) in oligometastatic/oligoprogressive lung adenocarcinoma.
Materials and MethodsPatients (n=176) with oligometastatic lung adenocarcinoma treated with LART were identified, and those treated with LART at the initial diagnosis of synchronous oligometastatic disease (OMD group) or treated with LART when they presented with repeat oligoprogression (OPD group) were included.
ResultsIn the OMD group (n=54), the 1- and 3-year progression-free survival (PFS) were 50.9% and 22.5%, respectively, whereas the 1- and 3-year overall survival in the OPD group were 75.9% and 58.1%, respectively. Forty-one patients (75.9%) received LART at all gross disease sites. Tyrosine kinase inhibitor (TKI) use and all-metastatic site LART were significant predictors of higher PFS (p=0.018 and p=0.046, respectively). In patients treated with TKIs at the time of LART (n=23) and those treated with all-metastatic site LART, the 1-year PFS was 86.7%, while that of patients not treated with all-metastatic site LART was 37.5% (p=0.006). In the OPD group (n=122), 67.2% of the patients (n=82) maintained a systemic therapy regimen after LART. The cumulative incidence of changing systemic therapy was 39.6%, 62.9%, and 78.5% at 6 months, 1 year, and 2 years after LART, respectively.
ConclusionAggressive LART can be an option to improve survival in patients with oligometastatic disease. Patients with synchronous oligometastatic disease receiving TKI and all-metastatic site LART may have improved PFS. In patients with repeat oligoprogression, LART might potentially extend survival by delaying the need to change the systemic treatment regimen.
IntroductionOligometastasis is biologically defined as an intermediate state between locally confined and widely spread metastatic disease and is characterized by a limited number of metastases (e.g., ≤ 5) [1]. Patients with oligometastasis may benefit from combination treatment at the primary site and/or the metastatic lesions as well as systemic therapy. Many systematic and retrospective reviews have presented the long-term survival benefits in patients treated with surgical resection, stereotactic body radiotherapy, or radiofrequency ablation (RFA) of oligometastases [2-7]. Additionally, recent phase II clinical trials in patients with oligometastatic non–small cell lung cancer (NSCLC) demonstrated that combining local ablative radiotherapy (LART) with systemic therapy prolonged progression-free survival (PFS) and overall survival (OS), compared with treatment with systemic therapy alone [8-10]. Although such good treatment outcomes can be expected supporting the use of active local treatment in oligometastatic disease, many unknowns remain regarding patient selection, which metastatic lesions should be treated, or whether treating all-metastatic sites provides clinical benefit.
Furthermore, considering LART for oligoprogressive disease, where one or a few metastatic lesions are growing while all other lesions remain stable on systemic treatment, is increasingly gaining attention [11]. Such a clinical setting falls into the “repeat oligoprogression” category in the oligometastatic disease classification system described by Guckenberger et al. [12] In contrast to the de novo oligometastatic disease setting, repeat oligoprogression occurs in the presence of a high tumor volume with multiple metastatic lesions [11,13,14]. The European Society for Radiotherapy and Oncology (ESTRO)–American Society for Radiation Oncology (ASTRO) Oligometastatic Disease Consensus document, published in 2020, also stated that oligoprogression on systemic therapy is a clinical entity that differs from oligometastasis, possibly with worse prognosis than that of de novo or isolated metastatic disease [15-20]. Therefore, the major goals of treatment should differ between these, and treatment of only the growing lesions in patients with oligoprogression may be performed to delay the need to change the systemic therapy.
Most studies on multisite LART for metastatic cancer have included various types of cancer and histologies. Therefore, the results may have been diluted by the use of various systemic therapies and different natural disease courses [4,21]. To the best of our knowledge, no such studies have addressed the outcomes of LART for specific lung cancer histological subtypes. Nevertheless, this is crucial given that lung cancers of different histological types are completely different disease entities. For instance, lung adenocarcinoma shows different gene mutation profiles from other squamous cell carcinomas or small cell carcinomas and, thus, demonstrates a completely different therapeutic response. Patients with epidermal growth factor receptor (EGFR) mutations can survive for an extended period using tyrosine kinase inhibitors (TKIs) [22]. As patients diagnosed with lung adenocarcinoma are relatively younger and in better condition than those diagnosed with other types of lung cancer, there is a greater need for active treatment [23]. Thus, identifying suitable candidates that can benefit from LART is of clinical importance. Therefore, this study aimed to determine the role of LART in oligometastatic/oligoprogressive lung adenocarcinoma and identify the predictive factors for the survival of this cohort.
Materials and Methods1. Patient eligibilityPatients presenting to a single institution between 2013 and 2020, who were initially diagnosed with stage IV lung adenocarcinoma, with ≤ 3 synchronous metastases at the time of initial diagnosis, and who were treated with LART to any lesion, were included for analysis. Among these patients, those who were treated with LART at the initial diagnosis of synchronous oligometastatic disease and those who were treated with LART when they presented with repeat oligoprogression, as described by Guckenberger et al. [12] were eligible for inclusion. Patients who presented with repeat oligorecurrence or repeat oligopersistence were excluded from the analysis. Additionally, patients who did not undergo any systemic therapy at the time of LART and those who had uncontrolled brain metastases were excluded.
Patients diagnosed with a synchronous oligometastatic disease and who received LART as the first-line treatment were allocated to the oligometastatic disease (OMD) group. The remaining patients who received LART when they presented with repeat oligoprogression while under active systemic treatment were classified as the oligoprogression (OPD) group. Since repeat oligoprogression is a distinct disease entity from de novo oligometastasis, in that it follows a more aggressive course due to the high background tumor volume, we decided to analyze the results of these groups separately. Referring to the ASTRO Oligometastatic Disease Consensus document, oligoprogression was defined as ≤ 3 sites of metastatic disease progression while the other sites remained stable [15].
Cases were retrospectively identified by searching the registry data of the institution. All patient records were manually reviewed to determine eligibility for inclusion based on the above criteria. Patients were staged at the time of diagnosis using brain magnetic resonance imaging (MRI), wholebody positron emission tomography–computed tomography (PET-CT), and contrast-enhanced chest/abdomen CT. We used the American Joint Committee on Cancer, 8th edition definitions for staging patients. Intrathoracic nodal disease was counted as one site; however, multiple lesions within a single organ were counted as individual, discrete sites, as in previous studies [24,25].
2. LART detailsLART was administered to the primary and/or metastatic lesions at the discretion of the radiation oncologist. Single-fraction cumulative doses ≥ 12 Gy, 2-fraction doses ≥ 20 Gy, 3-fraction doses ≥ 24 Gy, 4-fraction doses ≥ 48 Gy, 5-fraction doses ≥ 30 Gy, and 10-fraction doses ≥ 50 Gy prescribed to the planning tumor volume (PTV) were regarded as LART. An alternative acceptable prescription was 45 Gy in 15 fractions. A prescription for an 80%-90% isodose line was preferred for the treatment of bone metastases, and a prescription for a 70%-90% isodose line was most commonly used for other treatment sites. Immobilization devices, such as vacuum locks and thermoplastic masks, were appropriately used. Continuous positive airway pressure or an abdominal compressor was also used to reduce tumor motion when necessary. Gross tumor volumes (GTVs) were contoured from CT simulation scans. For spine metastases, MRI was fused with the planning CT to aid in GTV delineation. No clinical target volume expansion for microscopic disease extension was used, except for bone metastases. A uniform PTV expansion of 3-5 mm was used in all cases.
Treatment was delivered using linear accelerators with daily cone-beam CT and tomotherapy with daily megavoltage CT. As for CyberKnife M6 (Accuray, Sunnyvale, CA), orthogonal radiographs that visualized fiducial markers and/or the spine were acquired for image-based guidance before every treatment. For patients who were candidates for CyberKnife M6 treatment, fiducial markers were implanted near the tumor for tracking. Spine tracking without fiducials was also allowed in patients whose tumor was adjacent to the spine.
3. EndpointsThe primary endpoint was PFS, while the secondary endpoints were OS, the cumulative incidence of local failure, local failure-free survival (LFFS), and toxicity. The cumulative incidence of changing systemic therapy (CST) was also analyzed as a secondary endpoint in the OPD group. PFS was defined as the time from commencing LART to the time of disease progression or death, whichever occurred first. Progression was defined by the radiologist, based on the Response Evaluation Criteria in Solid Tumor (RECIST) criteria 1.1. OS was defined as the time from commencing LART to the date of last contact for surviving patients or death. Local failure was defined as recurrence or progression within the treatment field after radiotherapy (RT). For determining the cumulative incidence of CST, switching systemic therapy to the next-line regimen was counted as the event in patients in the OPD group.
4. Follow-up and response assessmentThe first follow-up was performed 6-8 weeks after LART, using contrast-enhanced CT and/or MRI or PET-CT. Thereafter, follow-up was performed every 2-3 months with CT for the first 2 years after LART and every 6 months starting from the third year. Additional imaging studies, including MRI and/or PET-CT, were performed in cases of suspected progressive disease. Acute and late radiation treatment-related toxicities were scored according to Common Terminology Criteria for Adverse Events v4.0.
5. Statistical analysisUnivariate and multivariate Cox proportional-hazards regression modeling were performed to identify factors associated with PFS, OS, and LFFS. Variables with a p < 0.05 in univariate analysis were subsequently tested in the multivariate model. Kaplan-Meier curves were generated to analyze survival trends in the cohorts. Death was censored and patients alive at the end of the study period were censored at the time of the last follow-up. The equivalent doses in 2 Gy fractions (EQD2) calculation was performed using an alpha/beta ratio of 10. A chi-square test was used to compare categorical variables. Statistical calculations were performed using SPSS ver. 26.0 (IBM Corp., Armonk, NY).
Results1. Patients and treatmentTwo-hundred and eighty-one patients who were diagnosed with stage IV lung adenocarcinoma with ≤ 3 metastases at initial diagnosis and were treated with LART were included for analysis. Patients with repeat oligorecurrence or repeat oligopersistence (n=51) were excluded. Of the 87 patients initially diagnosed with synchronous oligometastasis, 23 patients who did not undergo systemic therapy and 10 who had uncontrolled brain metastases were excluded. Thus, 54 patients were finally included in the OMD group. Among 143 patients treated with LART presenting with repeat oligoprogression, 21 who had uncontrolled brain metastases were excluded; hence, 122 patients were included in the OPD group (S1 Fig.).
The median follow-up time for the entire cohort was 18.2 months (range, 0.3 to 71.2 months). The median EQD2 for RT treatments to the tumor was 43.9 Gy (range, 22.0 to 150.0 Gy). Therefore, an EQD2 cut-off value of 43.9 Gy was used to dichotomize this variable. Ninety-two patients were treated with volumetric-modulated arc therapy (52.3%), 63 were treated with CyberKnife M6 (35.8%), 14 were treated with three-dimensional conformal RT (8.0%), and seven patients were treated with tomotherapy (4.0%). Table 1 describes the patient, tumor, and treatment characteristics. In the OMD group, 25 patients (46.3%) presented with EGFR mutation, four patients (7.4%) with anaplastic lymphoma kinase (ALK)–positive status, and nine patients (16.7%) with c-ros oncogene 1 (ROS1)–positive status. Thirty-seven patients (68.5%) had a single metastatic lesion, 12 patients (22.2%) had two lesions, and five patients (9.3%) had three lesions. Bone metastasis was the most common site of LART (63.6%). Among these patients, 41 (75.9%) received LART at all-metastatic gross disease sites, and this strategy was defined as all-metastatic site RT. In the OPD group (n=122), 58 patients (47.5%) presented with EGFR mutations, seven patients (5.7%) with ALK-positive status, and seven patients (5.7%) with ROS1-positive status. ROS1-positive status was the only factor that showed a significant difference between the two groups (ROS1-positive 16.7% in OMD group vs. 5.7% in OPD group, p=0.020).
The median PFS of the oligometastasis and oligoprogression groups were 12.3 and 4.0 months, respectively (p=0.001) (S2A Fig.). The median OS of the oligometastasis and oligoprogression groups were 32.5 and 14.3 months, respectively (p=0.043) (S2B Fig.). In the univariate and multivariable analysis, oligoprogression was significantly associated with worse PFS (p=0.002) but not OS (p=0.056). In contrast, EGFR mutation status was a significant predictor of OS (p=0.014), but not of PFS (p=0.425). These results are shown in S3 Table.
2. Prognosis of the OMD groupThe median PFS and OS were 12.3 months (95% confidence interval [CI], 7.5 to 17.1) and 32.5 months (95% CI, 23.0 to 42.1), respectively, in the OMD group (Fig. 1A and B). The 1- and 3-year PFS in this group were 50.9% and 22.5%, respectively, whereas their 1- and 3-year OS were 75.9% and 58.1%, respectively. The 1-year and 3-year cumulative incidence of local failure were 4.8% and 24.0%, respectively.
On univariate analysis, the use of TKIs (as opposed to no-TKI) as the first-line systemic regimen and of all-metastatic site LART (as opposed to not all-metastatic site LART) predicted an improved PFS (hazard ratio [HR], 0.48; 95% CI, 0.27 to 0.83; p=0.018; and HR, 0.68; 95% CI, 0.53 to 0.83; p=0.041, respectively). Multivariate analysis was subsequently performed using the significant variables: use of TKI (vs. no-TKI) and all-metastatic site LART (vs. not all-metastatic site LART) remained significant predictors of PFS (HR, 0.44; 95% CI, 0.22 to 0.87; p=0.018, and HR, 0.78; 95% CI, 0.36 to 0.94; p=0.046, respectively) (Table 2). On univariate analysis for OS, EGFR mutation status, the number of metastatic lesions, and the use of TKI over no-TKI predicted improved OS (HR, 0.37; 95% CI, 0.17 to 0.79; p=0.011; HR, 1.72; 95% CI, 1.07 to 2.77; p=0.025; and HR, 0.44; 95% CI, 0.20 to 0.99; p=0.049, respectively). Multivariable analysis was subsequently performed using the significant variables. EGFR mutation status and the number of metastatic lesions remained significant predictors of OS in this analysis (HR, 0.37; 95% CI, 0.17 to 0.79; p=0.011, and HR, 1.70; 95% CI, 1.07 to 2.63; p=0.024, respectively) (Table 2). In the univariate analysis, no variable was found to be significantly associated with LFFS (S4 Table).
In the subset of patients who were administered TKI at the time of LART (n=23) and who were treated with LART to all-metastatic sites, the 1-year PFS was 86.7%, whereas that of the patients who were not treated with LART to all-metastatic sites was 37.5% (p=0.006) (Fig. 2A). All-metastatic site LART did not yield PFS benefits in patients who were treated with immunotherapy or cytotoxic chemotherapy (CTx) as the first-line systemic therapy. In the OMD group (n=54), the 1-year PFS was 69.1% for patients treated with TKI, and 36.4% for patients not treated with TKI (p=0.015) (Fig. 2B).
3. Prognosis of the OPD groupThe median PFS and OS in the OPD group were 4.0 months (95% CI, 3.1 to 4.9 months) and 14.3 months (95% CI, 8.9 to 19.6 months), respectively (S5 Fig.). The 1- and 3-year PFS were 22.0% and 11.6%, respectively, whereas the 1- and 3-year OS were 57.4% and 26.4%, respectively. The 1-year and 3-year cumulative incidence of local failure were 23.4% and 36.5%, respectively. In univariate analysis, no variable was found to be significantly associated with PFS. Programmed death-ligand 1 (PD-L1) status was a significant risk factor for OS in univariate analysis (HR, 1.66; 95% CI, 1.08 to 2.57; p=0.022) but not in multivariate analysis (S6 Table). In the univariate analysis, no variable was found to be significantly associated with LFFS (S4 Table).
At the time of LART, 50 patients were being treated with TKIs (41.7%), 45 patients with immunotherapy (35.8%), and 27 patients with cytotoxic CTx (22.5%). Among these cases, 35 were on the first-line (28.7%), 44 on the second-line (36.1%), five on the third-line (20.5%), and the rest (14.8%) on the fourth-line or later systemic therapy. After LART, 67.2% of the patients (n=82) maintained the salvage systemic therapy regimen that was being administered at the time of LART (S7 Table). Among these patients, the cumulative incidence of CST was 39.6%, 62.9%, and 78.5% at 6 months, 1 year, and 2 years after LART, respectively (Fig. 3). The median delay in CST was 7.0 months (range, 0.4 to 43.0 months). In the subset of patients who were treated with TKI at the time of LART, the median delay of CST was 11.1 months (range, 0.4 to 43.0 months).
4. Toxicity of LARTTreatment-related toxicity was well-tolerated in the OMD group. None of the patients developed grade 3 or higher adverse effects such as cough, dermatitis, radiation pneumonitis, esophagitis, or radiation treatment-related death in the OMD group. One patient developed grade 2 radiation-induced dermatitis (1.9%) and another patient developed grade 2 esophagitis (1.9%). Grade 1 lung fibrosis was seen in one patient (1.9%). RT-related toxicities were similar in the subset of patients treated with TKIs in the OMD group. Grade 1 radiation pneumonitis developed in one of 15 patients (6.7%) in the all-metastatic site RT group and in none of the eight patients in the group not treated with all-metastatic site RT; hence, a non-significant difference in Fisher’s exact test was observed (p > 0.99).
In the OPD group, no patient experienced grade ≥ 3 cough, dermatitis, radiation pneumonitis, esophagitis, or radiation treatment-related death. Grade 2 dermatitis and grade 2 radiation pneumonitis were seen in one patient (0.8%). Grade 1 lung fibrosis was observed in one patient (0.8%).
DiscussionIn this study, patients with repeat oligoprogression had shorter PFS compared to those with synchronous oligometastasis. We also revealed that the use of TKIs and administration of LART to all-metastatic sites was associated with improved PFS in patients who presented with oligometastasis at initial diagnosis. Furthermore, all-metastatic site LART did not result in severe toxicity. Moreover, in oligoprogressive cases, the use of LART might have the benefit of delaying CST.
Interest in using local therapy at oligometastatic sites is increasing. A phase 2 trial that randomized patients with metastatic colorectal cancer with liver metastases to treatment with or without RFA found that RFA significantly decreased disease progression and improved OS [26]. Small randomized phase 2 trials have demonstrated that local ablative therapy in the setting of oligometastatic NSCLC can significantly improve PFS [8,25], and longer follow-up of one of these study cohorts has also revealed an OS benefit [9].
Several potential mechanisms may explain the benefit of all-metastatic site LART. First, treating all-metastatic tumors could reduce the burden of treatment-resistant cells. Second, treating multiple lesions with ablative doses may potentiate the effects of systemic therapy. LART to all sites could also alter the immune system and tumor microenvironment, rendering residual disease more sensitive to subsequent maintenance therapy, thereby delaying progression [27]. In addition, adding LART to a backbone of immunotherapy has been of great interest. RT and immunotherapy show excellent synergy and this combination has the potential of being enhanced by targeting multiple lesions rather than using single-target ablation. A single-arm study of patients with oligometastatic NSCLC who were treated with definitive local therapies at all sites followed by pembrolizumab found a median PFS of 19.1 months, which was significantly prolonged compared with the expected PFS of 6.6 months (p=0.005) based on historical data [28]. However, in the present study, all-metastatic site LART did not substantially benefit patients who were treated with immunotherapy as the first-line systemic therapy. One of the reasons may be the small sample size of the immunotherapy group. Further studies are needed to verify these findings in a larger number of patients.
The use of immunotherapy or cytotoxic CTx as the first-line systemic therapy was independently associated with poor PFS after LART, compared with patients who used TKIs as systemic therapy. Those treated with immunotherapy or cytotoxic CTx as the first-line therapy were EGFR wild-type; therefore, the poor prognosis of these patients could not be overcome despite the addition of LART. It is well known that patients with EGFR-mutant metastatic lung adenocarcinoma treated with TKIs demonstrate significantly better survival outcomes compared to those with EGFR wild-type metastatic lung adenocarcinoma [22]. However, as the number of patients in each group was small, it is difficult to draw firm conclusions; therefore, a large-scale prospective study should be conducted on each group by dividing the patient groups according to molecular types or the level of PD-L1 expression in the future.
Furthermore, we found that in patients who presented with repeat oligoprogression, intervention with LART might delay the need to change systemic therapy. The desire to delay the need to start or to change systemic therapy in some patients is increasing, particularly when the volume of progressing metastases is low. Patients who are older or have poor performance status, who are not good candidates for aggressive CTx, could be candidates in this regard. In addition, there may be a need for a “chemotherapy break” or “chemotherapy holiday” in some patients, while others may want to delay the need to change systemic therapy owing to the limited systemic therapy options left to use. Some studies reported the use of LART to delay the time to change systemic therapy in NSCLC cases. Gan et al. demonstrated that patients with ALK-positive NSCLC could be maintained on TKIs for extended periods when using LART [29]. The benefit of LART in terms of delaying the change in systemic therapy has been realized in patients with metastatic colorectal cancer: the cumulative incidence of starting or CST in these patients was 41.7% at 2 years post-LART, implying that more than half of the patients did not require a change in systemic treatment strategy in that period [30]. In the present study, more than two-thirds of the patients in the OPD group maintained a salvage systemic therapy regimen using LART. Notably, more than one-fifth of patients were able to maintain systemic therapy for ≥ 2 years after LART, which suggests that there might be a potential benefit of using LART in patients with repeat oligoprogression. In the subset of patients who were treated with TKIs at the time of LART, CST was delayed even further. Thus, the potential benefit of delaying CST should be investigated more comprehensively in future prospective studies.
Since the evidence for using local ablative therapy for oligometastatic disease is increasing, identification of those patients likely to benefit from such therapy is essential and has been strongly debated. We aimed to identify the prognostic factors that are associated with clinical outcomes to facilitate identification of such patients. However, neither the number of metastatic lesions nor the site of metastases showed a significant association with disease progression in patients who presented with synchronous oligometastatic disease at initial diagnosis. EGFR mutation, ALK rearrangement, and PD-L1 expression status also demonstrated no significant association with disease progression after LART. This might be due to the small sample size of the patients included. Further studies with a larger number of patients addressing this issue will be required. Currently, there is no biological evidence supporting the maximal number of metastases or the maximal lesion size that should be treated to provide clinical benefit [15].
EQD2 was not an independent predictor of improved PFS, OS, or LFFS in this study. This may be due to the low median dose of EQD2 (43.9 Gy), which might have been insufficient to achieve good local control. In a case-control study of local consolidative therapy for oligometastatic NSCLC, there was a dose-response effect, such that delivery of > 75 Gy biologically effective dose (BED) (α/β=10) in patients with synchronous oligometastatic NSCLC was associated with improved local control, PFS, and OS [31]. A multi-institutional study of patients with stage I NSCLC showed that LART regimens with BED > 100 Gy are needed to improve local control and OS [32]. A recent analysis of the National Cancer Data Base suggests that achieving BED > 130 Gy may further improve survival rates [33]. Another reason might be the heterogeneous dose prescriptions used in our study, which may have made it difficult to draw a firm conclusion regarding the dose-response relationship. Further efforts to identify the factors that are associated with the clinical benefit of LART are required.
LART was well-tolerated by our patients. None of the patients in the OMD and OPD groups developed grade ≥ 3 cough, dermatitis, radiation pneumonitis, esophagitis, or radiation treatment-related death. Grade 2 radiation-induced dermatitis, esophagitis, and radiation pneumonitis rarely occurred in either of the groups. The addition of LART could be an effective treatment strategy that can be used in both the synchronous oligometastasis and repeat oligoprogression setting, without compromising safety outcomes.
Our study has several limitations. First, this was a retrospective study. Other untested variables could present confounding effects. A wide range of LART doses were used in the present study. In general, our institutional policy on LART dosing is based mainly on tumor location and the proximity of organs at risk. Additionally, the patients were treated for almost a decade, implying that there could have been a marked heterogeneity in terms of the comprehensive medical care they received, which possibly affected the survival outcomes and patterns. Only the patients treated with LART were included in this study. Further studies comparing systemic therapy alone vs. LART combined with systemic therapy in patients diagnosed with oligometastatic lung adenocarcinoma are required. Although all-metastatic site LART increased PFS, it did not improve OS. Further studies with larger samples and longer follow-up periods are needed to elucidate the potential benefits of all-metastatic site LART.
Aggressive LART could be proposed as an option to improve survival in patients with oligometastatic disease. Patients with synchronous oligometastatic disease receiving TKI and all-metastatic site LART may show improved PFS. In patients with repeat oligoprogression, LART might potentially extend the survival by delaying the need to change the systemic treatment regimen.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the institutional review board of Severance Hospital, which waived the need for obtaining informed patient consent (IRB no. 4-2022-0500). Author Contributions Conceived and designed the analysis: Yang G, Cho Y. Collected the data: Yang G. Contributed data or analysis tools: Yang G, Kim KH, Lee CG, Hong MH, Kim HR, Cho Y, Yoon HI. Performed the analysis: Yang G, Cho Y. Wrote the paper: Yang G. Writing - reviewing and editing: Kim KH, Lee CG, Hong MH, Kim HR, Cho Y, Yoon HI. AcknowledgmentsThis study was supported by a faculty research grant of Yonsei University College of Medicine (6-2020-0225) and by National Research Foundation of Korea Grant funded by the Korean Government (NRF-2021R1A2C1007191).
Fig. 1.Probability of progression-free survival (A) and overall survival (B) in the oligometastasis group (n=54). LART, local ablative radiotherapy. 
Fig. 2.(A) Progression-free survival of the patients in the oligometastasis group treated with tyrosine kinase inhibitors (TKIs, n=23) with all-metastatic site local ablative radiotherapy (LART) or not all-metastatic site LART. (B) Progression-free survival of patients who were treated with or without TKIs in the oligometastasis group (n=54). 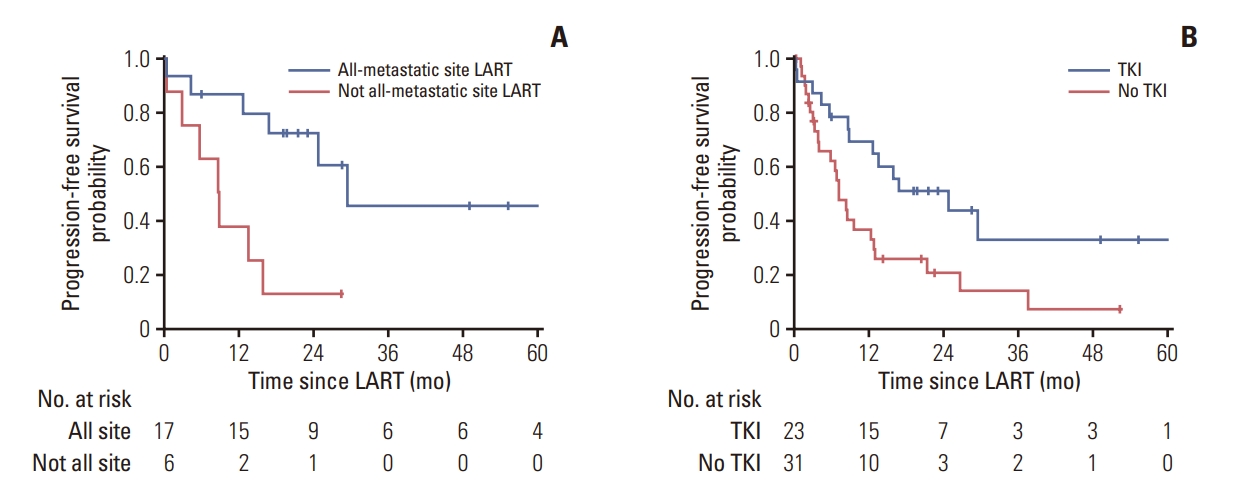
Fig. 3.Cumulative incidence of changing systemic therapy in the oligoprogression group (n=122). LART, local ablative radiotherapy. 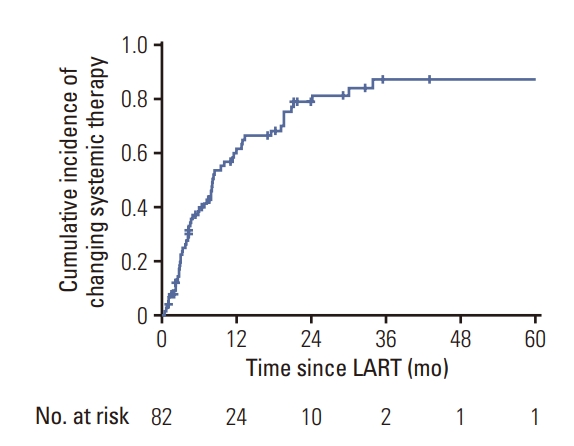
Table 1.Patient and treatment characteristics in the oligometastasis (n=54) and oligoprogression groups (n=122) Table 2.Univariate and multivariate analysis in the oligometastasis group (n=54) ALK, anaplastic lymphoma receptor tyrosine kinase; CI, confidence interval; EGFR, epidermal growth factor receptor; EQD2, equivalent doses in 2 Gy fractions; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; ROS1, c-ros oncogene 1; RT, radiotherapy; TKI, tyrosine kinase inhibitor. References2. Casiraghi M, De Pas T, Maisonneuve P, Brambilla D, Ciprandi B, Galetta D, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the “international registry of lung metastases”. J Thorac Oncol. 2011;6:1373–8.
3. Aitken K, Tree A, Thomas K, Nutting C, Hawkins M, Tait D, et al. Initial UK experience of stereotactic body radiotherapy for extracranial oligometastases: can we change the therapeutic paradigm? Clin Oncol (R Coll Radiol). 2015;27:411–9.
4. Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–86.
5. Rim CH, Cho WK, Lee JH, Kim YS, Suh YG, Kim KH, et al. Role of Local treatment for oligometastasis: a comparability-based meta-analysis. Cancer Res Treat. 2022;54:953–69.
6. Kroese TE, van Laarhoven HW, Nilsson M, Lordick F, Guckenberger M, Ruurda JP, et al. Definition of oligometastatic esophagogastric cancer and impact of local oligometastasis-directed treatment: a systematic review and meta-analysis. Eur J Cancer. 2022;166:254–69.
7. Gutiontov SI, Pitroda SP, Tran PT, Weichselbaum RR. (Oligo) metastasis as a spectrum of disease. Cancer Res. 2021;81:2577–83.
8. Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4:e173501
9. Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–65.
10. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–8.
11. Cheung P. Stereotactic body radiotherapy for oligoprogressive cancer. Br J Radiol. 2016;89:20160251.
12. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–28.
13. Patel PH, Palma D, McDonald F, Tree AC. The Dandelion dilemma revisited for oligoprogression: treat the whole lawn or weed selectively? Clin Oncol (R Coll Radiol). 2019;31:824–33.
14. Tree AC, Khoo VS, Eeles RA, Ahmed M, Dearnaley DP, Hawkins MA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–37.
15. Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–66.
16. Lazzari R, Ronchi S, Gandini S, Surgo A, Volpe S, Piperno G, et al. Stereotactic body radiation therapy for oligometastatic ovarian cancer: a step toward a drug holiday. Int J Radiat Oncol Biol Phys. 2018;101:650–60.
17. Merino Lara T, Helou J, Poon I, Sahgal A, Chung HT, Chu W, et al. Multisite stereotactic body radiotherapy for metastatic non-small-cell lung cancer: delaying the need to start or change systemic therapy? Lung Cancer. 2018;124:219–26.
18. Santini D, Ratta R, Pantano F, De Lisi D, Maruzzo M, Galli L, et al. Outcome of oligoprogressing metastatic renal cell carcinoma patients treated with locoregional therapy: a multicenter retrospective analysis. Oncotarget. 2017;8:100708–16.
19. Pembroke CA, Fortin B, Kopek N. Comparison of survival and prognostic factors in patients treated with stereotactic body radiotherapy for oligometastases or oligoprogression. Radiother Oncol. 2018;127:493–500.
20. Triggiani L, Alongi F, Buglione M, Detti B, Santoni R, Bruni A, et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer. 2017;116:1520–5.
21. Sutera P, Clump DA, Kalash R, D’Ambrosio D, Mihai A, Wang H, et al. Initial results of a multicenter phase 2 trial of stereotactic ablative radiation therapy for oligometastatic cancer. Int J Radiat Oncol Biol Phys. 2019;103:116–22.
22. Lin JJ, Cardarella S, Lydon CA, Dahlberg SE, Jackman DM, Janne PA, et al. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol. 2016;11:556–65.
23. Charloux A, Quoix E, Wolkove N, Small D, Pauli G, Kreisman H. The increasing incidence of lung adenocarcinoma: reality or artefact?: a review of the epidemiology of lung adenocarcinoma. Int J Epidemiol. 1997;26:14–23.
24. Mitchell KG, Farooqi A, Ludmir EB, Corsini EM, Zhang J, Sepesi B, et al. Improved overall survival with comprehensive local consolidative therapy in synchronous oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2020;21:37–46.
25. Gomez DR, Blumenschein GR Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–82.
26. Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109:djx015.
27. Shirvani SM, Huntzinger CJ, Melcher T, Olcott PD, Voronenko Y, Bartlett-Roberto J, et al. Biology-guided radiotherapy: redefining the role of radiotherapy in metastatic cancer. Br J Radiol. 2021;94:20200873.
28. Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic non-small cell lung cancer: a phase 2 trial. JAMA Oncol. 2019;5:1283–90.
29. Gan GN, Weickhardt AJ, Scheier B, Doebele RC, Gaspar LE, Kavanagh BD, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014;88:892–8.
30. Thompson R, Cheung P, Chu W, Myrehaug S, Poon I, Sahgal A, et al. Outcomes of extra-cranial stereotactic body radiotherapy for metastatic colorectal cancer: dose and site of metastases matter. Radiother Oncol. 2020;142:236–45.
31. Farooqi A, Ludmir EB, Mitchell KG, Antonoff MB, Tang C, Lee P, et al. Increased biologically effective dose (BED) to the primary tumor is associated with improved survival in patients with oligometastatic NSCLC. Radiother Oncol. 2021;163:114–8.
|
|
|||||||||||||||||||||||||||||||||||||||||||