AbstractPurpose
BRCA1/2 mutations are well-known risk factors for breast and ovarian cancers in women. Risk-reducing salpingo-oophorectomy (RRSO) is the standard treatment for preventing ovarian cancer with BRCA mutations. Postmenopausal syndrome (symptoms after RRSO can be alleviated by hormone replacement therapy (HRT); however, the use of HRT in carriers of BRCA mutations has been controversial because of the concern that HRT increases the risk of breast cancer. This study aimed to evaluate the effects of HRT in BRCA mutation carriers who underwent RRSO.
Materials and MethodsA total of 151 carriers, who underwent RRSO between 2013 and 2020 after the diagnosis of BRCA1 or BRCA2 mutations were selected and followed up for a median of 3.03 years. Patients were divided into two groups: those who received HRT after RRSO (n=33) and those who did not (n=118). We compared the incidence of breast cancer over time between these two groups.
ResultsThere was no significant difference in the incidence of breast cancer between women who received HRT and those who did not (p=0.229). Multivariate logistic regression analysis, adjusted for age and parity revealed no significant difference in the risk of breast cancer between these two groups (hazard ratio, 0.312; 95% confidence interval, 0.039 to 2.480; p=0.278).
Introduction
BRCA1 and BRCA2 gene mutations are well-known risk factors for breast and ovarian cancers in women [1,2]. Riskreducing salpingo-oophorectomy (RRSO) is the standard prophylactic treatment for preventing ovarian cancer in women with BRCA mutations [3]. However, since most cases of RRSO are performed before menopause, those who undergo RRSO suffer from postmenopausal syndrome and adverse effects on cognition, mood, cardiovascular, bone, and sexual health [4-8]. These symptoms can be alleviated by hormone replacement therapy (HRT); however, the use of HRT in carriers of BRCA mutations has been controversial owing to the concern that HRT increases the risk of breast cancer.
In a previous study, the effect of HRT on increase in the risk of breast cancer in patients who underwent RRSO was conducted mostly in Caucasian [9-11]. There is no research conducted in Asia. The frequency of BRCA1/2 mutations in early-onset breast cancer in Asian and Caucasian populations is similar [12-14], and previous studies have found differences in breast cancer characteristics between Asians and Caucasians. The age of onset of breast cancer in Asians is lower than that in Caucasians [12], and the hormone receptor status in Asians also differs from that in Caucasians [15]. The goal of our study was to estimate the risk of breast cancer in Korean carriers of BRCA mutations who underwent HRT after undergoing RRSO.
Materials and Methods1. Healthcare system in KoreaIn the year 2000, the health insurance systems in South Korea were merged into a single system run by the Korea National Health Insurance Service (KNHIS). Consequently, most people living in South Korea are covered by the KNHIS. Study data were collected from the Korean National Health Insurance (KNHI) claims database between 2007 and 2015. In Korea, 97% of the population is obliged to enroll in the KNHI program, and the remaining 3% enroll under the Medical Aid Program [16]. The Health Insurance Review & Assessment Service (HIRA) is a government-affiliated organization that performs claims reviews and quality assessments for the KNHI. The data for the remaining 3% of the population covered by the Medical Aid Program were also reported to the HIRA. Therefore, the HIRA claims that the database contains information on all claims of approximately 50 million Koreans. Nearly all information regarding the disease can be obtained from this centralized database, except for the procedures not covered by insurance, such as cosmetic surgeries. The claims data of the HIRA include patient diagnosis, treatment, procedures, surgical history, and prescription drugs, which are valuable resources for clinical research. However, HIRA data are incomplete and do not include medical records such as laboratory results and cancer stage.
2. Study populationUsing the HIRA claims database, we retrieved all Korean women who were newly diagnosed with a BRCA mutation (Z40.0) and underwent RRSO between January 1, 2013, and December 31, 2021. Exclusion criteria were women diagnosed with breast cancer before RRSO, those who underwent mastectomy for any reasons and those who had not undergone RRSO. Patients diagnosed with BRCA mutation in 2021 and 2022 were excluded from the analysis because of the short follow-up period.
3. OutcomesFrom the HIRA database, women diagnosed with breast cancer after RRSO were identified according to the principal or secondary diagnosis by searching for the relevant International Classification of Diseases, 10th revision (ICD-10) codes. Women were classified as having breast cancer if they were newly diagnosed with breast cancer (ICD-10 codes: C50 malignant neoplasm of breast) from RRSO to December 31, 2021. The timing of each patient’s initial diagnosis was confirmed by the lack of a breast cancer diagnosis prior to the RRSO.
4. Measurement of patients’ characteristicsCharacteristics, such as age, parity, and postoperative HRT exposure, were obtained from the HIRA claims database. Disease stage was not available in the HIRA claims database.
5. Statistical analysisContinuous and categorical variables were expressed as means±standard deviations and percentages, respectively. Clinical characteristics were compared using the t test for continuous variables and the chi-square test for categorical variables. Cox proportional hazards models were used to estimate the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of occurrence of breast cancer. All tests were two-sided, and statistical significance was set at p < 0.05. Statistical analyses were performed using SAS Enterprise Guide ver. 6.1 (SAS Institute Inc., Cary, NC).
ResultsData from the HIRA were used with ICD codes from 2013 to 2020. Among the 8,122 diagnosed with BRCA mutation, 1,486 who underwent RRSO were selected. Among them, 151 patients had not been previously diagnosed with C50 (malignant neoplasm of the female breast), or C56 (malignant neoplasm of ovary) (Fig. 1). Thirty-three patients received HRT and 118 did not. Comparing the characteristics of the two groups, the group that received HRT had more patients under the age of 45 (p < 0.001), while the group that did not receive HRT had a significantly higher proportion of nulliparous patients (p=0.030). Of the patients who received HRT, six received progesterone alone (P), seven received estrogen alone (E), 18 received tibolone (L), and two received estrogen plus progesterone (E+P) (Table 1).
A total of 12 (7.9%, 12/151) patients were newly diagnosed with breast cancer, with a follow-up period of median 3.03 years. Among them, one patient (3.0%, 1/33) in the HRT group was diagnosed with breast cancer, while 11 patients (9.3%, 11/118) in the non-HRT group were diagnosed with breast cancer.
Regarding the risk of breast cancer according to HRT, there was no significant difference in the incidence of breast cancer between women who received any HRT regimen, and those who did not (p=0.229) (Fig. 2). When categorized by the age of 45 years, there were no significant differences in the incidence of breast cancer, regardless of whether the patient received HRT, in both, the group under 45 years (p=0.807), and above age 45 years (p=0.253) (Fig. 2). In the multivariate logistic regression analysis adjusted for age and parity, there was no significant difference in the risk of breast cancer between those who received HRT and those who did not receive HRT after RRSO (HR, 0.312; 95% CI, 0.039 to 2.480; p=0.278) (Table 2). There was also no significant difference in the risk of breast cancer according to the type of HRT (progesterone) (HR, 2.658; 95% CI, 0.266 to 26.539; p=0.975) (Table 2).
DiscussionIn this retrospective study of 151 BRCA mutation carriers who underwent RRSO, the short-term effects of HRT after RRSO did not increase the risk of breast cancer. The incidence and risk of breast cancer did not differ according to HRT use when stratified by age and parity in the RRSO group.
Our findings are consistent with the previous studies, demonstrating that HRT does not increase the risk of breast cancer in BRCA mutation carriers who underwent RRSO. Eisen et al. [17] performed a matched case-control study of 472 postmenopausal women with BRCA1 mutation, and reported that HRT was associated with decreased risk of breast cancer (odds ratio, 0.58; 95% CI, 0.35 to 0.96). Another study by Rebbeck et al. [10] was conducted to evaluate the effects of HRT on breast cancer risk in those who received RRSO among BRCA1/2 mutation carriers, and it showed that HRT use after RRSO did not invalidate the protective effect of HRT on breast cancer (HR, 0.37; 95% CI, 0.14 to 0.96). A case-control analysis of 432 women with a BRCA1 mutation by Kotsopoulos et al. reported that HRT after RRSO was not associated with an increased risk of breast cancer (HR, 0.97; 95% CI, 0.62 to 1.52) [18]. Regarding the types of HRT after RRSO, the cumulative incidence of breast cancer after 10 years of follow-up was shown to be 12% for estrogenalone therapy and 22% for estrogen-progesterone combination therapy, indicating that the effect varies depending on the type of HRT [18]. In this study, there were limitations in assessing the risk of breast cancer associated with types of HRT after RRSO due to occurrence of breast cancer in only one patient who received progesterone therapy among the 33 patients who received HRT. Therefore, future research should focus on investigating the impact of types of HRT on breast cancer risk after RRSO.
RRSO is the most effective way to reduce the risk of ovarian cancer and all-cause mortality in BRCA mutation carriers [19]. In our study population, the number of cases in which BRCA mutations were found through genetic counseling tended to increase annually, and the rate of RRSO among BRCA mutation carriers also increased. However, as shown in our findings, only 18.2% of carriers underwent RRSO, which may be due to patients’ fear or hesitation regarding surgical menopause. This reluctance may be due to the concern that surgery leads to menopause and a decreased sexual function. Indeed, as demonstrated by Terra et al. [20], patients who underwent premenopausal RRSO experienced increased discomfort during sexual intercourse and were more likely to experience vaginal dryness than those who underwent postmenopausal RRSO. Stan et al. [21] revealed that BRCA mutation carriers who underwent RRSO experienced difficulties with vasomotor symptoms, such as hot flashes or night sweats.
The fact that the use of HRT is beneficial for menopausal symptoms, quality of life, and sexual function has already been established. It also has a protective effect against bone diseases, such as osteopenia, osteoporosis, and cardiovascular disease [22]. Although HRT can improve quality of life, this study indicates that only 21.9% of patients received HRT due to concerns regarding the risk of developing breast cancer. Based on our finding, clinicians can provide detailed explanations about the effects of HRT to BRCA mutation carriers who are hesitant to undergo RRSO HRT. In addition, these results could also encourage women who hesitate to undergo RRSO due to concerns about surgically induced menopause.
The strength of the current study is that it is the first report in Korea to evaluate the risk of breast cancer in carriers with BRCA mutations who underwent HRT after receiving RRSO. HRT data were obtained from the KNHIS database, and not from the history provided by patients; therefore, we could exclude the recall bias. In addition, by utilizing data from the KNHIS database, this study boasts the absence of follow-up loss as a notable advantage. Despite these strengths, our study had some limitations. We had a small sample size of 151 BRCA mutation carriers; among them, the number of patients who received HRT was very small at 33. According to recent Women’s Health Initiative data of postmenopausal women, HR for the effect of estrogen plus progesterone HRT on breast cancer was 1.24 (HR, 1.24; 95% CI, 1.01 to 1.53), and HR for the estrogen-only HRT was 0.79 (HR, 0.79; 95% CI, 0.61 to 1.02) [15]. However, our findings only showed the effect of progesterone-only HRT because there was no incidence of breast cancer after RRSO among BRCA carriers who received estrogen alone, or estrogen plus progesterone, or tibolone. Since the median follow-up time was 3.03 years which is a relatively short time to evaluate the risk of developing breast cancer, further studies with longer follow-up periods are needed. Because the KNHIS database did not discriminate between BRCA1 and BRCA2 mutation carriers, it is unknown whether the results of this study correspond to each BRCA mutation carrier. Further research with a longer follow-up period and larger sample size for each HRT medication is needed, and should include information on HRT duration, so that we can provide evidence for the effects of different HRT durations on breast cancer risk.
In conclusion, our findings showed that the use of short-term post-RRSO HRT does not increase the risk of breast cancer in populations with BRCA mutations. Therefore, healthcare providers may consider alleviation of symptoms of postmenopausal syndrome through HRT in BRCA mutation carriers who undergo RRSO. Further studies with longer follow-up periods and larger study populations are required to establish specific guidelines.
NotesEthical Statement This study was approved by the Institutional Review Board of the Korea University Medical Center (2021GR0339), and the need for informed consent was waived as the study was based on routinely collected insurance claims data. Author Contributions Conceived and designed the analysis: Kim HY, Park J, Cho HW. Collected the data: Kim HY, Park J, Moon SJ, Jeong S, Hong JH, Lee JK, Cho HW. Contributed data or analysis tools: Kim HY, Moon SJ, Jeong S, Cho GJ, Cho HW. Performed the analysis: Park J, Jeong S, Hong JH, Lee JK, Cho GJ, Cho HW. Wrote the paper: Kim HY, Park J, Cho HW. AcknowledgmentsWe thank Korea National Health Insurance for their cooperation in providing access to the claims database.
Fig. 1.Flow chart of the study. HIRA, Health Insurance Review & Assessment Service; RRSO, risk-reducing salpingo-oophorectomy. 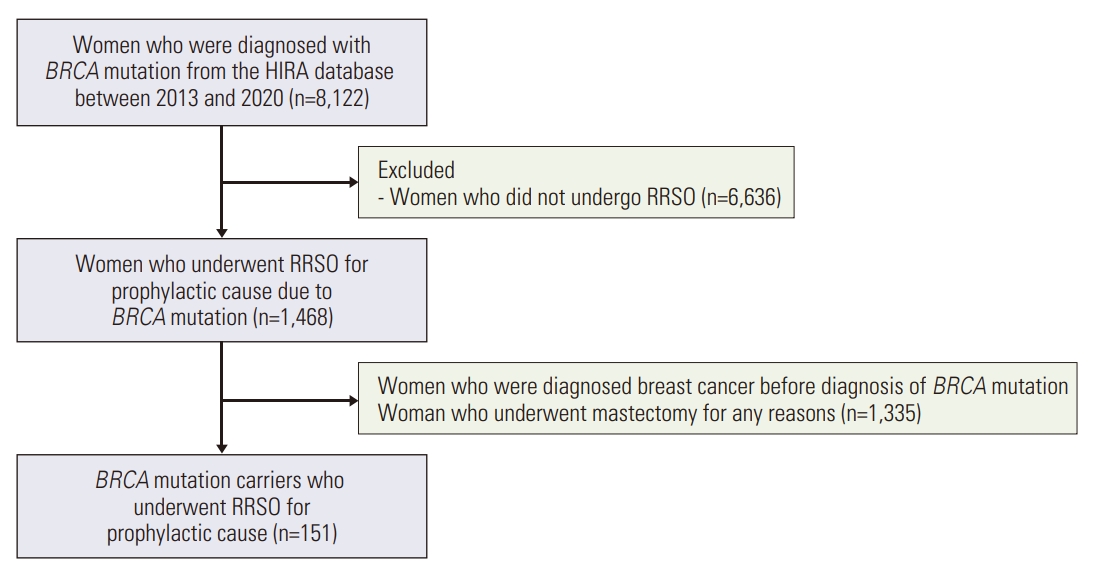
Fig. 2.Breast cancer incidence among BRCA mutation carriers by hormone replacement therapy (HRT) use. (A) Breast cancer incidence among BRCA mutation carriers who used any HRT compared with women who did not use HRT. (B) Breast cancer incidence among BRCA mutation carriers under the age of 45, who used any HRT compared with women who did not use HRT. (C) Breast cancer incidence among BRCA mutation carriers over the age of 45 who used any HRT compared with women who did not use HRT. 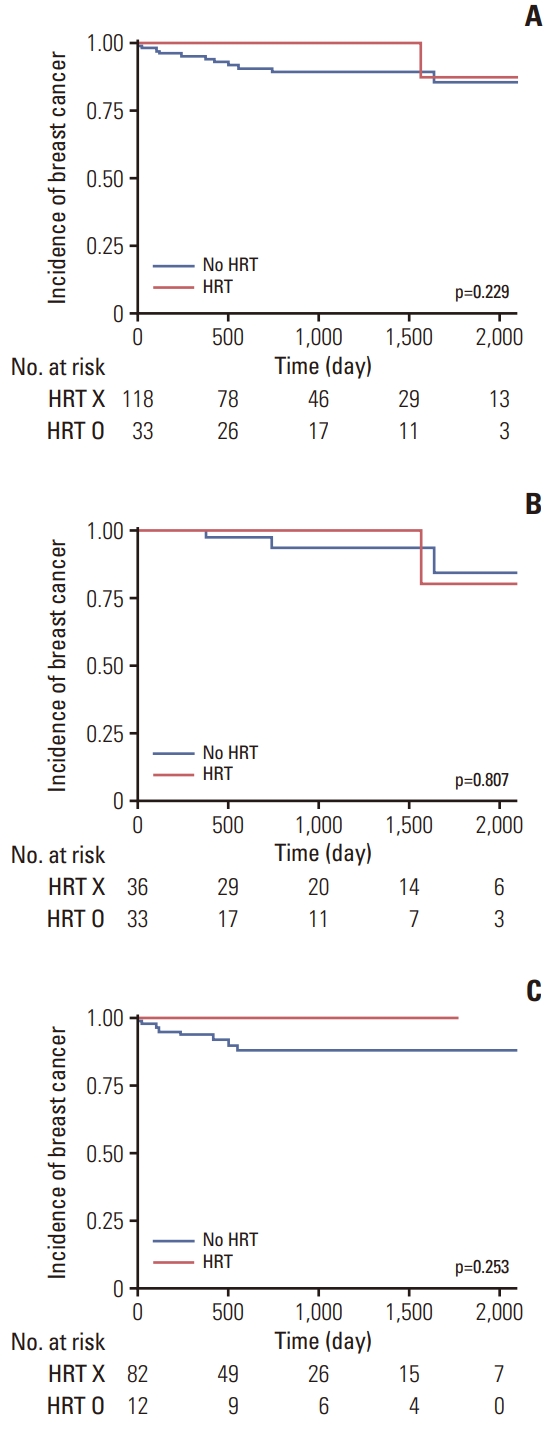
Table 1.Baseline characteristics of study population according to history of HRT use Table 2.Risk of breast cancer among BRCA mutation carriers according to age, parity, and HRT
References1. Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71.
2. Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692–5.
3. Daly MB, Pilarski R, Berry M, Buys SS, Farmer M, Friedman S, et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9–20.
4. Gallicchio L, Whiteman MK, Tomic D, Miller KP, Langenberg P, Flaws JA. Type of menopause, patterns of hormone therapy use, and hot flashes. Fertil Steril. 2006;85:1432–40.
5. Madalinska JB, Hollenstein J, Bleiker E, van Beurden M, Valdimarsdottir HB, Massuger LF, et al. Quality-of-life effects of prophylactic salpingo-oophorectomy versus gynecologic screening among women at increased risk of hereditary ovarian cancer. J Clin Oncol. 2005;23:6890–8.
6. Finch A, Metcalfe KA, Chiang JK, Elit L, McLaughlin J, Springate C, et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol Oncol. 2011;121:163–8.
8. Yoshida T, Takahashi K, Yamatani H, Takata K, Kurachi H. Impact of surgical menopause on lipid and bone metabolism. Climacteric. 2011;14:445–52.
9. Michaelson-Cohen R, Gabizon-Peretz S, Armon S, SrebnikMoshe N, Mor P, Tomer A, et al. Breast cancer risk and hormone replacement therapy among BRCA carriers after riskreducing salpingo-oophorectomy. Eur J Cancer. 2021;148:95–102.
10. Rebbeck TR, Friebel T, Wagner T, Lynch HT, Garber JE, Daly MB, et al. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–10.
11. Kotsopoulos J, Gronwald J, Karlan BY, Huzarski T, Tung N, Moller P, et al. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol. 2018;4:1059–65.
12. Kim H, Choi DH. Distribution of BRCA1 and BRCA2 mutations in Asian patients with breast cancer. J Breast Cancer. 2013;16:357–65.
13. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72–8.
14. Haffty BG, Choi DH, Goyal S, Silber A, Ranieri K, Matloff E, et al. Breast cancer in young women (YBC): prevalence of BRCA1/2 mutations and risk of secondary malignancies across diverse racial groups. Ann Oncol. 2009;20:1653–9.
15. Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601–7.
16. Kim HA, Kim S, Seo YI, Choi HJ, Seong SC, Song YW, et al. The epidemiology of total knee replacement in South Korea: national registry data. Rheumatology (Oxford). 2008;47:88–91.
17. Eisen A, Lubinski J, Gronwald J, Moller P, Lynch HT, Klijn J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361–7.
18. Kotsopoulos J, Huzarski T, Gronwald J, Moller P, Lynch HT, Neuhausen SL, et al. Hormone replacement therapy after menopause and risk of breast cancer in BRCA1 mutation carriers: a case-control study. Breast Cancer Res Treat. 2016;155:365–73.
19. Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547–53.
20. Terra L, Beekman MJ, Engelhardt EG, Heemskerk-Gerritsen BA, van Beurden M, Roeters van Lennep JE, et al. Sexual functioning more than 15 years after premenopausal risk-reducing salpingo-oophorectomy. Am J Obstet Gynecol. 2023;228:440.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||