AbstractPurposeTamoxifen showed individual differences in efficacy under different CYP2D6*10 genotypes. Our study evaluated the prognosis of tamoxifen or toremifene in hormone receptor (HR)–positive breast cancer patients under different genotypes.
Materials and MethodsCYP2D6*10 genotypes of HR-positive breast cancer patients were determined by Sanger sequencing, and all the patients were divided into tamoxifen group or toremifene group.
ResultsA total of 268 patients with HR-positive breast cancer were studied. The median follow-up time was 72.0 months (range, 5.0 to 88.0 months). Of these, 88 (32.9%), 114 (42.5%), and 66 (24.6%) patients had C/C, C/T, and T/T genotypes, respectively. Among patients who received tamoxifen (n=176), the 5-year disease-free survival (DFS) rate in patients with C/C and C/T genotype was better than that in patients with T/T genotype, and the difference was statistically significant (p < 0.001 and p=0.030, respectively). In patients receiving toremifene, CYP2D6*10 genotype was not significantly associated with DFS (p=0.325). Regardless of genotypes, the 5-year DFS rate was higher in patients treated with toremifene than in patients with tamoxifen (91.3% vs. 80.0%, p=0.011). Compared with tamoxifen, toremifene remained an independent prognostic marker of DFS in multivariate analysis (hazard ratio, 0.422; p=0.021). For all the 180 patients with CYP2D6*10 C/T and T/T genotypes, the 5-year DFS rate was significantly higher in the toremifene group than in the tamoxifen group (90.8% vs. 70.1%, p=0.003).
IntroductionBreast cancer is a hormone-dependent tumor. Its occurrence and development are affected by hormone levels in the body. About 65%-75% of breast cancer patients express estrogen receptor (ER) or progesterone receptor (PR) [1]. Tamoxifen (TAM) is a selective estrogen receptor modulator (SERM) used for adjuvant endocrine therapy of hormone receptor (HR)–positive breast cancer, whose structure is similar to estrogen and competitively binds to ER, blocking the activation of ER, and then affecting the transcription and expression of downstream related genes [2]. TAM is a widely used adjuvant endocrine treatment option among premenopausal and postmenopausal breast cancer patients, with therapy durations of up to 10 years [3], which has been shown to reduce breast cancer mortality by 31% [4] and recurrence by 50% [5]. However, within 15 years of initial surgery, one-third of breast cancer patients treated with TAM will have relapsed [5].
Differences in clinical response to TAM among patients are thought to be related to enzyme conversion to active metabolites. The relatively poor affinity of the original TAM for ER resulted in their inability to fully exert their anti-estrogenic activity. Initially, TAM was metabolized into 4-hydroxy TAM and N-demethyl TAM (NDM-TAM), which were then bio-converted into endoxifen, the most active metabolite. However, CYP2D6 is considered to be the key rate-limiting enzyme for NDM-TAM conversion into endoxifen [6].
CYP2D6 gene is characterized by polymorphism, and more than 100 allele mutants have been found. These alleles are classified into normal function (CYP2D6*1 and *2), decreased function (CYP2D6*9, *10, *17, and *41) and no function (CYP2D6*3, *4, *5, and *6) on the basis of the level of activity [7]. Due to the ethnic differences, CYP2D6*10 is the type with the highest distribution frequency in Asian population, and its allele frequency in Chinese population is 30%-50%, but it is very low in Caucasians. In contrast, CYP2D6*4 is most common in Caucasians, with an allele frequency of 20%-25% [8]. Mutation of CYP2D6 gene will lead to changes in enzyme activity, resulting in wide differences in the level of endoxifen, further affecting the therapeutic effect of TAM. Since Goetz et al. [9] initially found that patients with CYP2D6 *4/*4 had a poor prognosis in the treatment of TAM, the influence of CYP2D6 polymorphism on the clinical effect and prognosis of TAM in the treatment of breast cancer has been debated until now.
Toremifene (TOR) is another SERM for the treatment of HR-positive breast cancer. Its structure is only one chlorine atom different from TAM. In postmenopausal patients, TOR has similar efficacy and side effects as adjuvant endocrine therapy as TAM, and may even reduce the occurrence of the events such as endometrial cancer and liver disease [10]. Relevant clinical trial results, meta-analysis and pharmacological studies have shown that TOR is an effective and well-tolerated drug for the treatment of early and advanced breast cancer compared with TAM [11].
Human liver microsomes tests showed that CYP2D6 activity was significantly correlated with TAM hydroxylation, but not with TOR hydroxylation [12]. In addition, in vitro inhibition tests to detect the effects of recombinant human CYP450 subtype on TOR and TAM metabolism found that the hydroxylation and demethylation of TOR were mainly catalyzed by CYP3A4, and did not depend on CYP2D6 [13]. The contribution of CYP2D6 in the TOR bioactivation pathway may be lower than that of TAM. Therefore, TOR may be a viable alternative for CYP2D6*10 gene mutant patients with poor prognosis of TAM.
Materials and Methods1. Study populationA total of 268 patients with breast cancer treated in the Harbin Medical University Cancer Hospital from 2016 to 2018 were selected. Inclusion criteria is as follows: (1) all patients were pathologically confirmed as primary malignant breast tumors; (2) ER+ (ER ≥ 1%) and or PR+ (PR ≥ 1%), standard TAM (20 mg/day) or TOR (60 mg/day) adjuvant endocrine therapy for at least 5 years after surgery, and for patients who were high risk of recurrence at initial diagnosis, we extend treatment up to at least 10 years according to individual tolerance of treatment. During treatment period of adjuvant phase, if recurrence or distant metastasis occur, we discontinued adjuvant endocrine therapy. All patients received standard local and systemic therapy. Clinical and pathological characteristics were collected, including age at diagnosis, menopause, surgery, pathological type, histological grade, tumor diameter, axillary lymph node metastasis, TNM stage, adjuvant chemotherapy, adjuvant radiotherapy, and ER, PR, and human epidermal growth factor receptor 2 (HER-2) status.
2. Trial designPatients were divided into TAM (n=176) and TOR (n=92) groups based on prior medication. The CYP2D6*10 gene test was performed after endocrine therapy for 6 months, and the test results were obtained from our Precision Medicine Center. We isolated approximately 10 ng genomic DNA from 3 mL peripheral blood samples of patients using the TIANamp Genomic DNA kit (TIANGEN Biotech, Beijing, China), amplified the sample DNA by multiplex polymerase chain reaction, and finally sequenced it using the Applied Biosystems 3130Dx sequencer. The CYP2D6*10 gene phenotypes were classified into wild-type (C/C), heterozygous (C/T), and mutant (T/T) types. It should be noted that none of the patients in this study changed endocrine medications based on genetic test results. Patients were followed up by phone or in clinic. Disease-free survival (DFS) is defined as the time from surgery to disease progression or death. The median follow-up time was 72.0 months (range, 5.0 to 88.0 months).
3. Statistical analysisThe data was processed using IBM SPSS ver. 26.0 software (IBM Corp., Armonk, NY). A χ2 test was used to compare the baseline characteristics of patients between different CYP2D6*10 genotypes and between different treatment groups. The Kaplan-Meier method was used to create survival curves, and the log-rank method was used in statistical testing. Cox regression analysis was used to determine independent predictors. A p-values < 0.05 were considered statistically significant.
Results1. Study characteristicsThe results of CYP2D6 genotype showed that among 268 patients with breast cancer, there were 88 cases of C/C genotype (32.9%), 114 cases of C/T genotype (42.5%), and 66 cases of T/T genotype (24.6%). The frequency of C allele was 54.15%, and that of T allele was 45.85%. A total of 51 cases (19.0%) of disease progression occurred. The χ2 test showed no significant difference in baseline characteristics between different CYP2D6*10 genotypes and between the two treatment groups (TAM vs. TOR) (p > 0.05) (Table 1).
2. Association between CYP2D6*10 genotypes and survival in patients who received adjuvant TAM or TOR treatmentKaplan-Meier curve estimates that in patients receiving TAM (n=176), the CYP2D6*10 genotype was significantly related to DFS. Patients with CYP2D6*10 C/C and C/T genotype showed a better DFS than patients with T/T genotype, and the difference was statistically significant (p < 0.001 and p=0.030 respectively) (Fig. 1A). However, significant differences in DFS were not noted in patients receiving TOR (n=92) among the three CYP2D6*10 genotypes (p=0.325) (Fig. 1B), and the 5-year DFS rates were similar among C/C, C/T, and T/T genotypes (93.8% vs. 93.2% vs. 87.5%).
3. Comparison of DFS between TAM and TOR groupsKaplan-Meier curve estimates indicated that 5-year DFS rate was higher in patients treated with TOR (n=92) than in patients treated with TAM (n=176) (91.3% vs. 80.0%, p=0.011) (Fig. 2). In univariate Cox proportional risk analysis for DFS, age, menopause, tumor diameter, histological grade, axillary lymph node metastasis, TNM stage, HER-2 status, PR, and adjuvant chemotherapy were significant variables. When they were included in multivariate cox regression analysis (Table 2), it was found that adjuvant TOR therapy remained an independent prognostic factor for DFS compared with TAM (hazard ratio, 0.422; 95% confidence interval, 0.202 to 0.879; p=0.021).
4. Comparison of DFS between TOR and TAM in CYP2D6*10 patients with different genotypesIn patients with CYP2D6*10 C/C genotype (n=88), no statistically significant difference in DFS was observed between the two treatment groups (p > 0.05) (Fig. 3A). However, among the patients with C/T genotype (n=114), DFS in TAM group was significantly worse than TOR (80.0% vs. 93.2%, p=0.019) (Fig. 3B), as did patients with T/T genotype (61.8% vs. 87.5% p=0.029) (Fig. 3C). Thus, patients with C/T or T/T genotypes exhibit worse DFS when treated with TAM than with TOR.
5. Comparison of DFS between TOR and TAM in CYP2D6*10 C/T and T/T patientsFor all the 180 patients with CYP2D6*10 C/T and T/T genotypes, the 5-year DFS rate was significantly higher in patients receiving TOR (n=76) than in patients receiving TAM (n=104) (90.8% vs. 70.1%, p=0.003) (Fig. 4).
DiscussionBreast cancer has become the most common malignancy in the world and the highest morbidity and mortality rate among women worldwide [14]. The age distribution of breast cancer patients differs significantly between east and west. The median age of onset of breast cancer in the United States is 62-64 years old, while in China and other East Asian countries, the median age of onset is about 45-49 years old [15]. TAM is the standard treatment for premenopausal breast cancer with low recurrence risk. Therefore, the reasonable application of TAM is of great significance for Chinese breast cancer patients. However, until now, we still do not have a good biomarker to predict the efficacy of TAM.
In this study, it was found that among the people who treated with TAM for adjuvant endocrine therapy, the 5-year DFS rate of patients with CYP2D6*10 T/T genotype was significantly lower than that of patients with C/C and C/T genotype, which was similar to the research results of Chinese scholar Lan et al. [16] published in the International Journal of Cancer in 2018. In addition, this research also confirmed that T/T genotype remained an independent poor prognostic factor of DFS in this patient cohort [16]. In 2009, a large retrospective analysis of 1,325 German and American patients with early breast cancer found that there was an association between CYP2D6 variation and clinical outcomes during TAM treatment, and patients with two functional CYP2D6 alleles had better clinical outcomes than patients with nonfunctional or reduced-function alleles [17]. Many studies have also confirmed the correlation between CYP2D6 gene polymorphism and TAM drug metabolism. Lim et al. [18] in Korea confirmed that CYP2D6*10/*10 is associated with lower steady-state plasma concentrations of active TAM metabolites, which could possibly influence the clinical outcome by TAM in Asian breast cancer patients. Helland et al. [19] conducted CYP2D6 allele typing among 99 HR-positive breast cancer patients treated with TAM, and grouped their metabolic functions according to allele combinations: ultrafast metabolizers, extensive/normal metabolizers, intermediate metabolizers, and poor metabolizers. It was found that CYP2D6 genotype was related to the concentration of TAM active metabolites, and low concentration of endoxifen would lead to poor survival outcome [19]. He et al. [20] calculated hazard ratio to determine the association between CYP2D6 metabolic status and breast cancer mortality, and found that compared with patients with normal metabolism, patients with poor TAM metabolism and ultrafast metabolizers had a worse prognosis, and the reason for the poor prognosis of ultrafast metabolizers might be related to stronger side effects and higher discontinuation rate. In contrast, a prospective study conducted in 2019 by Sanchez-Spitman et al. [21] found no association between endoxifen levels and relapse-free survival, but its study design and statistical power have been criticized by various researchers. Firstly, the patients included in this study were not treated with TAM monotherapy, and some patients received additional systemic therapy or switched to aromatase inhibitors during adjuvant therapy, which altered the hazard of both early and late breast cancer events [22]. Secondly, in view of the long time to recurrence of breast cancer, the study by Sanchez-Spitman did not have the power to determine the association between CYP2D6 genotype or endoxifen concentrations with RFS either in terms of treatment time or follow-up time [23]. Furthermore, it was noted that the anticipated effect size of hazard ratio=2.0 at 3 years may have been overestimated, as the hazard ratio was based on studies with a more extended clinical follow-up of 5 and 10 years [24]. Jorge-Aaron et al. [25] also reported the lack of effect of CYP2D6 genotype on breast cancer-free survival, which may be related to the limited sample size.
As early as the 1990s, studies have evaluated the efficacy of TOR and TAM in adjuvant endocrine therapy for breast cancer. The International Breast Cancer Research Group IBCSG conducted two randomized trials (Trials 12-93 and 14-93), combining the results of the two trials (n=1,035) and comparing TOR 60 mg/day with TAM 20 mg/day as adjuvant in combination with chemotherapy in perimenopausal and postmenopausal patients with node-positive breast cancer. It was found that the 5-year DFS and overall survival (OS) of TOR and TAM were similar (DFS: 72% TOR, 69% TAM; OS: 85% TOR, 81% TAM), and both treatment groups were similar in terms of toxicity and quality of life [26]. A similar conclusion was reached by FBCG, which compared the efficacy and safety of TOR 40 mg/day and TAM20 mg/day, and found that TOR had similar side effects to TAM and was no less effective than TAM after an average follow-up of 3.4 years [27]. The NAFTA trial further demonstrated that TOR is a safe and effective alternative to TAM for adjuvant endocrine therapy in perimenopausal and postmenopausal HR-positive breast cancer [28]. A meta-analysis involving data from 3,747 breast cancer patients also confirmed the results more precisely [29].
There is limited clinical data on the use of TOR in the treatment of breast cancer in premenopausal women. A Korean registry study analyzed the survival outcomes of young (age ≥ 50 years) breast cancer patients given adjuvant endocrine therapy after chemotherapy. Of the 3,489 patients treated, 2,856 (82%) received TAM and 632 (18%) received totamifen, but no drug-specific survival data was reported [30]. A retrospective cohort study in China of TOR endocrine therapy in premenopausal women with HR-positive early invasive breast cancer compared the outcomes of premenopausal women receiving TOR (n=212) versus TAM (n=240). The relapse-free survival rate in the TOR group was significantly better than that in the TAM group (TOR 97.2% vs. TAM 90.4, p=0.022), and the toxic side effects were similar between the two groups [31]. Similarly, a study by Lan et al. [32] compared the survival of 230 breast cancer patients (86% of whom were younger than 50 years old) receiving adjuvant endocrine therapy TAM with TOR 1:1, and found that without considering CYP2D6*10 genotype, the 5-year DFS rate was significantly higher in the TOR group than in the TAM group (89.6% vs. 80.9% p=0.009). Therefore, TOR may be an effective and safe alternative to TAM for Chinese premenopausal HR-positive breast cancer patients.
Our study found that the efficacy of patients treated with TOR was not affected by CYP2D6*10 genotype, and the 5-year DFS rate was better than TAM (91.3% vs. 80.0%, p=0.011). In multivariate analysis, adjuvant TOR therapy was still an independent prognostic factor for DFS.
Finally, we respectively compared the DFS of TOR and TAM under different CYP2D6*10 genotypes, and found that the 5-year DFS rate of patients treated with TOR was significantly better than that of TAM in C/T and T/T genotypes (90.8% vs. 70.1% p=0.003). Different from the research results of Lan et al. [32], our study included patients with C/T and T/T genotypes in different treatment groups for comparison, and the results were more targeted. Due to the high mutation frequency of CYP2D6*10 alleles in China, patients with C/T and T/T genotypes accounted for 67.1% in this study. Therefore, this result indicates that TOR has a better prognosis than TAM in at least over half of the population in China.
Helland et al. [19] suggest that direct measurement of serum concentrations of active metabolites of TAM to predict prognosis of breast cancer is a simple and applicable method for patients with poor TAM metabolism. However, the results of a prospective clinical trial indicate that endoxifen drug-monitoring is not clinically valuable in patients receiving TAM [31]. Khalaj et al. [33] increased TAM dose in patients with low CYP2D6 enzyme activity, and found that plasma concentration of the active metabolite endoxifen significantly increased. Similarly, a prospective phase II clinical trial in Japan randomly assigned CYP2D6 homozygous and heterozygous mutant patients to an increased TAM dose group (ID group, 40 mg/day) and regular dose group (RD group, 20 mg/day), the results showed that the plasma endoxifen dose in ID group was significantly higher than that in RD group, but increasing TAM dosing did not achieve a higher progression-free survival rate at 6 months. Therefore, the CYP2D6 genotype solely cannot explain individual variability in the efficacy of TAM [34]. In 2018, CPCI, Clinical Pharmacogenetics Implementation Consortium, issued guidelines for clinical medication of TAM based on CYP2D6 gene test [35]. However, due to insufficient evidence, European Society for Medical Oncology (ESMO) of the European Society of Internal Medicine of Oncology in 2019 considered that CYP2D6 gene test could not determine the medication decision of TAM [36], which is consistent with the current National Comprehensive Cancer Network (NCCN) breast cancer guidelines. The reason why we are against NCCN and ESMO guidelines because of the clinical trials they are referring to is from randomized controlled trials with mainly postmenopausal and Western countries (Schroth et al. [17] and BIG 1-98), while our study population was mainly premenopausal patients from Asian, so our data are more targeted. The TEXT and SOFT trials found that increasing ovarian suppression could significantly reduce the recur-rence in premenopausal patients with HR-positive early breast cancer, but combining ovarian suppression may lead to a set of additional side effects [37]. However, our results confirm that TOR may be a good choice for adjuvant endocrine therapy in HR-positive breast cancer, at least to reverse the outcome in patients with CYP2D6*10 mutant genotypes, but this result needs to be confirmed by clinical trials with larger sample sizes.
NotesEthical Statement This study was carried out using the opt-out method for the case series of our hospital. The study was approved by the Ethics Committee of the Harbin Medical University Cancer Hospital (approval no. KY2019-14) and was conducted in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Participants provided written informed consent prior to taking part in the study. Author Contributions Conceived and designed the analysis: Li X, Li Z (Zehao Li), Li L, Liu T, Qian C, Ren Y, Li Z (Zhigao Li), Zhang M, Chen K, Ji D, Wang J. Collected the data: Li X, Li Z (Zehao Li), Li L, Chen K, Ji D, Wang J. Contributed data or analysis tools: Li X, Li Z (Zehao Li), Li L, Liu T, Qian C, Ren Y, Li Z (Zhigao Li), Zhang M, Wang J. Performed the analysis: Li X, Li L, Wang J. Wrote the paper: Li X, Wang J. Fig. 1.Association between CYP2D6 *10 genotypes and disease-free survival in patients who received adjuvant tamoxifen (A) or toremifene (B) treatment. 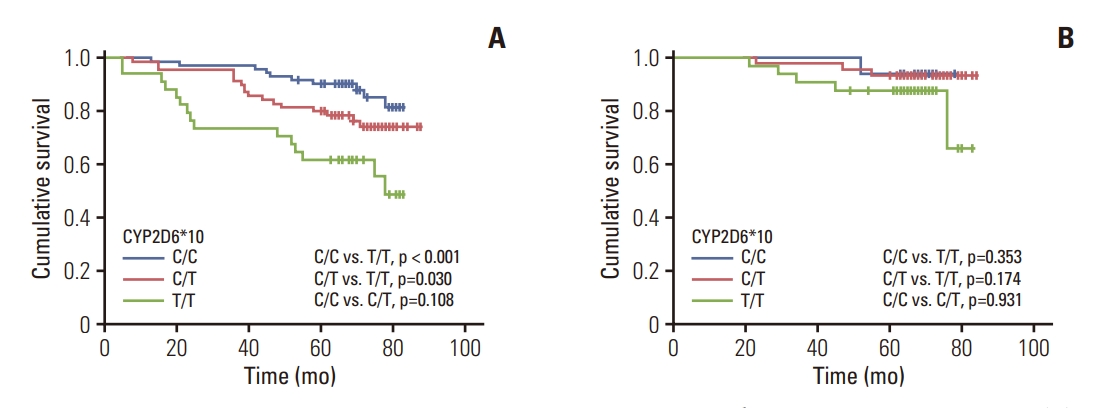
Fig. 3.Comparison of disease-free survival between toremifene (TOR) and tamoxifen (TAM) in CYP2D6*10 patients with different genotypes. (A) CYP2D6*10 C/C. (B) CYP2D6*10 C/T. (C) CYP2D6*10 T/T. 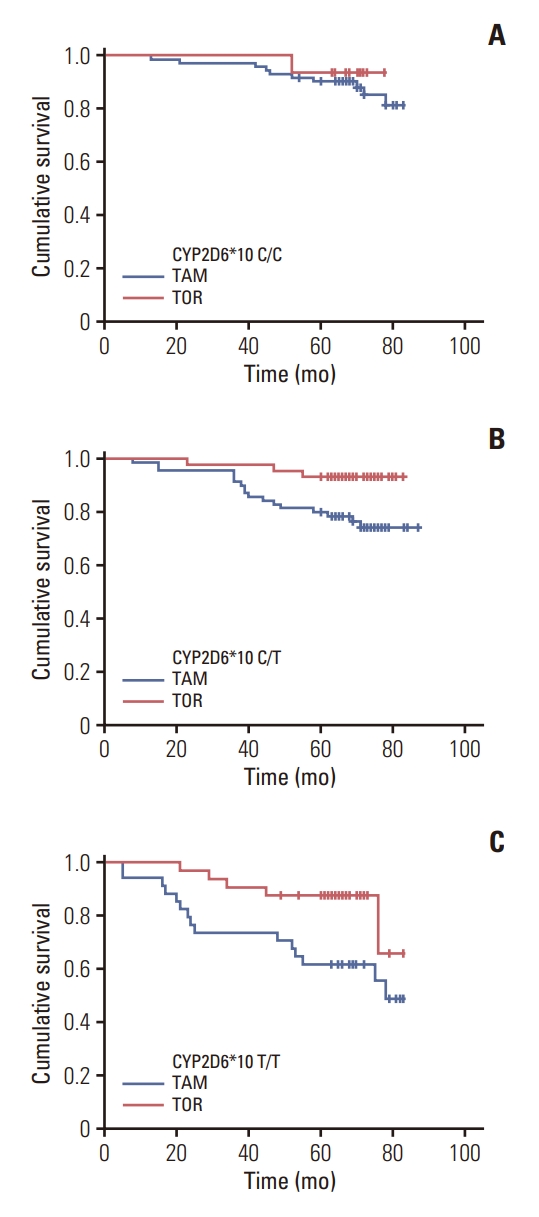
Fig. 4.Comparison of disease-free survival between toremifene (TOR) and tamoxifen (TAM) in CYP2D6 *10 C/T and T/T patients. 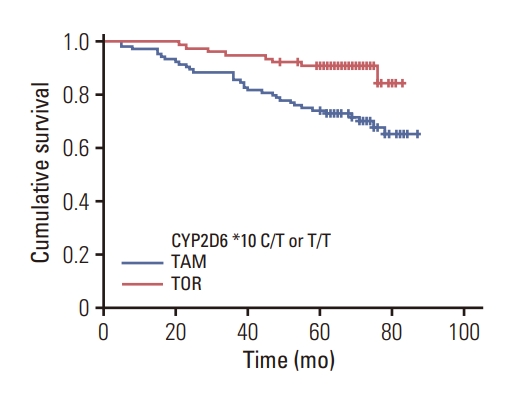
Table 1.Relationship between different CYP2D6*10 genotypes and between treatment groups Table 2.Multivariate analysis of DFS in breast cancer patients receiving TOR or TAM treatment References1. O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–10.
2. Dezentje VO, Guchelaar HJ, Nortier JW, van de Velde CJ, Gelderblom H. Clinical implications of CYP2D6 genotyping in tamoxifen treatment for breast cancer. Clin Cancer Res. 2009;15:15–21.
3. Jackisch C, Harbeck N, Huober J, von Minckwitz G, Gerber B, Kreipe HH, et al. 14th St. Gallen International Breast Cancer Conference 2015: evidence, controversies, consensus - primary therapy of early breast cancer: opinions expressed by German experts. Breast Care (Basel). 2015;10:211–9.
4. Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62.
5. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
6. Blackburn HL, Ellsworth DL, Shriver CD, Ellsworth RE. Role of cytochrome P450 genes in breast cancer etiology and treatment: effects on estrogen biosynthesis, metabolism, and response to endocrine therapy. Cancer Causes Control. 2015;26:319–32.
7. Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95:376–82.
8. Qian JC, Xu XM, Hu GX, Dai DP, Xu RA, Hu LM, et al. Genetic variations of human CYP2D6 in the Chinese Han population. Pharmacogenomics. 2013;14:1731–43.
9. Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–8.
10. Ye QL, Zhai ZM. Toremifene and tamoxifen have similar efficacy in the treatment of patients with breast cancer: a meta-analysis of randomized trials. Mol Biol Rep. 2014;41:751–6.
11. Mustonen MV, Pyrhonen S, Kellokumpu-Lehtinen PL. Toremifene in the treatment of breast cancer. World J Clin Oncol. 2014;5:393–405.
12. Kim J, Coss CC, Barrett CM, Mohler ML, Bohl CE, Li CM, et al. Role and pharmacologic significance of cytochrome P-450 2D6 in oxidative metabolism of toremifene and tamoxifen. Int J Cancer. 2013;132:1475–85.
13. Watanabe M, Watanabe N, Maruyama S, Kawashiro T. Comparative metabolic study between two selective estrogen receptor modulators, toremifene and tamoxifen, in human liver microsomes. Drug Metab Pharmacokinet. 2015;30:325–33.
15. Yap YS, Lu YS, Tamura K, Lee JE, Ko EY, Park YH, et al. Insights into breast cancer in the East vs. the West: a review. JAMA Oncol. 2019;5:1489–96.
16. Lan B, Ma F, Zhai X, Li Q, Chen S, Wang J, et al. The relationship between the CYP2D6 polymorphisms and tamoxifen efficacy in adjuvant endocrine therapy of breast cancer patients in Chinese Han population. Int J Cancer. 2018;143:184–9.
17. Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–36.
18. Lim HS, Lee HJ, Lee KS, Lee ES, Jang IJ, Ro J. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007;25:3837–45.
19. Helland T, Henne N, Bifulco E, Naume B, Borgen E, Kristensen VN, et al. Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res. 2017;19:125.
20. He W, Grassmann F, Eriksson M, Eliasson E, Margolin S, Thoren L, et al. CYP2D6 genotype predicts tamoxifen discontinuation and prognosis in patients with breast cancer. J Clin Oncol. 2020;38:548–57.
21. Sanchez-Spitman A, Dezentje V, Swen J, Moes D, Bohringer S, Batman E, et al. Tamoxifen pharmacogenetics and metabolism: results from the prospective CYPTAM study. J Clin Oncol. 2019;37:636–46.
22. Goetz MP, Suman VJ, Nakamura Y, Kiyotani K, Jordan VC, Ingle JN. Tamoxifen metabolism and breast cancer recurrence: a question unanswered by CYPTAM. J Clin Oncol. 2019;37:1982–3.
23. Gusella M, Pasini F, Corso B, Bertolaso L, De Rosa G, Falci C, et al. Predicting steady-state endoxifen plasma concentrations in breast cancer patients by CYP2D6 genotyping or phenotyping: which approach is more reliable? Pharmacol Res Perspect. 2020;8:e00646
24. Brauch H, Schroth W, Murdter T, Schwab M. Tamoxifen pharmacogenetics and metabolism: the same is not the same. J Clin Oncol. 2019;37:1981–2.
25. Jorge-Aaron RM, Rodrigo RC, de Jesus AI, Esther MR. CYP2D6 does not impact on breast cancer-free survival in Southeast Mexican patients under tamoxifen treatment. Per Med. 2020;17:261–70.
26. International Breast Cancer Study Group; Pagani O, Gelber S, Price K, Zahrieh D, Gelber R, et al. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12-93 and 14-93. Ann Oncol. 2004;15:1749–59.
27. Holli K; Finnish Breast Cancer Group. Tamoxifen versus toremifene in the adjuvant treatment of breast cancer. Eur J Cancer. 2002;38 Suppl 6:S37–8.
28. Lewis JD, Chagpar AB, Shaughnessy EA, Nurko J, McMasters K, Edwards MJ. Excellent outcomes with adjuvant toremifene or tamoxifen in early stage breast cancer. Cancer. 2010;116:2307–15.
29. Zhou WB, Ding Q, Chen L, Liu XA, Wang S. Toremifene is an effective and safe alternative to tamoxifen in adjuvant endocrine therapy for breast cancer: results of four randomized trials. Breast Cancer Res Treat. 2011;128:625–31.
30. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea: a report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25:2360–8.
31. Gu R, Jia W, Zeng Y, Rao N, Hu Y, Li S, et al. A comparison of survival outcomes and side effects of toremifene or tamoxifen therapy in premenopausal estrogen and progesterone receptor positive breast cancer patients: a retrospective cohort study. BMC Cancer. 2012;12:161.
32. Lan B, Ma F, Chen S, Wang W, Li Q, Fan Y, et al. Toremifene, rather than tamoxifen, might be a better option for the adjuvant endocrine therapy in CYP2D6*10T/T genotype breast cancer patients in China. Int J Cancer. 2018;143:2499–504.
33. Khalaj Z, Baratieh Z, Nikpour P, Schwab M, Schaeffeler E, Mokarian F, et al. Clinical trial: CYP2D6 related dose escalation of tamoxifen in breast cancer patients with Iranian ethnic background resulted in increased concentrations of tamoxifen and its metabolites. Front Pharmacol. 2019;10:530.
34. Tamura K, Imamura CK, Takano T, Saji S, Yamanaka T, Yonemori K, et al. CYP2D6 genotype-guided tamoxifen dosing in hormone receptor-positive metastatic breast cancer (TARGET-1): a randomized, open-label, phase II study. J Clin Oncol. 2020;38:558–66.
35. Goetz MP, Sangkuhl K, Guchelaar HJ, Schwab M, Province M, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin Pharmacol Ther. 2018;103:770–7.
|
|
|||||||||||||||||||||||||||||||||||||||