AbstractPurposeDespite numerous studies on the optimal treatments for oligometastatic disease (OMD), there is no established interdisciplinary consensus on its diagnosis or classification. This survey-based study aimed to analyze the differential opinions of colorectal surgeons and radiation oncologists regarding the definition and treatment of OMD from the colorectal primary.
Materials and MethodsA total of 141 participants were included in this study, consisting of 63 radiation oncologists (44.7%) and 78 colorectal surgeons (55.3%). The survey consisted of 19 questions related to OMD, and the responses were analyzed using the chi-square test to determine statistical differences between the specialties.
ResultsThe radiation oncologists chose “bone” more frequently compared to the colorectal surgeons (19.2% vs. 36.5%, p=0.022), while colorectal surgeons favored “peritoneal seeding” (26.9% vs. 9.5%, p=0.009). Regarding the number of metastatic tumors, 48.3% of colorectal surgeons responded that “irrelevant, if all metastatic lesions are amendable to local therapy”, while only 21.8% of radiation oncologist chose same answer. When asked about molecular diagnosis, most surgeons (74.8%) said it was important, but only 35.8% of radiation oncologists agreed.
ConclusionThis study demonstrates that although radiation oncologists and colorectal surgeons agreed on a majority of aspects such as diagnostic imaging, biomarker, systemic therapy, and optimal timing of OMD, they also had quite different perspectives on several aspects of OMD. Understanding these differences is crucial to achieving multidisciplinary consensus on the definition and optimal management of OMD.
IntroductionOligometastatic disease (OMD), which is a stage of cancer between local disease and disseminated disease, has gained a lot of attention recently [1]. Previous studies suggested that patients with OMD showed more favorable survival outcomes when compared to those with disseminated metastatic disease [2,3]. In addition to the advancements in systemic therapy, such as target therapy and immunotherapy agents, which have also improved survival outcomes of the patients with systemic metastasis, the practical benefits of applying locally aggressive treatment modalities, such as metastasectomy and ablating radiation therapy, to the OMD patients have attracted significant interest.
A reasonable definition and classification of OMD is highly required before the oncologists determine the optimal treatment strategy. Despite previous enthusiastic efforts to reflect the consensus, the definition of OMD still needs to be comprehensively understood and shared among the oncologists. While the number of metastatic lesions has been the most commonly discussed criterion in defining OMD, the number of metastatic organs and the size of metastatic lesions, however, need to be considered. The latest consensus by European Organization for Research and Treatment of Cancer (EORTC) group has sub-classified OMD into nine categories based on the history of polymetastatic disease, disease-free interval (DFI), active systemic therapy, and disease progression [4]. After the publication of this consensus, there is an agreement that de novo OMD or oligorecurrence is defined as OMD. However, there are still conflicts on induced oligometastasis, which refers to OMD that had previously been a polymetastatic disease [5]. Minimum imaging requirements and the use of serum biomarkers in the diagnosis of OMD are also controversial.
Colorectal cancer has a long history of OMD [6]. In selected patients with single lung or liver metastasis, the survival benefit of metastasectomy has been reported [7–9]. However, research on the definition, diagnosis, and management of OMD from colorectal cancer is still required and far from the concrete conclusion. Recently, new treatment modalities for metastatic tumors such as stereotactic body radiation therapy (SBRT) and radiofrequency ablation, which are much less invasive compared to surgical resection, have been introduced to the clinical practice setting. In addition, clinicians have limited expertise in the curative management of OMD occurring in the lymph node (LN), peritoneal mass, or bone.
The aim of this survey-based study by K-OWG (Korean Oligometastasis Working Group), a research group affiliated with the Korean Cancer Association, is to investigate the perspectives of colorectal surgeons and radiation oncologists regarding the definition and treatment approach for OMD from colorectal primaries.
Materials and MethodsAn online survey was performed from August 2022 to September 2022. The survey questionnaire was distributed to the colorectal surgeons, members of the Korean Society of Coloproctology, and the radiation oncologists, members of the Korean Society of Radiation Oncology, and the informed consent form was signed by all participants. The survey questionnaire consisted of total of 15 questions: the participant’s demographics (Q1–2); definition (Q3–7); diagnosis (Q8–11); local therapy issue (Q12–14); and endpoint (Q15), respectively (Table 1). Radiation oncologists were asked four questions on radiation dose and fractionation additionally, if local radiation therapy was to be considered for lung and liver metastases (Q16–19). The questionnaire comprised of both multiple-choices and open-ended questions, whose distribution and collection was done using the Survey Monkey (Palo Alto, CA). This research was conducted in accordance with the principle of the Declaration of Helsinki and the approval of institutional review board for this study was waived because of the non-identifiable nature of the survey data. The differences in the responses between radiation oncologists and surgeons were compared using the chi-square test.
Results1. Participants’ demographics (Q1–2)Two questions were asked on the demographic information of the respondents (Table 2). A total of 141 participated in this survey: 78 (55.3%) were colorectal surgeons; and 63 (44.7%) were radiation oncologists, respectively (Q1). The lengths of the participants’ career after their respective board certification were ≤ 5 years in 24 (17.1%), > 5 and ≤ 10 years in 41 (29.1%), > 10 and ≤ 20 years in 42 (29.8%), and > 20 years in 34 (24.1%), respectively (Q2).
2. Definition of OMD (Q3–7)Five questions were asked on the definition of OMD (Table 1). Two-thirds of the respondents (93, 78.8%) answered that they considered the sites of metastatic lesions (Q3), while 25 (21.2%) answered that they did not. Surgeons considered the metastatic sites more frequently than radiation oncologists (85.7% vs. 70.9%, p=0.050). As for the metastatic sites (Q3-1), the liver and the lung were most commonly considered organs (63.8% and 63.1%), followed by the abdominal LN (39.7%), pelvic LN (35.5%), bone (27.0%), brain (22.7%), and peritoneal space (19.1%), respectively. Surgeons included the peritoneal space (26.9% vs. 9.5%, p=0.009), while radiation oncologists did the bone (19.2% vs. 36.5%, p=0.022), more frequently than their counterparts, respectively. Three-fourths (n=106, 89.8%) answered that they considered the maximum number of OMD (Q4), and 12 (10.2%) answered they did not. Radiation oncologists considered the maximum number of OMD more frequently than surgeons (84.1% vs. 96.4%, p=0.028). Regarding the maximum number of OMD (Q4-1), 41 respondents (35.7%) chose “3 or less”, 33 (28.7%) did “5 or less”, and 41 (35.7%) did “irrelevant, if all metastatic lesions are amendable to local therapy”. Surgeons chose “irrelevant, if all metastatic lesions are amendable to local therapy” more frequently than radiation oncologists (48.3% vs. 21.8%, p < 0.001).
About half of the respondents considered the maximum size of OMD (Q5: 47.5% vs. 52.5%). As for the maximum size (Q5-1), 32 (47.1%) considered “3 cm or smaller”, 33 (48.5%) did “5 cm or smaller”, and three (4.4%) did “10 cm or smaller”, respectively, where no surgeons chose “10 cm or smaller”. Ninety-five participants (80.5%) answered in affirmative for the consideration of the minimum length of DFI (Q6), where surgeons chose this more frequently (87.3% vs. 72.7%, p=0.046). The most commonly chosen answer was “6–12 months” (n=63, 63.0%), followed by “< 6 months” (n=10, 10.0%), and “≥ 12 months” (n=27, 27.0%), respectively (Q6-1). Surgeons chose “≥ 12 months” more frequently than radiation oncologists (37.3% vs. 12.2%, p=0.017). Three types of OMD, according to the ETRO-EORTC classification, were asked whether they conformed to the definition of OMD (Q7), and 83.7%, 62.4%, and 40.4% were affirmative for de novo, repeat, and induced types, respectively. Radiation oncologists more frequently chose repeat OMD (48.7% vs. 79.4%, p < 0.001) and induced OMD (23.1% vs. 62.0%, p < 0.001) than surgeons, respectively.
3. Diagnosis of OMD (Q8–11)Four questions were asked on the diagnosis of OMD (Table 1). For the necessity of liver magnetic resonance imaging (MRI) (Q8), 37.4% answered that liver MRI was mandatory, 51.3% answered that it was mandatory in case of liver metastasis, and 11.3% thought that it was not mandatory, respectively. For the necessity of 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography (Q9), 95 respondents (82.6%) answered that it was mandatory. For the tumor biomarkers representing the occult polymetastasis (Q10), 69 respondents (60.0%) answered negatively, where carcinoembryonic antigen and carbohydrate antigen 19-9 (Q10-1) were to be considered in 36.2% and 16.3% of the respondents, respectively. For the necessity of molecular diagnosis (Q11) (e.g., mismatch repair deficiency/microsatellite instability, NRAS, KRAS, etc.), 66 respondents (57.4%) answered affirmatively, and the majority of surgeons answered affirmatively (75.8% vs. 35.8%, p < 0.001).
4. Role of local therapy in relation to systemic therapy (Q12–14)Three questions on the role of local therapy in relation to systemic therapy (Q12–Q14) were asked. For the types of OMD in which metastasis-directed local therapy should be considered (Q12), 79.4%, 64.5%, and 40.4% of the respondents were affirmative on de novo OMD, repeat OMD, and induced OMD, respectively. While there was no difference between specialties on the role of local therapy in de novo OMD (76.9% vs. 82.5%, p=0.412), radiation oncologists more frequently answered affirmatively in repeated OMD (55.1% vs. 76.2%, p=0.009) and induced OMD (23.1% vs. 61.9%, p < 0.001), respectively. For the possibility of delaying systemic therapy following local control by local therapy (Q13), most respondents (61.6%) answered negatively. For the optimal timing of metastasis-directed local therapy (Q14), “no optimal timing” was the most frequent answer (25.9%), followed by “before systemic therapy” in 23.2%, “after systemic therapy” in 22.3%, “concurrently” in 18.8%, and “others” in 9.8%, respectively.
5. Endpoint (Q15)For the appropriate endpoint to evaluate the local therapy role (Q15), overall survival, cancer-specific survival, progression-free survival, and local control were chosen in 31.2%, 36.9%, 47.5%, and 44.7%, respectively. Radiation oncologists chose progression-free survival (37.2% vs. 60.3%, p=0.006) and local control (29.5% vs. 63.5%, p < 0.001) as the important endpoints more frequently than surgeon, respectively.
6. Radiation dose for lung and liver oligometastasis of colorectal cancer (Q16–19)For both lung and liver oligometastases, SBRT was the most favored treatment option (89.4% and 84.4%, respectively) among radiation oncologists (Table 3). Regarding lung metastasis, prescription dose of 60 Gy (29.1%) was the most frequently chosen for 10-fractions scheme (Fig. 1A). For four-fractions scheme, 60 Gy (30.5%) was also the most common dose followed by 48 Gy (28.8%) (Fig. 1B). In case of liver metastasis, the most common prescription dose was 50 Gy (35.9%) for 10 fractions scheme (Fig. 2A). Favored prescription dose for four-fractions scheme was 48 Gy (31.0%) followed by 60 Gy (22.4%) (Fig. 2B).
DiscussionRecent research has revealed that the patients with OMD may benefit from local treatment. However, neither the definitions nor the management policies of OMD are yet concrete. In clinical practice, treatment of cancer patients requires participation of a multidisciplinary team, which includes surgeons, medical oncologists, radiation oncologists, and various types of other caregivers. In order to determine the optimal treatment options for the OMD patients, it would be essential for the multidisciplinary team members to have a consensus on the definition and diagnosis of OMD. This study showed that the colorectal surgeons and radiation oncologists had differences in several aspects regarding the OMD from colorectal cancer.
Regarding the metastatic sites, the radiation oncologists were more likely to include bone metastases than the surgeons (19.2% vs. 36.5%). In contrast, the surgeons were more likely to select a peritoneal space than the radiation oncologists (26.9% vs. 9.5%). Peritoneal metastasis is not a “one size fits all” concept when it comes with colorectal cancer. From a classical oncologic view point, peritoneal metastasis is presumed disseminated disease because the peritoneal cavity is an open cavity, and the radiation oncologists may be reluctant to apply local radiation therapy for peritoneal lesions which are uneasy to be included within the radiation target volume. Meanwhile, the surgeons might have been influenced by the Japanese surgeons’ effort of performing R0 resection in the patients with resectable peritoneal metastases, which were defined as metastases confined to the adjacent peritoneum or a few distant peritoneal metastases [10]. On the other hand, the bone is a metastatic site that the radiation oncologists commonly encounter. Bone metastasis usually does not result in mortality by itself in most cancer types such as lung, breast, and prostate cancers. However, the patients with bone metastases from colorectal cancer are known to carry dismal prognosis, with the expected survival of mere 7–10 months [11,12]. This might result in the difference in answers between the two groups. However, the European Society for Medical Oncology (ESMO) guideline for metastatic colorectal cancer states that single bone metastasis should be classified as OMD. Thus, further studies are needed to define whether bone metastasis of colorectal cancer could be classified as OMD [13]. LNs are chosen by majority of respondents in both groups. O’Cathail et al. [14] have shown that nodal metastasis is associated with better survival than visceral metastases particularly when patients have not been treated with a prior chemotherapy. According to their findings, nodal metastases had the best local control despite the lowest prescription dose (BED10 60–93.3 Gy). Kalapurackal Mathai et al. [15] have also reported the benefit of curative treatment in patients with non-regional nodal metastases from colorectal cancer in overall survival (73 vs. 23.2 months, p=0.007).
Regarding the maximum number of tumors, the radiation oncologists were stricter. In particular, approximately half of the colorectal surgeons responded that the number itself was very important if the metastatic lesions could be treated with local treatment modalities. A recent ESTRO-ASTRO consensus also preferred more flexible criteria based on the treatability rather than the number per se [16]. The number criterion is, in fact, rather a simple, while a comprehensive decision-making based on the tumor biology, DFI, or availability of local treatment could be more realistic, and far more complicated.
Two previous studies reported that the DFI shorter than 12 months was a favorable prognostic factor for survival in colorectal liver metastases [17,18]. Recently proposed guidelines, however, did not specify the minimum DFI for OMD despite of its potential of practical significance, particularly for oligorecurrence cases. In a recent ESTRO-ASTRO consensus, mainly driven by the radiation oncologists, 91% agreed that the minimum DFI was not necessary in defining OMD [16]. Similarly, the ESMO guideline [13] did not suggest any DFI criteria either. In the current survey, although “6–12 months” was the most frequent response in both groups, 37.3% of the surgeons chose “≥ 12 months”, which was chosen by only 12.2% of the radiation oncologists. There was also a discrepancy in the classification of OMD. In the current survey, the radiation oncologists tended to have a more generous view of applying local treatment to repeated OMD (55.1% vs. 79.4%) and induced OMD (23.1% vs. 62.0%). Additional research seems to be required in order to identify the minimum DFI and the clinical significance of OMD categorization in colorectal primaries.
The greatest disparity in response between the two specialty groups was observed for the issue of molecular diagnosis. In colorectal cancer, molecular diagnosis is recommended to predict prognosis and determine optimal systemic therapy [13,19]. KRAS mutations are detected in 40%–50% of the colorectal cancer patients and known as poor prognostic indicators [20,21]. BRAF mutations account for approximately 4%–10% of the colorectal cancer patients and are also associated with poor prognosis [22]. Another widely reported biomarker for colorectal cancer is MSI or TP53 [23]. Sean et al. [24] revealed integrated molecular subtypes for characterizing oligometastatic liver metastases. Based on such classification, the survival rate of the low-risk and high-risk groups were 94% and 19%, respectively. Regarding the molecular biomarkers and radiation response, there were still no confirmed data [14,25]. A phase II trial of proton therapy for liver metastases from colorectal cancer demonstrated that KRAS and TP53 mutations were associated with inferior local control [26]. However, KRAS was not associated with local control following radiation therapy in another study [14]. Although the effect of molecular subtype of colorectal cancer on the response to radiation has not been established, the radiation oncologists should be aware of the potential implication of this in order to predict prognosis, and more importantly, to improve the multidisciplinary team communication.
In Korea, while the radiation oncologists usually care for the patients with various types of cancers, rather than focusing on only colorectal cancer cases, the colorectal surgeons are dedicated in treating the colorectal cancer patients. The findings of this survey-based study could indicate that the surgeons have the concept of OMD that is very specific to colorectal cancer, whereas the radiation oncologists have more general definition of OMD that apply to all types of cancer. The results of randomized trials on OMD have revealed that the role of local therapy may vary depending on the subtype of cancer [27–30]. The SABR-COMET trial results provided the radiation oncology society with an optimism on the potential of wider utilization of radiation therapy in treating the OMD patients in general. However, it is believed that further investigations are needed to validate the optimal disease-specific approach. To achieve this goal, understanding the different viewpoints of the participating caregivers would be prerequisite.
This study revealed that although radiation oncologists and colorectal surgeon agreed on the several aspects of OMD such as diagnostic imaging, biomarker, systemic treatment, and optimal timing of the treatment, they had quite different viewpoints in several aspects of OMD. Such different viewpoints might be inevitable because they experience different situations in their real clinical practice settings. However, it is necessary to have an awareness of many caregivers’ viewpoints as part of a multidisciplinary team, which was demonstrated in the current study, in order to achieve optimal management for the OMD patients. In future studies, the inclusion of not only radiation oncologists and surgeons but also medical oncologists and radiologists who are important multidisciplinary members taking care of oligometastatic patients should be considered imperative to ensure comprehensive understanding of multidisciplinary perspectives on oligometastases.
NotesEthical Statement This research was conducted in accordance with the principle of the Declaration of Helsinki and the approval of institutional review board for this study was waived because of the non-identifiable nature of the survey data. All participants provided written informed consent prior to their participation. Fig. 1Recommended total dose for lung metastasis from colon and rectum in 10-fractions (A) and 4-fractions (B) scheme. 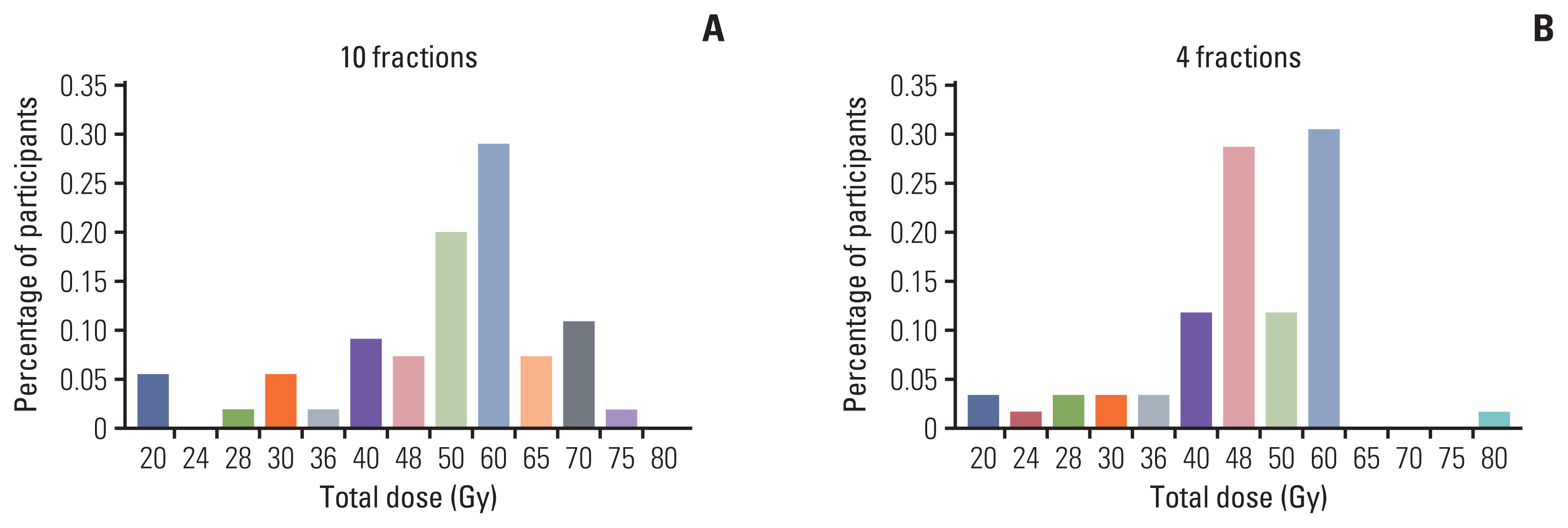
Fig. 2Recommended total dose for liver metastasis from colon and rectum in 10-fractions (A) and 4-fractions (B) scheme. 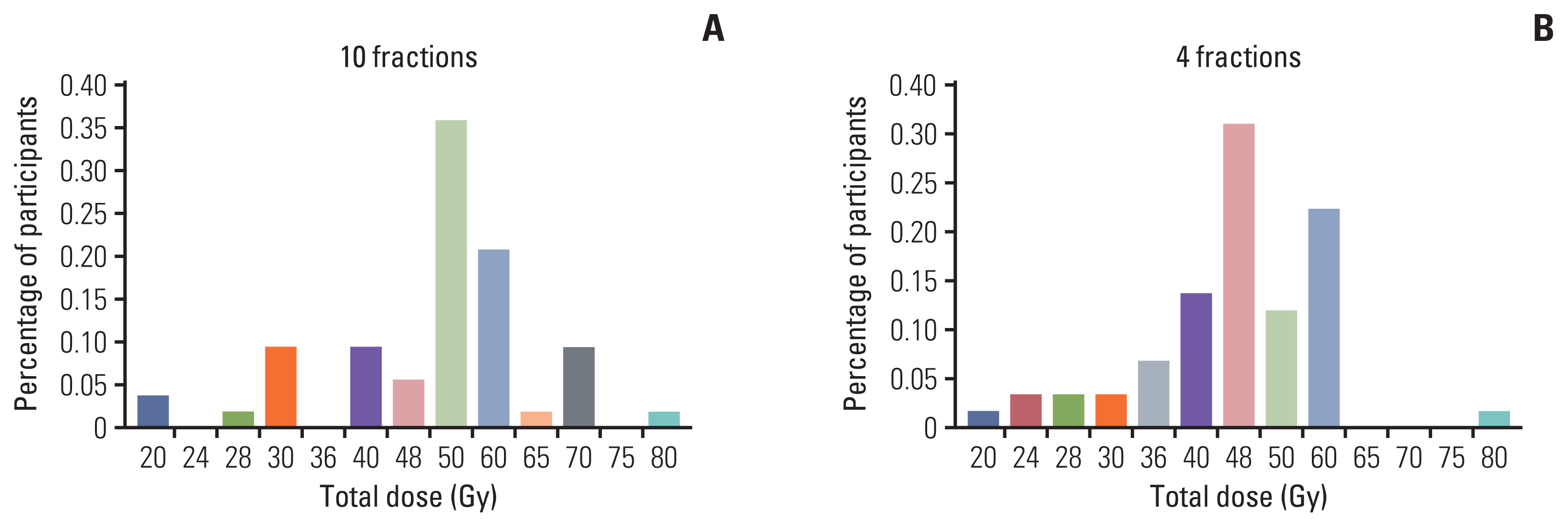
Table 1Survey results and distribution related to definition, diagnosis, treatment, and endpoint of OMD
Values are presented as number (%). CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CRC, colorectal cancer; DFI, disease-free inverval; FDG-PET/CT, 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography; MRI, magnetic resonance imaging; OMD, oligometastatic disease; Tx, therapy. Table 2Demographics of responders Table 3Radiation dose and fractions of metastasis-directed radiotherapy for radiation oncologists only References2. Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–66.
3. Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301.
4. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–28.
5. Willmann J, Vlaskou Badra E, Adilovic S, Ahmadsei M, Christ SM, van Timmeren JE, et al. Evaluation of the prognostic value of the ESTRO EORTC classification of oligometastatic disease in patients treated with stereotactic body radiotherapy: a retrospective single center study. Radiother Oncol. 2022;168:256–64.
6. Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174.
7. Hughes KS, Rosenstein RB, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of long-term survivors. Dis Colon Rectum. 1988;31:1–4.
8. Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49.
9. Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62.
10. Shida D, Kobayashi H, Kameyama M, Hase K, Maeda K, Suto T, et al. Factors affecting R0 resection of colorectal cancer with synchronous peritoneal metastases: a multicenter prospective observational study by the Japanese Society for Cancer of the Colon and Rectum. Int J Clin Oncol. 2020;25:330–7.
11. Santini D, Tampellini M, Vincenzi B, Ibrahim T, Ortega C, Virzi V, et al. Natural history of bone metastasis in colorectal cancer: final results of a large Italian bone metastases study. Ann Oncol. 2012;23:2072–7.
12. Christensen TD, Jensen SG, Larsen FO, Nielsen DL. Systematic review: incidence, risk factors, survival and treatment of bone metastases from colorectal cancer. J Bone Oncol. 2018;13:97–105.
13. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
14. O’Cathail SM, Smith T, Owens R, Zeniou A, Tsang Y, Holyoake DL, et al. Superior outcomes of nodal metastases compared to visceral sites in oligometastatic colorectal cancer treated with stereotactic ablative radiotherapy. Radiother Oncol. 2020;151:280–6.
15. Kalapurackal Mathai V, Aung SY, Wong V, Dunn C, Shapiro JD, Jalali A, et al. Outcomes of isolated distant lymph node metastases in colorectal cancer. J Clin Oncol. 2021;39(3 Suppl):84.
16. Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–66.
17. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18.
18. Kim KY, Kim NK, Cha IH, Ahn JB, Choi JS, Choi GH, et al. Novel methods for clinical risk stratification in patients with colorectal liver metastases. Cancer Res Treat. 2015;47:242–50.
19. Taieb J, Zaanan A, Le Malicot K, Julie C, Blons H, Mineur L, et al. Prognostic effect of BRAF and KRAS mutations in patients with stage III colon cancer treated with leucovorin, fluorouracil, and oxaliplatin with or without cetuximab: a post hoc analysis of the PETACC-8 trial. JAMA Oncol. 2016;2:643–53.
20. Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol. 2012;18:5171–80.
21. Vaughn CP, Zobell SD, Furtado LV, Baker CL, Samowitz WS. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer. 2011;50:307–12.
22. Li Y, Xiao J, Zhang T, Zheng Y, Jin H. Analysis of KRAS, NRAS, and BRAF mutations, microsatellite instability, and relevant prognosis effects in patients with early colorectal cancer: a cohort study in East Asia. Front Oncol. 2022;12:897548.
23. Singh MP, Rai S, Pandey A, Singh NK, Srivastava S. Molecular subtypes of colorectal cancer: An emerging therapeutic opportunity for personalized medicine. Genes Dis. 2021;8:133–45.
24. Pitroda SP, Khodarev NN, Huang L, Uppal A, Wightman SC, Ganai S, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018;9:1793.
25. Jethwa KR, Jang S, Mullikin TC, Harmsen WS, Petersen MM, Olivier KR, et al. Association of tumor genomic factors and efficacy for metastasis-directed stereotactic body radiotherapy for oligometastatic colorectal cancer. Radiother Oncol. 2020;146:29–36.
26. Hong TS, Wo JY, Borger DR, Yeap BY, McDonnell EI, Willers H, et al. Phase II study of proton-based stereotactic body radiation therapy for liver metastases: importance of tumor genotype. J Natl Cancer Inst. 2017;109:djx031.
27. Jia-Mahasap B, Madla C, Sripan P, Chitapanarux I, Tharavichitkul E, Chakrabandhu S, et al. Stereotactic radiosurgery for limited brain metastasis using three different techniques: helical tomotherapy, volumetric modulated arc therapy, and cone-based LINAC radiosurgery. Radiat Oncol J. 2022;40:232–41.
28. Tsai CJ, Yang JT, Guttmann DM, Shaverdian N, Eng J, Yeh R, et al. Final analysis of consolidative use of radiotherapy to block (CURB) oligoprogression trial: a randomized study of stereotactic body radiotherapy for oligoprogressive metastatic lung and breast cancers. Int J Radiat Oncol Biol Phys. 2022;114:P1061.
29. Chmura SJ, Winter KA, Woodward WA, Borges VF, Salama JK, Al-Hallaq HA, et al. NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). J Clin Oncol. 2022;40(16 Suppl):1007.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||