AbstractPurposeWe conducted a nationwide, multicenter, prospective registry study for newly diagnosed patients with peripheral T-cell lymphoma (PTCL) to better define the clinical characteristics, treatment patterns, survival outcomes, and the role of upfront autologous stem cell transplantation (ASCT) in these patients.
Materials and MethodsPatients with PTCL receiving chemotherapy with curative intent were registered and prospectively monitored. All patients were pathologically diagnosed with PTCL.
ResultsA total of 191 patients with PTCL were enrolled in this prospective registry study. PTCL, not otherwise specified (PTCL-NOS) was the most common pathologic subtype (n=80, 41.9%), followed by angioimmunoblastic T-cell lymphoma (AITL) (n=60, 31.4%). With a median follow-up duration of 3.9 years, the 3-year progression-free survival (PFS) and overall survival (OS) rates were 39.5% and 60.4%, respectively. The role of upfront ASCT was evaluated in patients who were considered transplant-eligible (n=59). ASCT was performed as an upfront consolidative treatment in 32 (54.2%) of these patients. There were no significant differences in PFS and OS between the ASCT and non-ASCT groups for all patients (n=59) and for patients with PTCL-NOS (n=26). However, in patients with AITL, the ASCT group was associated with significantly better PFS than the non-ASCT group, although there was no significant difference in OS.
IntroductionPeripheral T-cell lymphoma (PTCL) is a rare and heterogeneous group of diseases characterized by aggressive clinical behavior. It accounts for 5%–10% of all non-Hodgkin lymphomas (NHL) and 15%–20% of all aggressive NHLs [1]. There are 27 different subtypes of PTCL defined by the 2016 World Health Organization classification, including PTCL–not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL), and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) [2]. There are significant geographical variations in the incidence and proportions of PTCL subtypes, with PTCL-NOS being the most common subtype in North America and Europe. In contrast, extranodal natural killer (NK)/ T-cell lymphoma and AITL are the most common subtypes in Asia [3,4]. With the exception of several subtypes of PTCL, such as anaplastic lymphoma kinase–positive (ALK+) ALCL, the prognosis of most subtypes of PTCL is poor, with a 5-year survival rate of 30%–40% [5].
Treatment regimens for PTCL are generally extrapolated from those initially developed to treat aggressive B-cell lymphoma. Currently, there is no clear consensus on the optimal management of patients with newly diagnosed, relapsed, or refractory PTCL. Although CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like regimens are preferred as first-line treatment, the treatment outcomes are disappointing [6]. In this regard, upfront autologous hematopoietic stem cell transplantation (ASCT) has been proposed for patients achieving partial or complete remission after induction therapy with conflicting results; several retrospective studies and prospective single-arm phase II trials have reported encouraging results and others found no added survival benefit of ASCT as an upfront consolidation treatment [5,7–10]. Thus, in the absence of randomized clinical trial data, the role of upfront ASCT remains controversial.
We conducted a nationwide, multicenter, prospective registry study for newly diagnosed patients with PTCL to better define the clinical characteristics, treatment patterns, survival outcomes, and the role of upfront ASCT in patients with PTCL.
Materials and MethodsPatients with newly diagnosed PTCL receiving chemotherapy with curative intent between May 2015 and April 2018 from 20 academic centers in South Korea were registered and prospectively monitored. Eligible patients were adults (age > 18 years) with adequate tissue biopsy specimens for diagnosis and available clinical data, including baseline information on disease staging, laboratory parameters at diagnosis, and treatment regimens received. All patients were pathologically confirmed with PTCL, excluding extranodal NK/ T-cell lymphoma. This study was approved by the institutional review boards of all participating institutions, and the trial was registered at ClinicalTrials.gov, number NCT02364466.
Response to treatment was assessed according to the 2014 Lugano classification [11]. The best response was recorded at the end of the initial treatment, and any changes in disease status were captured during yearly follow-up intervals. Survival data were updated until the cutoff date of June 30, 2021. The choice of treatment regimen and consideration of upfront ASCT after first-line regimen was at the treating physician’s discretion. Patients with PTCL subtypes other than ALK+ ALCL or cutaneous T-cell lymphoma, who were younger than 65 years of age, and achieved at least a partial response to first-line treatment were considered eligible for ASCT.
Progression-free survival (PFS) was defined as the time from the start date of chemotherapy to the date of disease progression or death from any cause, whichever occurred first. Overall survival (OS) was calculated from diagnosis until death from any cause or was censored at the last follow-up. PFS2 was defined as the time from the start date of second-line chemotherapy to the date of disease progression or death from any cause, whichever occurred first. OS2 was defined as the time from the start date of second-line chemotherapy to the date of death from any cause or was censored at the last follow-up. Survival rates and corresponding standard errors were estimated using the Kaplan-Meier method, and survival curves were compared using the log-rank test. Baseline characteristics of the groups were compared using Pearson’s chi-square test or Fisher exact test for categorical variables and Student’s t test or the Mann-Whitney U test for continuous variables, as appropriate.
ResultsA total of 191 patients with PTCL were enrolled in this prospective registry study, and the baseline characteristics of the patients are presented in Table 1. The median age was 57 years (range, 18 to 86 years), 120 patients (62.8%) were male, and 162 (84.8%) had an Eastern Cooperative Oncology Group performance status of 0–1. PTCL-NOS was the most common pathologic subtype (n=80, 41.9%), followed by AITL (n=60, 31.4%), ALK− ALCL (n=17, 8.9%), ALK+ ALCL (n=15, 7.9%), and MEITL (n=12, 6.3%). The most frequently administered first-line regimen was CHOP or a CHOP-like regimen (n=165, 86.4%), followed by ICE (ifosfamide, carboplatin, and etoposide) (n=19, 9.9%).
With a median follow-up duration of 3.9 years (95% confidence interval [CI], 3.6 to 4.0), the 2-year and 3-year PFS rates were 43.5% (95% CI, 36.9 to 51.4) and 39.0% (95% CI, 32.4 to 47.0), respectively. The 2-year and 3-year OS rates were 64.9% (95% CI, 58.2 to 72.3) and 60.4% (95% CI, 53.5 to 68.1), respectively (Fig. 1A and B). Treatment response to first-line regimens were available for 174 patients. The overall response rate and the complete response rate among these patients were 71.3% (n=124) and 54.6% (n=95), respectively. The PFS and OS according to the five most common subtypes of PTCL (PTCL-NOS, AITL, ALK+ ALCL, ALK− ALCL, MEITL) are presented in Fig. 1C and D (n=184). ALK+ALCL patients had the highest 3-year PFS of 77.9%, followed by ALK− ALCL, AITL, PTCL-NOS, and MEITL with a 3-year PFS of 63.0%, 48.2%, 24.1%, and 0.0%, respectively (p < 0.001) (Fig. 1C). For OS both ALK+ ALCL and ALK− ALCL patients had the highest 3-year OS of 100.0%, followed by AITL, PTCL-NOS, and MEITL with a 3-year OS of 68.6%, 42.0%, and 22.9%, respectively (p < 0.001) (Fig. 1D).
The role of upfront ASCT was evaluated in patients with PTCL subtypes other than ALK+ ALCL or cutaneous T-cell lymphoma (n=172). Among 104 patients younger than 65, 59 patients achieved a partial or complete response to first-line treatment and were considered transplant-eligible (S1 Fig.). The baseline characteristics of the transplant-eligible patients are presented in Table 2. ASCT was performed as an upfront consolidative treatment in 32 (54.2%) of these patients, and there were no significant differences in baseline characteristics between patients who received upfront ASCT (ASCT group, n=32) and those who did not (non-ASCT group, n=27). The combination of busulfan, cyclophosphamide, and etoposide (BuCyE) was the most commonly used conditioning regimen for upfront ASCT (n=26, 81.3%) followed by etoposide, cyclophosphamide, and total body irradiation (VCT) (n=4, 12.5%). With a median follow-up duration of 4.2 years (95% CI, 3.6 to 4.5), there were no significant differences in PFS and OS between the ASCT (n=32) and non-ASCT (n=27) groups, with 3-year PFS rates of 53.5% vs. 41.7% (p=0.289) and 3-year OS rates of 70.2% vs. 70.8% (p=0.750), respectively (Fig. 2A and B).
Additional analyses were performed to explore the impact of ASCT on survival outcomes among various subgroups of patients with PTCL. There was no difference in PFS between the ASCT and non-ASCT groups among patients who achieved a complete remission and among patients who achieved partial remission to first-line chemotherapy (Fig. 3A and B). Among patients with advanced-stage disease, there was a trend toward improved PFS in the ASCT group compared with the non-ASCT group with 3-year PFS rates of 61.8% vs. 29.6% (p=0.060), while no difference in PFS was observed for upfront ASCT in patients with limited-stage disease (Fig. 3C and D). An analysis of International Prognostic Index (IPI) and Prognostic index for PTCL-unspecified (PIT) scores revealed that there was a trend toward improved PFS in the ASCT group compared with the non-ASCT group in patients with high IPI scores (high-intermediate or high-risk group) (3-year PFS rate, 68.4% vs. 33.3%; p=0.077) and high PIT scores (group 3 or 4) (3-year PFS rate, 62.5% vs. 27.8%; p=0.06), while no differences in PFS were observed according to upfront ASCT in patients with low IPI scores (low or low-intermediate risk group) or low PIT scores (group 1 or 2) (Fig. 3E–G). For OS, there was no subgroup demonstrating better OS in the ASCT group compared to the non-ASCT group (S2 Fig.).
We further evaluated the role of ASCT within patients with PTCL-NOS (n=26) and AITL (n=21) separately. There were no significant differences in PFS or OS between the ASCT (n=14) and non-ASCT (n=12) groups for PTCL-NOS, with 3-year PFS rates of 40.2% vs. 38.9% (p=0.618) and 3-year OS rates of 53.1% vs. 56.2% (p=0.979), respectively (Fig. 4A and B). In patients with AITL, however, the ASCT group (n=14) was associated with significantly better PFS compared with the non-ASCT group (n=7), with 3-year PFS rates of 77.4% vs. 28.6% (p=0.014), respectively, although there was no significant difference in OS, with 3-year OS rates of 85.1% vs. 83.3% (p=0.882), respectively (Fig. 4C and D).
A total of 83 patients were treated with second-line systemic chemotherapy. The most frequently administered second-line regimen was ICE (n=21, 25.3%), followed by GDP (gemcitabine, dexamethasone, and cisplatin) (n=18, 21.7%), and DHAP (dexamethasone, cytarabine, and cisplatin) (n=10, 12.0%). With a median follow-up duration of 2.5 years, the 3-year PFS2 rate of these patients was 26.0% (95% CI, 17.5 to 38.7), and the 3-year OS2 rate was 36.1% (95% CI, 25.7 to 50.7) (S3 Fig.). Treatment responses to second-line regimens were available for 66 patients. The overall response rate and the complete response rate among these patients were 47.0% (n=31) and 30.3% (n=20), respectively.
DiscussionAlthough several studies have evaluated the real-world outcomes of PTCL, the results are not uniform due to the heterogeneity of PTCL and the geographical differences in the relative frequency of its various subtypes [3–5,12]. In addition, differences in the proportion of PTCL subtypes exist within the same geographical region, which makes it even more challenging to compare and interpret the results of such studies [3,4,13,14]. In this context, this nationwide, multicenter, prospective registry study may provide additional insight into the real-world outcomes of PTCL patients.
In this study, the most common pathologic subtype was PTCL-NOS (41.9%) followed by AITL (31.4%). This is slightly different from a recently reported prospective registry study in Asia, which showed that AITL was the most common subtype of nodal PTCL, further supporting that there is a difference in the proportion of PTCL subtypes within the same geographical region [4]. In line with previous studies, a CHOP-based regimen was the most frequently administered first-line regimen. However, the survival outcome was poor in the entire group of patients, with a 3-year PFS rate of 39.0%. This poor outcome was mainly due to the poor survival of patients with PTCL-NOS and MEITL.
On the other hand, patients with ALK+ or ALK− ALCL showed favorable survival outcomes with a 3-year PFS rate of higher than 50.0%. Similar findings have been reported in several previous studies, which demonstrated that the survival outcome of ALCL patients was significantly better than that of PTCL-NOS [3–5]. Of note, patients with AITL showed better survival outcomes than patients with PTLC-NOS in the current study, which is in line with the results of a recent Asian prospective registry study [4]. In contrast, most of the previous studies that included patients in Western countries have demonstrated no significant difference in the survival outcomes between patients with PTCL-NOS and AITL [3,5,7]. Differences in patient clinical characteristics between these studies may have contributed to these discrepant results. Still, it may also be due to differences in the biological aspects of Asian and Western patients with AITL. In this regard, additional investigations, including genetic analysis, are necessary to explore the biological characteristics of AITL.
The role of upfront ASCT as a consolidative treatment for patients with PTCL is still controversial as there is currently no randomized trial addressing this issue. Although a small number of phase II single-arm trials have demonstrated promising survival outcomes with upfront ASCT in PTCL, these studies lacked direct comparisons with non-ASCT patients [8,9]. Several retrospective studies and prospective registry trials have recently compared the survival outcomes according to ASCT [4,7,10,15,16]. In the large multicenter retrospective study conducted by the LYSA group, there was no survival benefit of upfront ASCT for patients with PTCL-NOS, AITL, or ALK− ALCL [10]. Similar findings were observed in prospective registry studies conducted in Asia [4,16]. However, these studies pooled all PTCL-NOS, AITL, and ALK− ALCL patients together and evaluated the role of upfront ASCT as a whole, and did not investigate the potential role of ASCT in individual pathologic subtypes. In a prospective multicenter cohort study conducted in the United States (the COMPLETE study), significant improvement in survival was observed in patients with AITL [7], although there was no survival benefit of upfront ASCT in pooled patients with PTCL-NOS, AITL, and ALK− ALCL. Similar findings were observed in a prospective international cohort study of patients with AITL, which demonstrated that upfront ASCT was associated with improved survival outcomes in patients with AITL [15]. In line with these studies, the current study showed significantly better PFS in AITL patients who received upfront ASCT compared with those who did not, while no survival benefit was observed in patients with PTCL-NOS. In addition, upfront ASCT was associated with a trend towards better PFS in high-risk patients with advanced-stage disease, high IPI scores, or high PIT scores. These findings also align with the results from the COMPLETE study [7]. Taken together, the results of this study suggest that upfront ASCT may provide a survival benefit in subgroups of patients with PTCL, especially those with AITL and/or high-risk patients with advanced-stage disease, high IPI scores, or high PIT scores. Although the improved PFS with upfront ASCT in AITL or high-risk patients did not translate into an OS benefit, a longer follow-up is required to assess whether this PFS benefit translates into an OS benefit.
This study had several limitations. First, the follow-up duration of the study was relatively short, with a median follow-up duration of 3.9 years. However, a recent study demonstrated that event-free survival at 24 months or progression of disease within 24 months translates into OS and may be a clinically significant endpoint in PTCL, which supports the clinical significance of the current study [15,17]. Another limitation of this study is that the sample size of patients considered transplant-eligible was small, limiting the statistical power. In addition, as the decision for upfront ASCT in these patients was at the attending physician’s discretion, there may have been selection bias. Furthermore, higher proportion of patients in the non-ASCT group was ALK− ALCL, had low IPI scores (low or low-intermediate risk group), and had low PIT scores (group 1 or 2) compared with ASCT group, although the differences were not statistically significant. Despite these limitations, this study was a prospective cohort study, allowing for an objective assessment of real-world treatment approaches and outcomes of patients treated in the contemporary era, providing valuable information for designing future trials.
In conclusion, the current study demonstrated that the survival outcomes with the current treatment options remain poor for patients with PTCL-NOS and MEITL. In contrast, relatively favorable survival outcomes were noted in patients with ALK+ ALCL, ALK− ALCL, and AITL. Upfront ASCT may provide a survival benefit in patients with AITL and high-risk patients with advanced disease, high IPI scores, and high PIT scores. The full extent of the role of upfront ASCT in PTCL should be evaluated in prospective randomized trials. The current study will bridge future trials by better defining the target patient populations most likely to benefit from upfront ASCT.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the institutional review boards of all participating institutions, and the trial was registered at ClinicalTrials.gov, number NCT02364466. Informed consent was obtained from all patients for being included in the study. Author Contributions Conceived and designed the analysis: Yoon DY, Kim WS. Collected the data: Cho H, Yoon DY, Shin DY, Koh Y, Yoon SS, Kim SJ, Do YR, Lee GW, Kwak JY, Park Y, Kang HJ, Yi JH, Yoo KH, Lee WS, Park BB, Jo JC, Eom HS, Kim HJ, Jeong SH, Won YW, Sohn BS, Kwon JH, Suh C, Kim WS. Contributed data or analysis tools: Cho H, Shin DY, Koh Y, Yoon SS, Kim SJ, Do YR, Lee GW, Kwak JY, Park Y, Kim MK, Kang HJ, Yi JH, Yoo KH, Lee WS, Park BB, Jo JC, Eom HS, Kim HJ, Jeong SH, Won YW, Sohn BS, Kwon JH, Suh C, Kim WS. Performed the analysis: Cho H, Yoon DY, Kim WS. Wrote the paper: Cho H, Yoon DY. Fig. 1Progression-free survival (A) and overall survival (B) of all patients (n=191). Progression-free survival (C) and overall survival (D) according to the five most common subtypes of PTCL (PTCL-NOS, AITL, ALK+ ALCL, ALK− ALCL, and MEITL) (n=184). AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified. 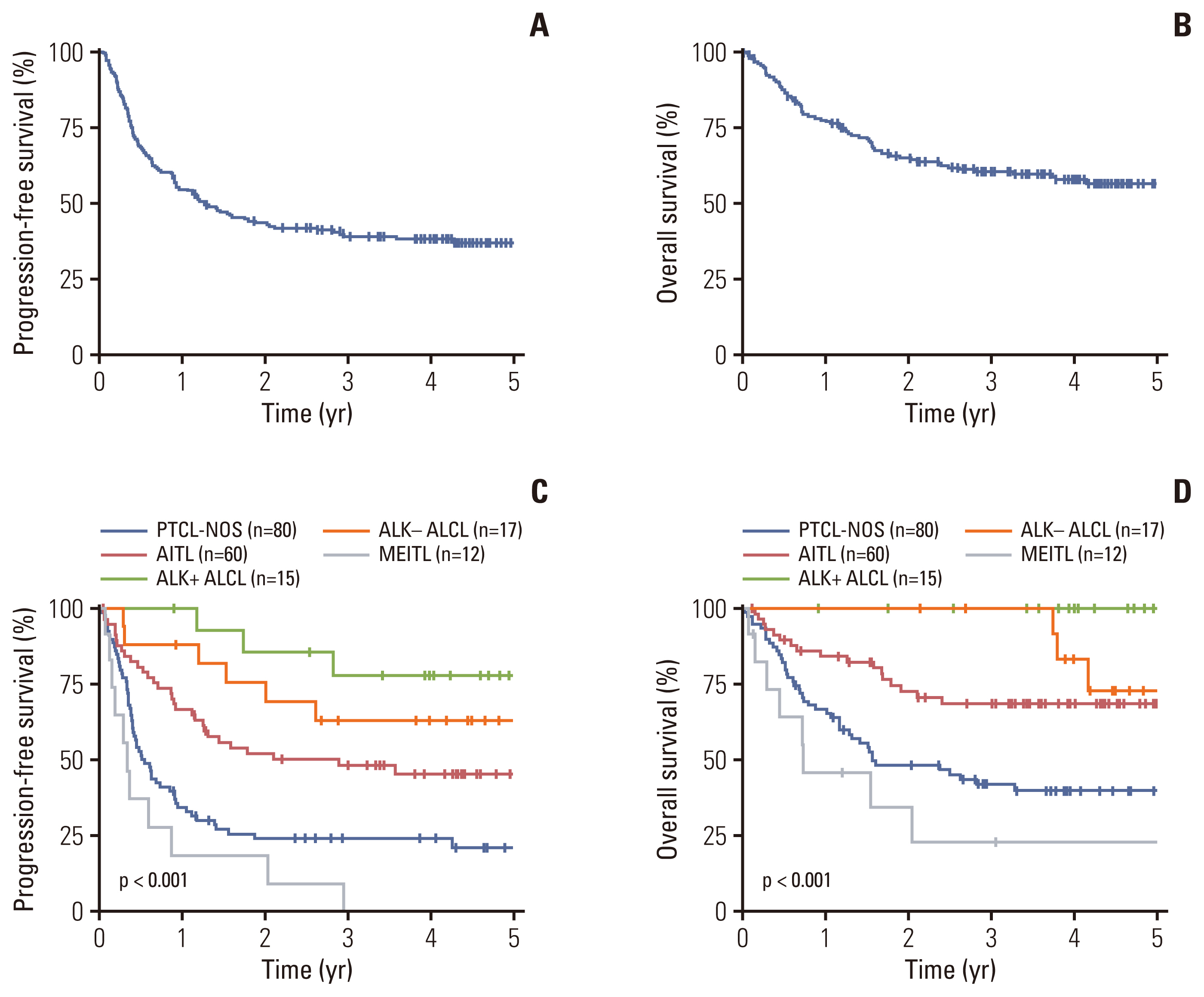
Fig. 2Progression-free survival (A) and overall survival (B) according to upfront autologous stem cell transplantation (ASCT) in transplant-eligible patients. 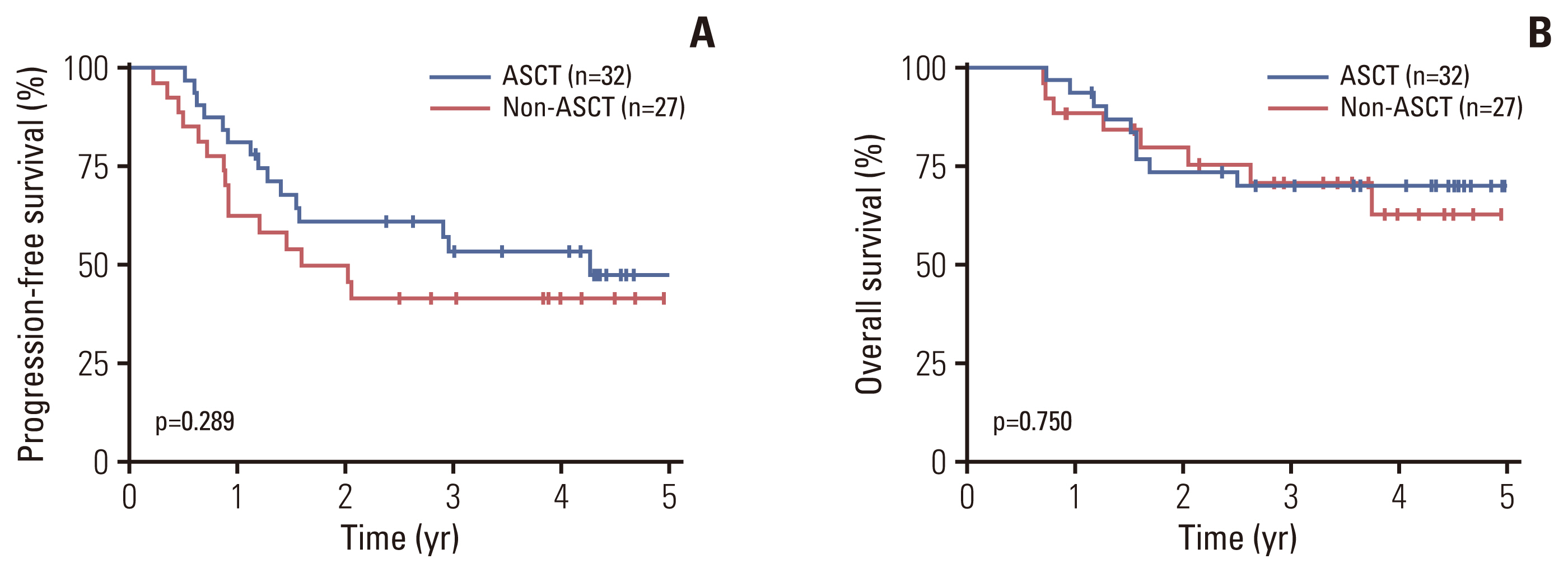
Fig. 3Progression-free survival according to upfront autologous stem cell transplantation (ASCT) in patients who achieved complete remission (A) and partial remission (B) from first-line systemic chemotherapy. Progression-free survival according to upfront ASCT in patients with limited-stage disease (C), advanced-stage disease (D), low International Prognostic Index (IPI) score (E) (low or low-intermediate risk group), high IPI score (high-intermediate or high-risk group) (F), low Prognostic Index for peripheral T-cell lymphoma–unspecified (PIT) score (group 1 or 2) (G), high PIT score (group 3 or 4) (H). 
Fig. 4Progression-free survival (A) and overall survival (B) according to upfront autologous stem cell transplantation (ASCT) in peripheral T-cell lymphoma not otherwise specified patients. Progression-free survival (C) and overall survival (D) according to upfront ASCT in angioimmunoblastic T-cell lymphoma patients. 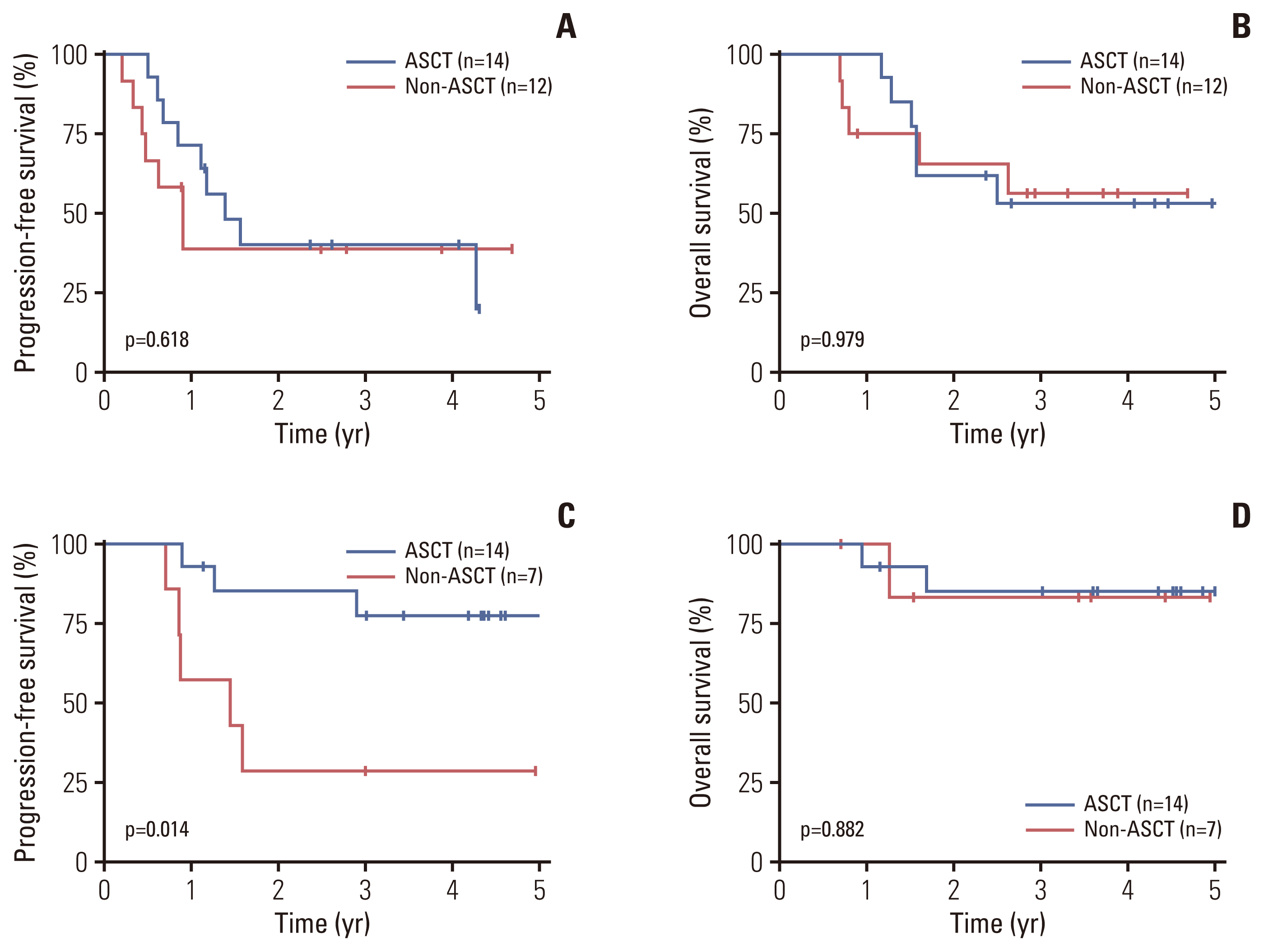
Table 1Baseline characteristics of the patients Table 2Baseline characteristics of upfront transplant-eligible patients Values are presented as number (%) unless otherwise indicated. AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; ASCT, autologous stem cell transplantation; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; ECOG PS, Eastern Cooperative Oncology Group performance status; ICE, ifosfamide, carboplatin, and etoposide; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; PTCL-U, peripheral T-cell lymphoma–unspecified. References1. Zain JM, Hanona P. Aggressive T-cell lymphomas: 2021 updates on diagnosis, risk stratification and management. Am J Hematol. 2021;96:1027–46.
2. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
3. Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–30.
4. Yoon SE, Song Y, Kim SJ, Yoon DH, Chen TY, Koh Y, et al. Comprehensive analysis of peripheral T-cell and natural killer/T-cell lymphoma in Asian patients: a multinational, multicenter, prospective registry study in Asia. Lancet Reg Health West Pac. 2021;10:100126.
5. Ellin F, Landstrom J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124:1570–7.
6. Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15:1467–75.
7. Park SI, Horwitz SM, Foss FM, Pinter-Brown LC, Carson KR, Rosen ST, et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study. Cancer. 2019;125:1507–17.
8. d’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–9.
9. Reimer P, Rudiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106–13.
10. Fossard G, Broussais F, Coelho I, Bailly S, Nicolas-Virelizier E, Toussaint E, et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncol. 2018;29:715–23.
11. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
12. Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral T-cell lymphoma incidence and survival in the United States. J Clin Oncol. 2016;34:963–71.
13. Shi Y. Current status and progress of lymphoma management in China. Int J Hematol. 2018;107:405–12.
14. Fuji S, Kida S, Nakata K, Morishima T, Miyashiro I, Ishikawa J. Increased incidence of adult T cell leukemia-lymphoma and peripheral T cell lymphoma-not otherwise specified with limited improvement in overall survival: a retrospective analysis using data from the population-based Osaka Cancer Registry. Ann Hematol. 2021;100:157–65.
15. Advani RH, Skrypets T, Civallero M, Spinner MA, Manni M, Kim WS, et al. Outcomes and prognostic factors in angioimmunoblastic T-cell lymphoma: final report from the international T-cell Project. Blood. 2021;138:213–20.
|
|
||||||||||||||||||||||||||||||||||||||||||||||