AbstractPurposeThis study was conducted to investigate the clinical characteristics of patients with advanced non–small cell lung cancer (NSCLC) harboring human epidermal growth factor receptor 2 (HER2) mutations and to evaluate response to standard treatment and HER2-targeted agents.
Materials and MethodsUsing tissue and/or blood next-generation sequencing, we identified 44 patients with NSCLC harboring HER2 mutations who were treated at Severance Hospital between December 2016 and February 2021. Clinical data, including patient characteristics, mutation status, incidence of metastasis for distant lesions, and response to chemotherapy, were retrospectively analyzed.
ResultsThe median age was 58 years, and 61% of the patients were female. Most patients (64%) were never-smokers. Adenocarcinoma was the most predominant subtype (98%). A total of 66% of the patients had extrathoracic metastatic lesions, and 32% had intracranial lesions at initial presentation. The median time to the development of brain metastasis was 15.6 months (range, 2.4 to 43.7). The most common type of HER2 mutation was 12 base pair in-frame insertion in exon 20, A775_G776insYVMA. Of the 44 patients, two had concomitant driver mutations, one with epidermal growth factor receptor (EGFR) mutation (V769M), and one with BRAF mutation (V600E). Patients treated with pemetrexed-based chemotherapy (75%) had an overall response rate (ORR) and progression-free survival (PFS) of 30% and 8.3 months (95% confidence interval [CI], 3.9 to 12.7), respectively. The ORR and PFS of HER2-targeted agent treated patients (14%) were 0.0% and 1.9 months (95% CI, 0.1 to 2.8), respectively.
IntroductionThe molecular and pathological classification of non–small cell lung cancer (NSCLC) has become considerably more complex over the past decade. A better understanding of the molecular pathways driving NSCLC has led to the development of drug agents that target specific molecular pathways. Patients with epidermal growth factor receptor (EGFR) mutation [1] or anaplastic lymphoma kinase (ALK) [2] or c-ros oncogene 1 (ROS1) [3] rearrangement are now commonly treated with oral tyrosine kinase inhibitors (TKIs), which exhibited improved therapeutic efficacy with decreased toxicity compared with conventional chemotherapies.
Alteration in human epidermal growth factor receptor 2 (HER2) gene has emerged as oncogenic drivers and therapeutic targets in lung cancers [4]. In addition to gene amplification, HER2 is also activated by specific mutations, most of which are placed in its extracellular domain or kinase domain. HER2 overexpression and HER2 mutations involve increased homodimerization or heterodimerization and the hyperactivation of downstream signaling cascades, such as the mitogen-activated protein kinase and phosphoinositide 3-kinase pathways, which drives proliferative oncogenic signaling. HER2 mutations and HER2 amplifications are reported in approximately 1%–4% and 2%–5% of lung adenocarcinomas, respectively [5].
Currently there are no approved HER2-targeted therapies for lung cancer [4]. HER2-targeted agents, including monoclonal antibodies, such as trastuzumab and pertuzumab, and TKIs, such as dacomitinib, afatinib, and neratinib, have been investigated in patients with lung cancers. Although clinical response was observed, neither class of drugs has presented practice-changing results in clinical trials. Antibody-drug conjugates (ADCs), such as ado-trastuzumab emtansine (T-DM1, Genentech/Roche) and trastuzumab deruxtecan (T-DXd, formerly DS8201a, Daiichi-Sankyo/AstraZeneca), are recently emerging antitumoral agents for HER2-mutated NSCLC; however, they have not yet been implemented in routine practice [6]. Moreover, information on the clinical outcomes of TKIs and ADCs in patients with HER2 mutations is limited [7]. Therefore, standard chemotherapy remains the first-line therapy for advanced lung cancer patients harboring HER2 mutations [8].
This study comprehensively analyzed the real-world, clinical characteristics of NSCLC patients with HER2 mutations detected by next-generation sequencing (NGS).
Materials and Methods1. Patients and samplesWe identified 44 NSCLC patients at the Yonsei Cancer Center between December 2016 and February 2021 with HER2 mutation detected by NGS. The numbers of blood and tissue samples tested were tissue only (n=16), blood only (n=16), and both (n=12). The following clinical data were retrospectively analyzed: patient characteristics; the incidence of brain metastasis; responses to pemetrexed-based treatment, immune checkpoint inhibitors, or HER2-targeted treatment. Because of a local health authority regulation that do not allow the off-label use of chemotherapeutic agents, the sequence of treatment was decided based on local health guidelines and the availability of clinical trials. Patients were assessed for treatment response by computed tomography. Computed tomography was performed after every three cycles. Besides regular follow-ups, imaging was conducted at the physician’s discretion. The response rate was calculated by adding the percentage of patients with a complete response and that with a partial response to the treatment.
2. Statistical analysisProgression-free survival (PFS) was calculated from the start of treatment until radiographic progression or death. Otherwise, patients were censored from analysis. Overall survival (OS) was defined as the interval between treatment initiation and the date of death. The OS and PFS times were calculated from the initiation of each line of treatment. To characterize the OS based on treatment exposure, the OS from the date of diagnosis to that of death was also measured. Kaplan-Meier curves and risk tables were used to predict treatment failure. The Cox proportional hazard progression model was used to calculate the hazard ratio. Statistical significance was set at p ≤ 0.05. The Response Evaluation Criteria in Solid Tumors ver. 1.1 were used to assess treatment response. SPSS software ver. 25 (IBM Corp., Armonk, NY) was used for statistical analysis.
3. Circulating tumor DNA analysisBlood samples were obtained, and circulating tumor DNA (ctDNA) was isolated from the plasma. Digital NGS of the ctDNA was carried out by Guardant Health (Redwood City, CA), a Clinical Laboratory Improvement Amendment–certified, College of American Pathologists–accredited, and New York State Department of Health–approved clinical laboratory. A targeted, hybrid-capture–based NGS panel detecting all four major types of genetic alterations in 73 genes (S1 Table) was used in patients. Protocols for isolation, sequencing, and analysis have been described elsewhere (Supplementary Methods) [9].
4. Tissue analysisTargeted RNA and DNA sequencing were done using TruSight Tumor 170 (Illumina, San Diego, CA). The TruSight Tumor 170 panel was designed to detect 170 cancer-related genes, including 151 genes with potential single nucleotide variants and indels, 59 with potential amplifications, and 55 with fusion and splice variants (S2 Table). Briefly, 40 ng of formalin-fixed paraffin-embedded (FFPE) tissue-derived DNA and RNA samples were extracted using a QIAGEN AllPrep DNA/RNA FFPE Kit (Qiagen, Hilden, Germany). After hybridization capture-based target enrichment, paired-end sequencing (2×150 bp) was performed on a NextSeq sequencer (Illumina) according to the manufacturer’s instructions. Variants with a total depth of > 100× and a variant allele frequency of > 3% were included for analysis. Variant interpretation was based on the recommendations of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists [10] (Supplementary Methods). Actionable genetic alterations were stratified into one of four levels based on the OncoKB website (http://www.OncoKB.org). Tier 1 variants, which include level 1 and level 2 genetic alterations, are Food and Drug Administration (FDA)–approved biomarkers and the standard of care, whereas tier two variants included alterations with compelling clinical or preclinical evidence to drug response.
Results1. Characteristics of the study populationA total of 44 patients with HER2-mutated NSCLC were included in this study (Table 1). The incidence of the HER2-mutataion were 5% within total NGS results. Most patients were female (n=27, 61%) and never-smokers (n=28, 64%). The majority of patients (n=43, 98%) had adenocarcinoma, and one patient with squamous cell carcinoma was identified. Interestingly, two patients had concurrent mutations one with the EGFR mutation (EGFR p.V769M), and the other (2%) with BRAF mutation (BRAF p.V600E). Most patients (84%) received palliative chemotherapy, whereas 11% received definitive therapies, including neoadjuvant chemotherapy, surgery, adjuvant chemotherapy, and definitive concurrent chemoradiation. Thirty patients (68%) initially presented as stage IV. Most common sites of metastasis were bone (n=18, 41%) and brain (n=14, 32%). At the time of data lock, 17 patients (39%) were alive, and 27 (61%) had died. The median follow-up duration was 14.8 months which was updated from last report [11].
The pemetrexed-based regimen included pemetrexed and platinum followed by pemetrexed maintenance. This treatment was administered in 75% of the patients. HER2-targeted agents (neratinib, afatinib, herceptin, and trastuzumab emtansine) were administered to 14% of the patients. There were 17 patients (39%) treated with immune checkpoint inhibitors and 10 (23%) with immune checkpoint inhibitor (ICI) and cytotoxic chemotherapy combination. HER2 genomic alteration was identified by NGS form tissue samples (n=27), blood samples (n=19), or both (n=12) (Table 1).
2. Heterogeneity of HER2 mutationsWe identified the following mutation types. The exon 20 mutation was found in 24 (55%) of 44 patients. The A775_G776insYVMA exon 20 insertion mutation was most frequently detected (n=14, 32%). Other mutations included G776delinsVC (exon 20 deletion and insertion), P780_Y781-insGSP (exon 20 insertion), G776delinsVV (exon 20 deletions and insertion), and G778_S779insCPG (exon 20 insertion). Exon 19 mutations were detected in five patients, of which four (9%) had the L755P mutation and one (2%) had the L755_N758delinsPST mutation (exon 19 deletion). The following exon 17 mutations were present in five patients: substitution V659E missense mutation (n=2, 5%), substitution G660D missense mutation (n=1, 2%), V659D mutation (n=1, 2%), and R678Q missense mutation with S310F (exon 8) missense mutation (n=1, 2%). For else, all the mutation landscape found by NGS are described in Fig. 1.
3. Treatment outcomesThe median OS (calculated from the date of relapse or metastasis to the date of death) was 17.2 months (95% confidence interval [CI], 9.3 to 24.9) for total study population (Fig. 2A). The median OS (calculated from the date of relapse or metastasis to the date of death) for patients with a mutation at exon 20 which is the most common site (n=19) was 21.7 months (95% CI, 6.2 to 37.3), whereas that for those who did not have this exon 20 mutation (n=19) was 12.2 months (95% CI, 6.7 to 17.7) showing no significant difference (Fig. 2B). The patients who were treated with pemetrexed-based chemotherapy (n=33), with a median OS of 18.9 months (95% CI, 9.1 to 28.7); that for patients who did not receive pemetrexed-based chemotherapy (n=5) was 10.6 months (95% CI, 6.1 to 15.1). There was no significant difference in the OS between the two groups (p=0.289) (Fig. 2C). The median OS for patients treated with HER2-targeted agents (n=6) was 11.8 months (95% CI, 8.8 to 13.9), and that for those who did not receive HER2-targeted agents (n=32) was 17.6 months (95% CI, 6.7 to 28.5). Also, there was no significant difference in the OS between the two groups (p=0.15) (Fig. 2D).
The median OSt (calculated from the date treatment to the date of death) for those treated with pemetrexed was 17.9 months (95% CI, 5.7 to 30.2), and the median PFS was 8.3 months (95% CI, 3.9 to 12.7) (Fig. 3A and B). The following HER2-targeted agents were used: afatinib (n=4), neratinib plus trastuzumab (n=1), and trastuzumab-emtansine (n=1). The median OSt for those treated with a HER2-targeted agents was 1.9 months (95% CI, 0.2 to 2.7), and the median PFS was 1.9 months (95% CI, 0.1 to 2.8) (Fig. 3C and D).
The treatment duration and best response to HER2-targeted agents are shown in Table 2. Overall, four patients were treated with afatinib, with a median PFS of 1.2 months (95% CI, 0.0 to 3.1). One patient was treated with neratinib plus trastuzumab and had a PFS of 3.68 months. One patient was administered trastuzumab emtansine; however, his condition worsened, and he died on the day of administration. This patient was treated as an outlier in the calculation of the overall PFS. However, this data was not excluded from the study because it was considered a real-world case given the fact that HER2-targeted agent is only used in a dead-end situation.
There was a unique subgroup of patients treated with ICI alone (n=17) and in combination with other cytotoxic chemotherapy (n=10). No patients in the former group showed a partial response, with a median PFS of 1.6 months (95% CI, 0.5 to 2.8). Two patients in the latter group showed a treatment partial response, with a median PFS of 5.0 months (95% CI, 2.1 to 7.8).
4. Patterns of brain metastases in HER2-positive NSCLC patientsOverall, 14 patients (32%) had intracranial metastases at the time of initial diagnosis or recurrence. Among the 30 patients without brain metastasis, 10 experienced development of brain metastasis during follow-up. The median time to brain metastasis was 15.6 months (95% CI, 1.5 to 29.7) (Fig. 4).
When patients were divided according to treatment, the most common site of metastasis in those receiving pemetrexed-based treatment was the lung (50%) followed by extrathorasic metastasis in the liver (37%), bone metastasis (23%), lymph node metastasis (20%), and brain metastasis (13%). This trend was similarly observed in patients receiving other treatments (Table 2).
DiscussionAlterations in HER2 have been identified as an oncogenic driver mutation for NSCLC. However, the HER2 mutation test is not regularly performed as the routine test, limiting their application in clinical practice. This is due to lower incidence of HER2 mutations and not enough data to support the biomarker driven treatment for this mutation [4,12]. However, recently NGS is increasingly being performed at initial diagnosis to detect the rare targetable mutation, simultaneously making HER2 mutation to be the next emerging target. In a previous study by Lee et al. [13] the incidence of HER2 alterations which include HER2 exon 20 insertion mutation and HER2 amplification was 3% (36/1,108) [13]. Other study by Oh et al. [14] showed frequency of 8.1% of HER2 alterations by NGS which was conducted only on specimens that were positive in direct sequencing [14]. These previous studies showed similar incidence with our study (5%). However, our study analyzed HER2 mutated NSCLC patients conduct by NGS on both tissue and blood samples, not relying on immunohistochemical (IHC) staining or direct sequencing results like the previous studies. The median OS time of HER2-mutant patients was 14.8 months (95% CI, 7.8 to 21.8), and the response to HER2-targeted agents was not seen. Altogether, this real-world data of HER2- mutant NSCLC patients shows that there is a huge unmet need for developing new HER2-targeted agents promptly.
In our study population, HER2-targeted agents were used in a very small number of patients (n=6), with limited therapies (afatinib, neratinib plus trastuzumab, and trastusumab-emtamsine). Trastuzumab, a HER2 targeting monoclonal antibody, have improved the outcome of HER2-positive gastric cancer and breast cancer. Trastuzumab, however, showed clinical response with an overall response rate (ORR) of only 13% in NSCLC patients with HER2 amplification/overexpression [15]. The therapeutic values of TKIs, including dacomitinib, afatinib, neratinib, poziotinib, and pyrotinib were reported [16]. In a preclinical study, afatinib inhibited the growth of HER2-altered NSCLC cells [17]. Afatinib showed antitumor activity in pretreated HER2-mutant NSCLC patients, with an ORR and a disease control rate of 19% (3/16) and 69% (11/16), respectively [12,18]. Neratinib is another pan-HER TKI that has been reported to improve the OS of post-operative patients with HER2-positive breast cancer previously treated with trastuzumab-based adjuvant therapy [19]. In the PUMA-NER-4201 study and NSCLC patients at SUMMIT trial [20], neratinib monotherapy showed limited activity in HER2-mutated NSCLC.
Recently, ADC has demonstrated promising antitumor effects in clinical studies. Two ADCs (T-DM1 and T-DXd) were mainly studied for NSCLC harboring HER2 mutations. T-DM1, an ADC made of the HER2-targeted monoclonal antibody trastuzumab with the cytotoxic microtubule inhibitor DM1 was shown to be effective and tolerable in patients with HER2-mutant NSCLC in a clinical trial proceeded in the United States, with an ORR of 44% and a median PFS of 5 months. However, a phase II study of T-DM1 alone in relapsed HER2-positive NSCLC was terminated early, due to limited efficacy. This trial showed that among 15 HER2-positive (IHC 2+/3+ and fluorescence in situ hybridization–positive or exon 20 mutation) patients, only one patient with mutation reached a partial response [21]. T-DXd is a novel ADC composed of an anti-HER2 antibody, cleavable tetrapeptide-based linker, and a topoisomerase I inhibitor payload. In a phase II trial, HER2-mutant NSCLC patients with T-DXd treatment had an ORR of 72.7% (8/11) and a median PFS of 11.3 months. T-Dxd recently earned its breakthough designation by the FDA [22]. In the DESTINY-Lung01 Trial, responses to T-DXd were seen in patients with different mutation subtypes located across the extracellular and kinase domains of the HER2 protein [23]. Moreover, responses were more likely seen in patients with HER2 mutation than patients with HER2 overexpression. It is presumed that activating HER2 mutations enhance receptor internalization and intracellular uptake of the complex of HER2 receptor and ADC. This may explain of the lack of activity in HER2-overexpressing NSCLC patients.
ICIs targeting the programmed cell death protein 1 and programmed death-ligand 1, including pembrolizumab, nivolumab, and atezolizumab have demonstrated single-agent activity over conventional chemotherapy in NSCLC. Recently, one research showed that HER2-mutant subsets do not result similar benefit from ICIs: the ORR in NSCLC patients with HER2 alteration was 7%, which was lower than those of patients with KRAS (26%), BRAF (24%), ROS1 (17%), and EGFR (12%) driver mutations [24]. Chen et al. [25], also reported an ORR of 0% (0/6) for HER2-mutant NSCLC patients treated with ICIs.
Brain metastases occur in 18%–61% of lung cancer patients [26]. In previous studies of patients with positive HER2 mutation detected by IHC staining, the incidence of brain metastasis ranged from 6% to 29% [6,8,12]. In this study, we observed an incidence of 31.8%, which is higher than previously reported rates. These findings suggest that next-generation HER2-targeted agents should have brain-penetration activity. Several TKIs and ADC such as ado-trastuzumab emtansine were reported to be effective in brain metastasis patients with ERBB2 mutation [23]. Drugs, such as neratinib, lapatinib dacomitinib, afatinib, erlotinib, and pyrotinib, are also known to penetrate the blood-brain barrier and have been reported to be effective in patients with ERBB2-positive solid tumors and brain metastasis [24–26]. Therefore, future drug development in HER2-mutant lung cancer should prioritize the blood-brain barrier penetration activity.
This study has several limitations. First, the sample size (n=44) was small. This is because NGS analysis has not yet been widely implemented, and the incidence of HER2 mutations in lung cancer is not relatively high [4,12]. However, following the increasing implementation of NGS analysis in Korean cancer patients, future large-scaled studies based on NGS are expected. Second, HER2-targeted used in this study have not shown any meaningful clinical outcomes. This is probably due to the small number of patients with limited treatment options. Moreover, HER2-targeted agents were used in the heavily-treated patients who were devoid of available therapeutic options. In this regard, our study population had a higher rate of brain metastasis than those reported in the previous studies [6,8].
In conclusion, given that no targeted treatments are currently approved for HER2-mutant lung cancer, the clinical outcomes of these patients remain poor. Comprehensive genomic profiling at the time of diagnosis is essential to discover HER2 mutant NSCLC patients who may derive more clinical benefit if they were treated at an earlier point of the disease course. Currently, several targeted drugs have shown promising efficacy and many clinical trials for HER2-altered NSCLC patients are ongoing. The optimal management of HER2-altered NSCLC, requires further well designed, high-quality trials and the exploration of new treatment strategies such as combination of ADC with TKI, ICIs, or chemotherapies.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This retrospective study was approved by an Institutional Review Board (approval number 2021-0921-001) of Severance Hospital. The requirement for informed consent was waived owing to the retrospective nature of the study. Fig. 1Overall landscape of human epidermal growth factor receptor 2 (HER2) mutation patients detected by next-generation sequencing (n=44). 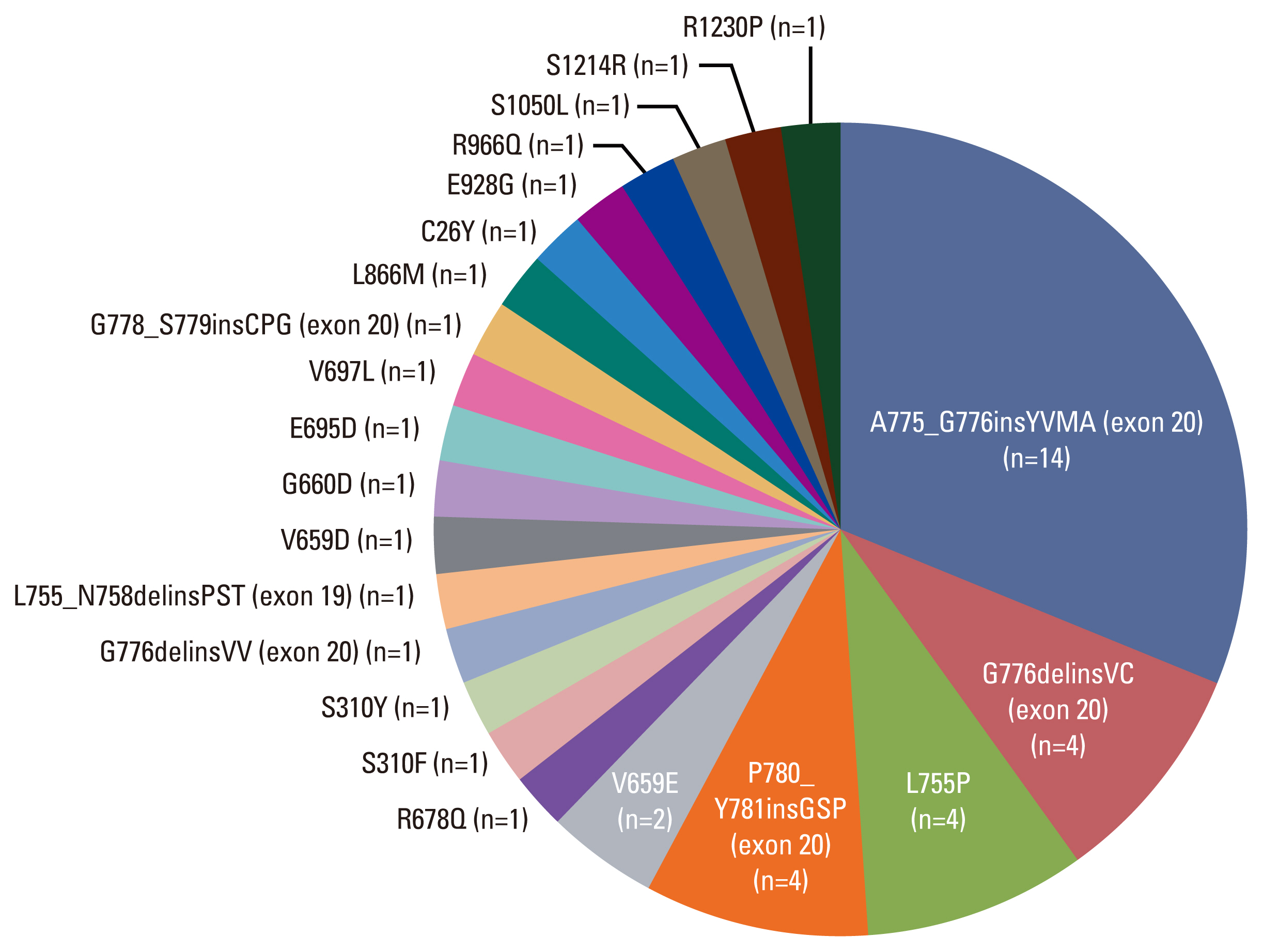
Fig. 2Kaplan-Meier plot of the median overall survival (mOS) analysis. (A) Overall survival for the entire study population calculated from the date of relapse or metastasis to the last follow-up date. Overall survival of patients stratified by presence of exon 20 mutation (B), treated with pemetrexed-based cytotoxic chemotherapy (C), and human epidermal growth factor receptor 2 (HER2) targeted therapy (Tx) (D). CI, confidence interval; mut, mutation. 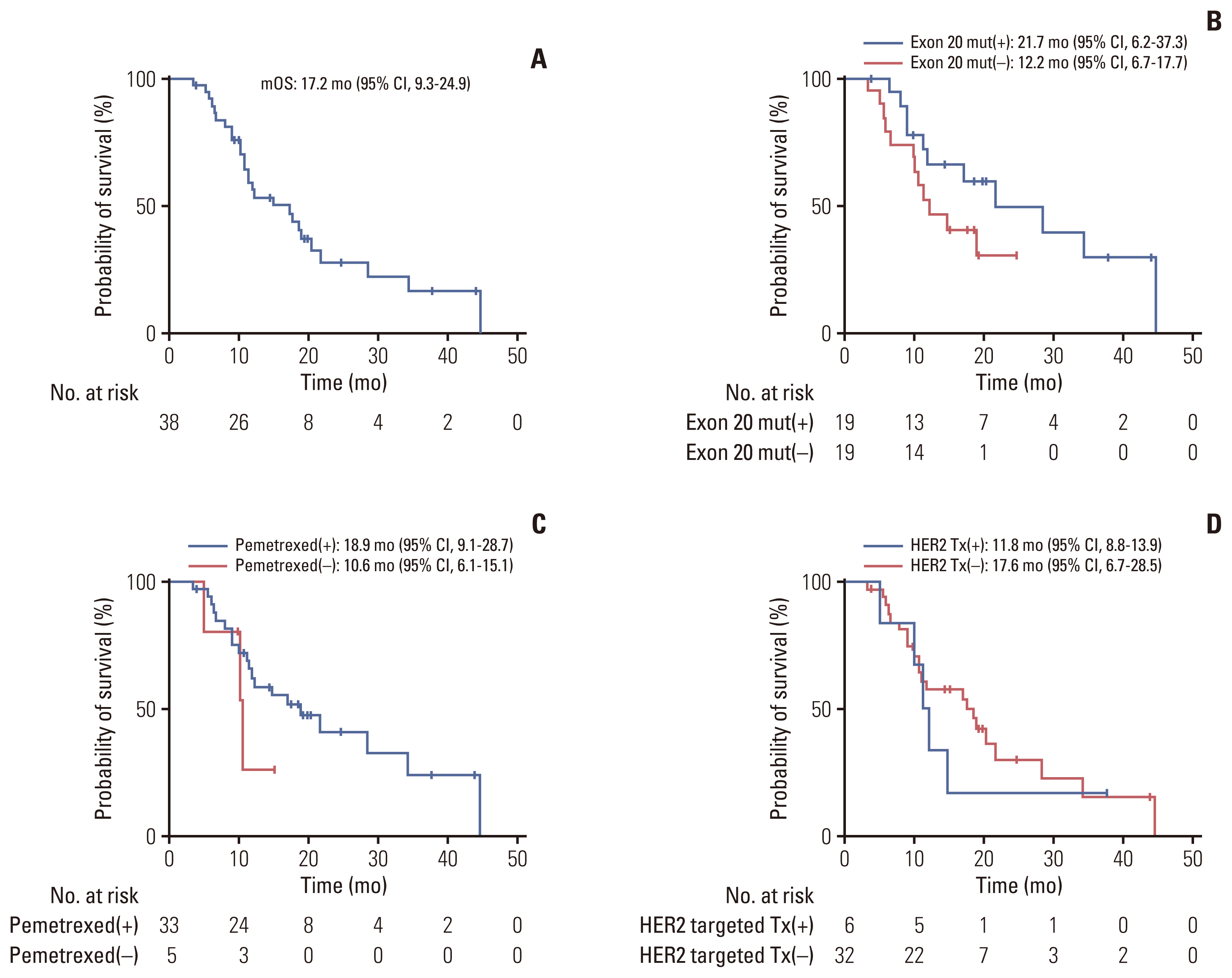
Fig. 3Kaplan-Meier plot for the median overall survival (mOS) and median progression-free survival (mPFS) from the beginning of treatment. (A) mOS of patients treated with pemetrexed-based cytotoxic chemotherapy. (B) mPFS with pemetrexed-based cytotoxic chemotherapy. (C) mOS with human epidermal growth factor receptor 2 (HER2) targeted therapy. (D) mPFS with with HER2 targeted therapy. CI, confidence interval; Tx, therapy. 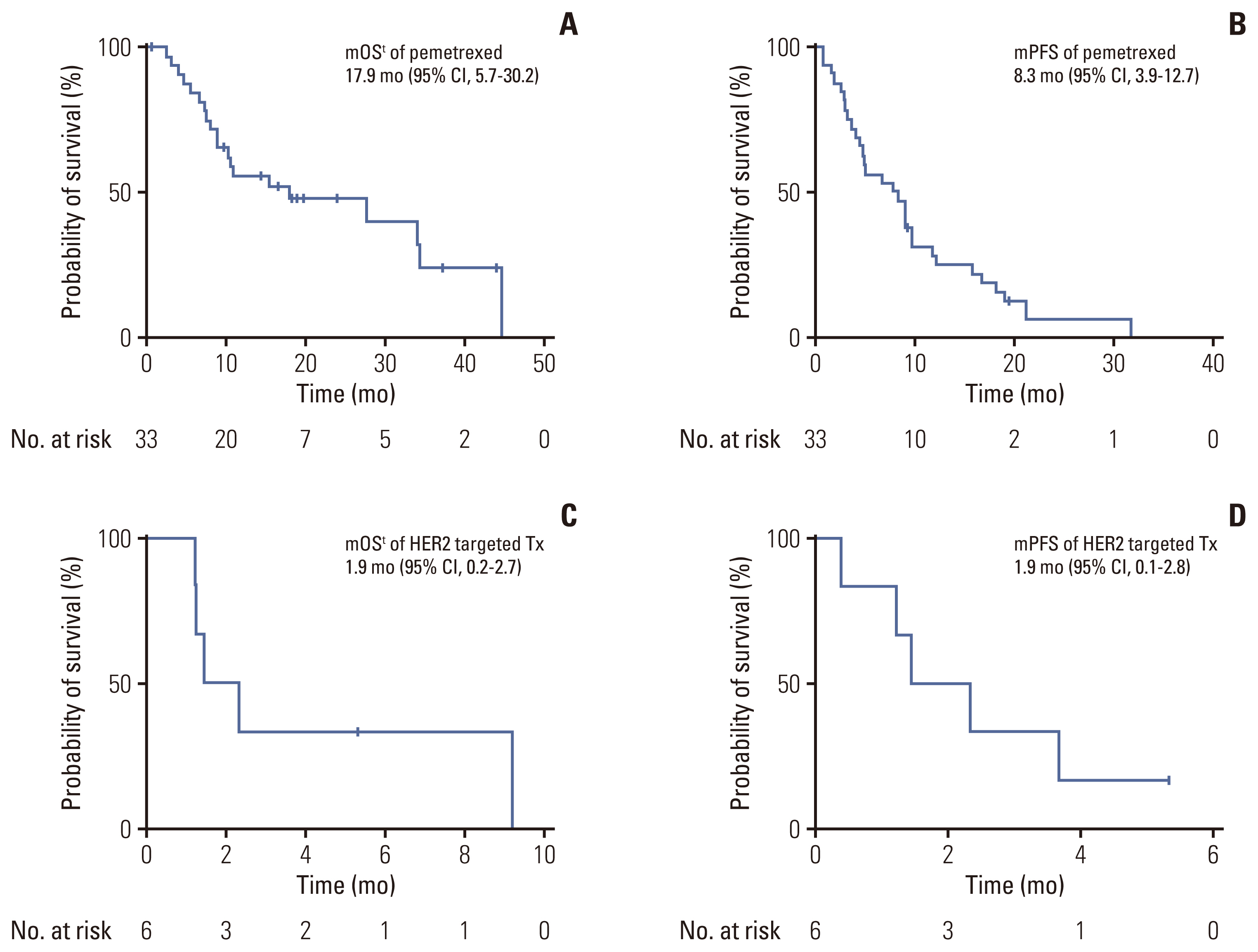
Fig. 4(A) Number of brain metastases when the cancer was first diagnosed. (B) Number of brain metastases that developed during the palliative treatment. (C) Kaplan-Meier plot for the time to brain metastases for patients without initial brain lesions (n=10). The median time to progression was 15.6 months (95% confidence interval [CI], 1.5 to 29.7). 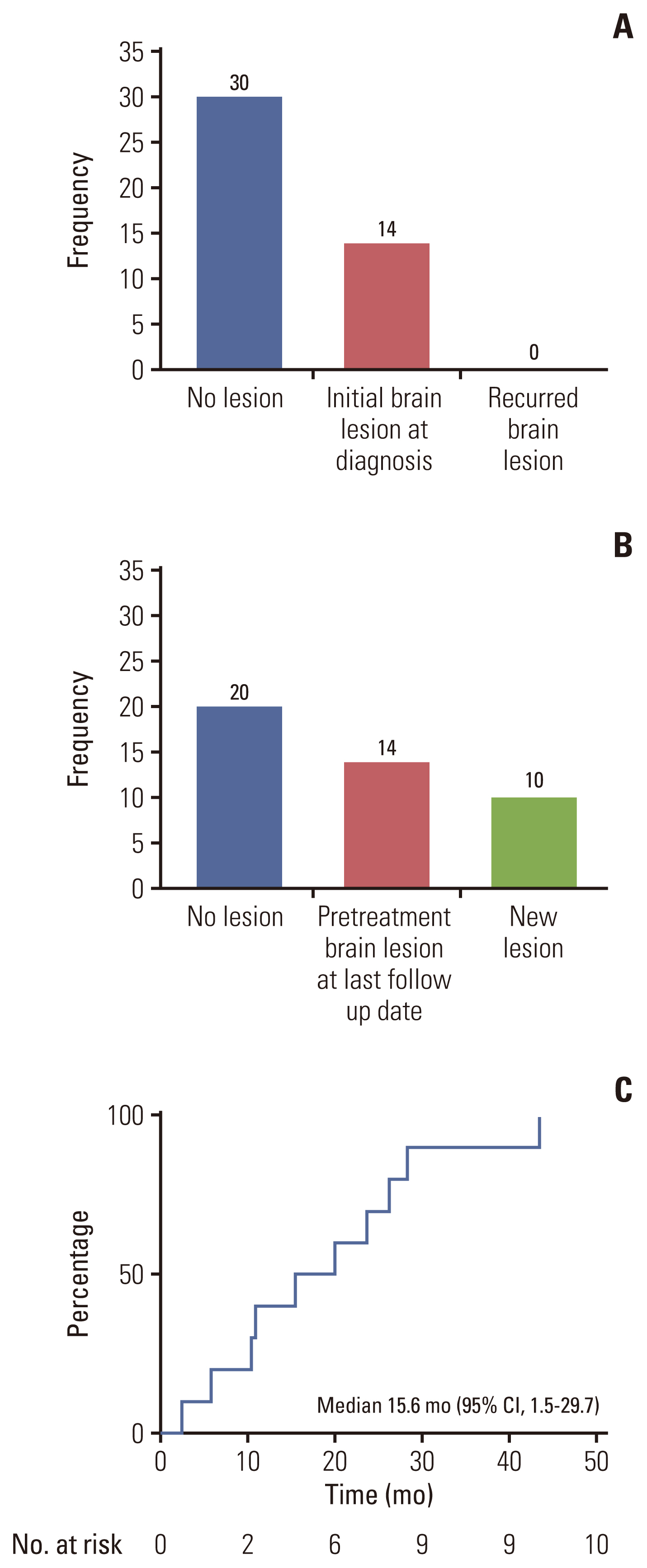
Table 1Baseline clinical characteristics BRAF, proto-oncogene B-Raf and v-Raf murine sarcoma viral oncogene homolog B; CCRT, concomitant chemotherapy and radiotherapy; CTx, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; LN, lymph node; NGS, next-generation sequencing. Table 2Overall response based on treatment CI, confidence interval; CTx, chemotherapy; DCR, disease control rate; DOT, duration of treatment; HER2, human epidermal growth factor receptor 2; ICI, immune checkpoint inhibitor; LN, lymph node; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; Tx, treatment. References1. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46.
2. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77.
3. Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–71.
4. Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O’Connell J, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amp-lified tumors. Ann Oncol. 2015;26:1421–7.
5. Li BT, Michelini F, Misale S, Cocco E, Baldino L, Cai Y, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 2020;10:674–87.
6. Patil T, Mushtaq R, Marsh S, Azelby C, Pujara M, Davies KD, et al. Clinicopathologic characteristics, treatment outcomes, and acquired resistance patterns of atypical EGFR mutations and HER2 alterations in stage IV non-small-cell lung cancer. Clin Lung Cancer. 2020;21:e191–204.
7. Zhou J, Ding N, Xu X, Zhang Y, Ye M, Li C, et al. Clinical outcomes of patients with HER2-mutant advanced lung cancer: chemotherapies versus HER2-directed therapies. Ther Adv Med Oncol. 2020;12:1758835920936090.
8. Mazieres J, Barlesi F, Filleron T, Besse B, Monnet I, Beau-Faller M, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016;27:281–6.
9. Odegaard JI, Vincent JJ, Mortimer S, Vowles JV, Ulrich BC, Banks KC, et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res. 2018;24:3539–49.
10. Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23.
11. Han Y, Lim SM, Kim HR, Hong MH, Cho BC, Ahn B. P50.09 Characteristics and clinical outcomes of HER2 mutated non-small cell lung cancer patients detected by NGS in routine clinical practice. J Thorac Oncol. 2021;16(10 Suppl):S1117–8.
12. Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997–2003.
13. Lee K, Jung HA, Sun JM, Lee SH, Ahn JS, Park K, et al. Clinical characteristics and outcomes of non-small cell lung cancer patients with HER2 alterations in Korea. Cancer Res Treat. 2020;52:292–300.
14. Oh IJ, Hur JY, Park CK, Kim YC, Kim SJ, Lee MK, et al. Clinical activity of Pan-HER inhibitors against HER2-mutant lung adenocarcinoma. Clin Lung Cancer. 2018;19:e775–81.
15. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. 2018;36:536–42.
16. Rolfo C, Russo A. HER2 mutations in non-small cell lung cancer: a herculean effort to hit the target. Cancer Discov. 2020;10:643–5.
17. Suzawa K, Toyooka S, Sakaguchi M, Morita M, Yamamoto H, Tomida S, et al. Antitumor effect of afatinib, as a human epidermal growth factor receptor 2-targeted therapy, in lung cancers harboring HER2 oncogene alterations. Cancer Sci. 2016;107:45–52.
18. De Greve J, Teugels E, Geers C, Decoster L, Galdermans D, De Mey J, et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer. 2012;76:123–7.
19. Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367–77.
20. Li B, Gandhi L, Besse B, Jhaveri K, Mazieres J, Boni V, et al. FP14.15 Neratinib-based combination therapy in HER2- mutant lung adenocarcinomas: findings from two internatio-nal phase 2 studies. J Thorac Oncol. 2021;16(3 Suppl):S234.
21. Peters S, Stahel R, Bubendorf L, Bonomi P, Villegas A, Kowalski DM, et al. Trastuzumab emtansine (T-DM1) in patients with previously treated HER2-overexpressing metastatic non-small cell lung cancer: efficacy, safety, and biomarkers. Clin Cancer Res. 2019;25:64–72.
22. Tsurutani J, Iwata H, Krop I, Janne PA, Doi T, Takahashi S, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10:688–701.
23. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazieres J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386:241–51.
24. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–8.
|
|
|||||||||||||||||||||||||||||||||||||||||||