AbstractPurposeEribulin is approved for advanced breast cancers refractory to anthracyclines and taxanes. Efficacy according to sensitivity to previous therapies has been poorly explored.
Materials and MethodsSafety data were collected prospectively and we retrospectively collected efficacy data from the five French centres that participated in the Eribulin E7389-G000-398 expanded access program. Our main objectives were exploration of safety and analysis of eribulin efficacy (progression-free survival [PFS] and overall survival [OS]) according to sensitivity to the last microtubule-inhibiting agent administered.
ResultsMedian eribulin treatment duration was 3.3 months for the 250 patients included in this prospective single-arm study. Two hundreds and thirty-nine patients (95.6%) experienced an adverse event (AE) related to treatment including 129 (51.6%) with grade ≥ 3 AEs. The most frequently observed toxicities were cytopenias (59.6% of included patients), gastro-intestinal disorders (59.2%), and asthenia (56.4%). The most frequent grade 3-4 AE was neutropenia (37.2% with 4.8% febrile neutropenia). Median PFS and OS were 4.6 and 11.8 months, respectively. Patients classified as responders to the last microtubule-inhibiting therapy had a longer OS (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.51 to 0.94; p=0.017), and tended to display a better PFS (HR, 0.78; 95% CI, 0.58 to 1.04; p=0.086). OS improvement was still significant in multivariate analysis (adjusted HR, 0.53; 95% CI, 0.35 to 0.79; p=0.002).
IntroductionBreast cancer remains the most frequent female cancer worldwide [1]. Even though most of early breast cancers can be cured, metastatic disease is not suitable for curative treatments [2]. However, therapies dedicated to metastatic breast cancer (MBC) have been dramatically improved in the last decade with the approval of human epidermal growth factor receptor 2 (HER2) inhibitors [3-6], mammalian target of rapamycin inhibitors [7], CDK4/6 inhibitors [8-10], and new chemotherapy agents [11]. One of these molecules, eribulin mesylate (EM) is approved by the Food and Drug Administration (FDA), the European Medicine Agency (EMA), and Asian regulatory authorities [12] since the results of a randomized phase III trial showing that EM improved overall survival (OS) versus treatments of physician choice after anthracyclines and taxane failure [13]. Another trial comparing EM to capecitabine in patients previously treated with taxanes and anthracyclines failed to show superiority, even though a trend in better progression-free survival (PFS) was suggested in triple-negative (TN) and HER2-negative subgroups [14]. Pooled analysis of these two trials, including 1,644 patients with 946 treated by EM, has recently been published and shows that OS (hazard ratio [HR], 0.85), PFS (HR, 0.87), and clinical benefit rate (30% vs. 27%), were all significantly improved with EM [15]. These results were similar to data from phase II trials [16-18], as prospective and retrospective cohorts [19,20].
EM is a synthetic analogue of the marine natural product halichondrin B that inhibits microtubule dynamics. Its mechanism of action is different from other microtubules inhibitors such as taxane and vinca alkaloids [21]. EM inhibits microtubule polymerisation without affecting depolymerisation, resulting in non-productive aggregates, leading to an irreversible mitotic block at the G2-M phase, resulting in cancer cell apoptosis [22]. Little is known about EM efficacy according to previous response to microtubules inhibiting agents. One can argue that tumours that have developed resistance to taxane and/or vinca alkaloids may be less sensitive to EM. Only few data are available to confirm or invalidate this hypothesis. It has been described that EM did not improve survival (median OS, 12.8 vs. 11.3 months; HR, 0.91; 95% confidence interval [CI], 0.78 to 1.06) for patients defined as refractory to taxane (progression within 60 days after their last taxane dose), whereas there was a 3.0 months difference in favour of EM in patients not refractory to taxane (median OS, 17.4 months vs. 14.4 months and HR, 0.81; 95% CI, 0.69 to 0.94) [23]. Similar results were found in a retrospective multicentre study with time to progression improvement for patients who achieved a clinical benefit with previous taxane-based regimen (HR, 1.50; 95% CI, 1.07 to 2.11) [19]. However, these results do not take into account the delay between taxane discontinuation and EM initiation, as well as vinorelbine use and microtubules inhibiting agents’ efficacy just before EM treatment.
Expanded access programs are usually initiated by a drug company when it becomes clear from previous prospective trials that a treatment can be given safely and that a clinical benefit may be derived from it, while no alternative therapy is available. In addition to providing drug access to patients before official registration, it allows collecting efficacy and safety data, from a perspective closer to real-world patients, whereas those included in clinical trials are a more selected subset, less frequently representative from patients treated in routine practice.
We proposed to analyze the patients included in the French prospective expanded access program to confirm EM safety and to analyse efficacy data according to sensitivity to the last microtubule-targeting agent received (docetaxel, paclitaxel, or vinorelbine).
Materials and Methods1. Study designThe E7389-G000-398 study (ClinicalTrials.gov Identifier: NCT01240421) was conducted as an open-label, multicentre, single-arm trial with EM for the treatment of heavily pretreated advanced breast cancer. Its primary objectives were to provide EM to patients with MBC who had no other treatment options in order to evaluate the safety profile of EM. The completion of this study occurred in the five French sites when EM was officially approved and available, i.e., in 2012. The sponsor collected safety data prospectively. We will present here these data as well as a retrospective analysis of efficacy data.
2. PatientsKey inclusion criteria for the E7389-G000-398 expanded access program were female gender, aged 18 years or older, prior treatment with anthracyclines, taxane, and capecitabine; prior treatment with trastuzumab for patients with HER2-positive MBC; Eastern Cooperative Oncology Group performance status ≤ 2; adequate hematological, liver and renal functions. Exclusions criteria were uncontrolled meningeal carcinomatosis and/or brain or subdural metastases; preexisting neuropathy of grade > 2; history of congestive heart failure, unstable angina, myocardial infarction within the past 6 months and serious cardiac arrhythmia.
3. Study and evaluation proceduresTreatment scheme, dose reductions, and safety assessments were done according to protocol recommendations. EM was administered at a dose of 1.4 mg/m2 as a 2- to 5-minute intravenous bolus on days 1 and 8 of a 21-day cycle. Two dose reductions were allowed (0.97 and 0.62 mg/m2) and were done in accordance to the EMA recommendations. Ancillary treatments were given as medically indicated. Radiotherapy was not allowed except for palliative treatment for bone metastases.
Safety assessments consisted of recording all adverse events (AEs) (according to National Cancer Institute Common Terminology Criteria for Adverse Events ver. 4.0) and serious AEs, monitoring hematology and blood chemistry, periodic measurement of vital signs, and physical examinations. The safety population was defined as the group of subjects who received at least a partial dose of EM.
Efficacy assessments were performed according to the site's standard of care. In France, efficacy assessments were performed every two cycles or if disease progression was suspected. These assessments could have involved (computed tomography, magnetic resonance imaging, and/or metabolic assessments [Tc99m-bone scan or flurodeoxyglucose positron emission tomography]). The same modalities of assessment that were used as baseline were used for the response evaluation. Tumour response was assessed using Response Evaluation Criteria In Solid Tumor (RECIST) 1.1 [24] in case of morphological assessment and Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) [25] in case of metabolic assessment.
4. Analysis of efficacy dataThe efficacy population was defined as the group of subjects for whom at least one radiological assessment was available, even if they did not complete one cycle of treatment. Best response rate and its 95% confidence limits were calculated, in all patients and in different subgroups. Complete (CR) and partial responses (PR), as well as stable disease (SD) and progressive disease were defined according to RECIST 1.1 or PERCIST criteria. Patients were described to have SD as best response if SD was confirmed more than 3 months after treatment initiation. Overall response rate (ORR) was defined as the sum of CR and PR. Disease control rate (DCR) was defined as the sum of ORR and SD.
The efficacy analysis case report form included patient and tumour characteristics, previous anti-cancer treatment history with a special focus on the last microtubule-inhibitor treatment (best response and PFS). Only efficacy data related to taxane-based regimen used in the metastatic setting were used. Patients were classified as “responder” to the last microtubules-inhibiting agent if they presented an objective response or a SD for more than 3 months with the last microtubules inhibitors administered. If they had a disease progression as best response or if they had disease stabilization for a maximum of 3 months, they were classified as "non-responder."
As response to the last microtubule-inhibiting treatment could just be a surrogate of chemo-sensitivity and not a specific marker of microtubules inhibitors efficacy, and since nearly all our patients had received capecitabine before EM introduction, we also explored the relationship between prior response to capecitabine and EM activity. Patients were classified as responders or non-responders to capecitabine using the same definition as used for characterizing sensitivity to the last microtubule-inhibiting therapy.
5. Statistical analysisThe number of subjects to be included in this expanded access program was undefined. Per protocol, no formal statistical analysis was required except simple summaries of AEs and serious AEs data (defined according to Common Terminology Criteria for Adverse Events ver. 4.0 classification). Descriptive statistics were used to summarise the frequency, severity, duration, and relationship to treatment for all AEs occurring after the initiation of treatment. Only AEs related or probably related to treatment will be described below. Categorical variables were described using counts and frequencies, and quantitative variables were described using medians and ranges. Patients’ characteristics were compared according to their sensitivity to the latest previous microtubule-inhibiting therapy using chi-square or Fisher exact tests for qualitative variables and rank-Wilcoxon’s tests for quantitative variables. Hazard ratios are provided with their bilateral confidence interval and Wald’s test p-value for significance. Follow-ups were estimated using the inverse Kaplan-Meier method. OS was defined as the time from inclusion to death or last follow-up. PFS was defined as the time from inclusion to progression or death, whatever occurred first. Patients lost to follow-up or without event were censored at the date of last news. Survival curves were estimated using the Kaplan-Meier method, and the median OS and PFS were calculated with their 95% CIs. Both univariate and multivariate analyzes were conducted using Cox’s proportional hazard regression models including age (≤ 35 years vs. > 35 years), hormone receptor status, HER2 status, TN phenotype, presence of visceral metastases, and response to the last microtubule inhibitor treatment (responder vs. non-responder) as categorical explanatory variables. The level of statistical significance was set at α=0.05. Statistical analyses were carried out with the SAS software ver. 9.4 (SAS Institute Inc., Cary, NC). This ancillary study was performed according to the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) criteria (see S1 Table) [26].
6. Ethical statementWritten informed consent was obtained from all patients before inclusion in the expanded access program, and all procedures were done in accordance with Good Clinical Practice standards, after approval of the responsible ethics committee (AFSSAPS approval A100578-21), and the 2008 Helsinki Declaration. Retrospective efficacy analyses were performed as an ancillary analysis of the initial protocol after approval from regulatory agencies (CNIL approval DR-2015-346, CCTIRS approval 14.576bis). A dedicated written informed consent from living patients at time of retrospective data collection.
Results1. Population descriptionTwo hundred and fifty patients were included from October 2009 to November 2012 (Table 1, Fig. 1). When focusing on the latest previous microtubule-inhibiting treatment received, we observed that docetaxel, paclitaxel and vinorelbine were given to 33 (13.6%), 94 (38.7%), and 116 (47.7%) patients, respectively.
2. Treatment administration and safetyMedian EM duration was 3.29 months (range, 0.03 to 27.48 months). Prospective collection of AEs showed that 239 patients (95.6%) experienced an AE related to treatment including 129 (51.6%) with grade ≥ 3 AEs and 33 (13.2%) with serious AEs (Table 2). The most frequently observed AEs of any grade related to treatment were cytopenias (59.6% of included patients), gastro-intestinal disorders (59.2%), asthenia (56.4%), and nervous system disorders (48.4%). Most frequent grade ≥ 3 AEs were neutropenia (37.2%), asthenia (8%), and peripheral neuropathy (4.8%). Even though neutropenia was frequent in this heavily pre-treated population, only 12 patients (4.8%) experienced grade 3-4 febrile neutropenia. Serious AEs led to EM dose reduction for six patients (2.4% of the entire population), treatment interruption for 10 patients (4%), and treatment discontinuation for three cases (1.2%). No death related to treatment was reported during study duration.
3. EfficacyMedian follow-up was 46.1 months for the whole population (range, 36.6 to 56.4). Median PFS and OS for the whole cohort were 4.6 months (95% CI, 4.2 to 5.7) and 11.8 months (95% CI, 10.7 to 13.2), respectively (Fig. 2). ORR was 17.8%, 64 patients (35.6%) had disease stabilization, and 84 (46.7%) had disease progression or non-confirmed SD as best response (Table 3). DCR was 53.4%.
4. Analysis according to response to the latest previous microtubule-inhibiting therapy receivedConsidering the definition detailed in the methods section, more than half (n=108, 52.4%) of the cases were classified as responders, versus 98 non-responders (47.6%) to the last microtubule-inhibiting therapy. There was no statistically significant imbalance between these two groups concerning usual clinicopathological features (Table 4). Treatments were slightly different between the two groups in the metastatic setting. More patients in the non-responders group had received taxanes whatever the treatment line (90.8% vs. 70.6%, p < 0.001), and less had received prior endocrine therapy (68.4% vs. 81.5%, p=0.029). More cases classified as responders received taxanes as last microtubules inhibiting therapy (p=0.010).
Median follow-up was similar between the two groups (46.2 months for responders vs. 38.5 months for non-responders, p=0.55). DCR was higher for the responders group (61.2% vs. 43.9%, p=0.020) (Table 3). Univariate analyses showed that a TN phenotype (HR, 1.58; 95% CI, 1.02 to 2.43; p=0.038) was the only parameter significantly associated to PFS (Table 5). Response to the last microtubule-targeting agent tended to be correlated to PFS (HR, 0.78; 95% CI, 0.58 to 1.04; p=0.086) (Fig. 3A). Four parameters were significantly associated with OS: hormone receptor-positivity (HR, 0.65; 95% CI, 0.44 to 0.97; p=0.036), a TN phenotype (HR, 1.89; 95% CI, 1.20 to 2.97; p=0.006), presence of visceral metastases (HR, 1.42; 95% CI, 1.04 to 1.94; p=0.028), and response to the last microtubule-targeting treatment (HR, 0.69; 95% CI, 0.51 to 0.94; p=0.017) (Table 6, Fig. 3B). Median OS was 10.9 months for non-responders versus 12.3 months for responders. Multivariate analysis of OS showed that only a TN phenotype (adjusted HR, 2.71; 95% CI, 1.51 to 4.86; p < 0.001) and response to the latest previous microtubule-inhibitor (adjusted HR, 0.53; 95% CI, 0.35 to 0.79; p=0.002) were independent prognostic features (Table 6). OS Cox regression multivariate analysis assessing the interaction between response to prior microtubule-inhibiting therapy and immunohistochemical subtypes showed that response to the last microtubule inhibitor received was prognostic for hormone receptor+/HER2- (n=98; HR, 0.62; 95% CI, 0.40 to 0.97; p=0.036) and TN cases (n=18; HR, 0.15; 95% CI, 0.05 to 0.48; p=0.001), but not for the few HER2+ tumours (n=8; HR, 0.94; 95% CI, 0.15 to 5.47; p=0.945).
Capecitabine efficacy data were available for 157 patients. Out of them 54 (34.4%) were classified as non-responders and 103 (65.6%) as responders to capecitabine. Median OS after EM initiation was not significantly different between these two groups (11.1 months; 95% CI, 8.64 to 13.37) for responders versus 11.9 months (95% CI, 9.56 to 17.38) for non-responders (p=0.24).
DiscussionWe present here the results from the French centres involved in the prospective expanded access program of EM as treatment of refractory MBC. We show that efficacy and safety profiles of the largest prospective “real-world” cohort ever published are consistent with data from pivotal trials. Moreover, we suggest for the first time that sensitivity to last microtubule-targeting agents received can have an impact on EM efficacy.
Prospective safety analysis of 250 heavily pre-treated MBC patients showed that the most frequent AE of any grade was asthenia (56.4%), and that the most frequent high grade AE was neutropenia (37.2%). The pooled analysis of the EMBRACE and 301 trials and another “real-world” Belgian prospective cohort showed similar results (45.5% and 73.8% for asthenia of any grade; 35.7% and 37.2% for grade 3-4 neutropenia) [15,20]. Even though this hematological toxicity is frequent under EM, febrile neutropenia is much less common in all published prospective cohorts, with a 3.4% to 9.2% rate. Our safety results are thus consistent with previously published prospective studies. Survival data (for both PFS and OS) are also in accordance to published data [13,14,17,20].
Concerning EM efficacy, median OS was slightly shorter in our cohort than for the eribulin arm of the EMBRACE trial (11.8 months vs. 13.1 months). This can be explained by the lower rate of HER2-positive tumours (7.3% vs. 16%) and higher rate of capecitabine pre-treated patients (93.9% vs. 73%) in our cohort. Moreover, only 20% of patients had received more than four lines of treatment before inclusion in the EMBRACE trial versus 37% in our set. This could have allowed administration of more treatment lines after eribulin failure, leading to OS prolongation. It is worth noting that details concerning post-progression therapies were available neither for our cohort nor for the EMBRACE trial. The key point of our work is that patients classified as responder to the last microtubules inhibiting agents display higher rates of disease control (61.2% vs. 43.9%), a 31% reduction of death risk, and tend to have a longer PFS (HR, 0.78; 95% CI, 0.58 to 1.04). The significant overall survival gain associated to the responder status was confirmed to be independent from other clinicopathological features in multivariate analysis, including TN status. In the EMBRACE study, even though there was no imbalance according to taxane resistance with more than 80% of taxane refractory patients in both study arms (as defined as a progression on or within 6 months of receiving treatment), efficacy data were not available according to taxane sensitivity. The pooled analysis of phase III studies showed that OS was not improved with EM (HR, 0.91; 95% CI, 0.78 to 1.06) for refractory patients, whereas patients not refractory to taxanes had a 3.0 months absolute gain (HR, 0.81; 95% CI, 0.69 to 0.94) [23]. The retrospective ERIBEX study showed similar results with time to progression [19]. Analysis of the Belgian expanded access program showed no difference between patients who responded to previous vinorelbine treatment (13% vs. 15%) and did not specified data related to the response to the last microtubuleinhibiting agent used. No data correlated to previous taxanes-based therapy was available in the E-301 study. An important result of our work is that we do not observe such positive results when looking at the relationship between capecitabine efficacy and EM activity. Overall survival under EM was similar between patients classified as responders to capecitabine and patients classified as non-responders (median OS of 11.1 vs. 11.9 months, p=0.24), suggesting that, contrary to what we observed for microtubules inhibiting treatment, sensitivity to capecitabine was not a predictive marker of survival after EM initiation. Our results suggest that EM efficacy may be correlated to sensitivity to therapies targeting microtubules and not cytotoxic treatments taken as a whole. Efficacy of the previous line of chemotherapy administered is not the only feature modulating EM activity [27]. The mechanism of action of the previous cytotoxic agents may also have an impact on this activity, suggesting that microtubule should be viewed as a specific biological target, and that there may be a cross-resistance between microtubule-inhibiting agents. However, this was not observed with vinorelbine for taxanes refractory patients. In a prospective single-arm study, some patients refractory to taxanes had been described to be able to respond to vinorelbine [28]. On a more general perspective, our data are in favour of considering cytotoxic chemotherapies as authentic targeted therapies, with specific determinants of sensitivity which remain to be identified in order to spare patients from inactive and potentially toxic treatment.
This descriptive analysis presents some limits. First, despite that the expanded access program was a prospective study; the current efficacy analysis is based on retrospective data possibly leading to data collection bias. For example, we could not collect data concerning therapies received after progression under eribulin, which would have been of interest to explain why there was only a trend for PFS improvement for the responder subset but a significant OS gain. Second, to confirm the fact that EM efficacy could be linked to previous sensitivity to taxanes and vinca alkaloids and not just with a less bad prognosis, efficacy of other cytotoxic or cytostatic agents than capecitabine should be analyzed. Nevertheless, this correlation is very unlikely to be performed in the next years because of current EM labels for MBC. Third, the limited sample size precluded to evaluate a putative differential prognostic impact of taxanes versus vinorelbine sensitivity. Indeed, even though EM displays a unique mechanism of action, it binds to the same vinca binding domain on tubulin as vinorelbine and it could be hypothesized more similar determinants of sensitivity than with taxanes, which bind on a different site located on the inner surface of microtubules. The limited sample size may have also led to underestimation of response rate and PFS improvements for the responder subgroup. Although this program was not designed to formally assess eribulin activity after microtubules targeting agents, the data about survival are of interest. Published randomized trials indeed did not prospectively collect data related to response to prior treatments and post progression therapies; and the probability that such trial will be launched in the next years is quite weak. Our cohort containing more than 200 patients gives important insights for this refractory population, and possibly allow the identification of EM refractory cases, thereby decreasing costs and specific toxicities associated to this molecule [29].
In conclusion, in this national multicentre expanded access program enrolling 250 poorly selected refractory MBC patients, safety and efficacy are very similar to phase III trials. For the first time, we suggest that sensitivity to microtubule-targeting agents used for MBC just before eribulin introduction could be a surrogate marker of eribulin efficacy, and confirm previous cell line and clinical data hypothesizing that microtubule could be a specific therapeutic target. Further prospective data are required to confirm that response to previous microtubule inhibitors has a predictive value specific to eribulin efficacy.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Conflicts of InterestEisai was the sponsor of the E7389-G000-398 trial and thus provided the drug and covered the costs of the prospective safety data collection. Eisai also provided a grant to the Institut Paoli Calmettes in order to perform this ancillary analysis. This work was also funded by SIRIC (INCa-DGOSInserm 6038). AcknowledgmentsThe authors would like to thank the patients and their families for their participation in this expanded access program. We also are grateful to Dr Marie-Justine Paillard for her help concerning data collection for the patients from Besançon.
Fig. 1.CONSORT flow diagram. a)Safety data were prospectively collected, b)Efficacy data were retrospectively collected. 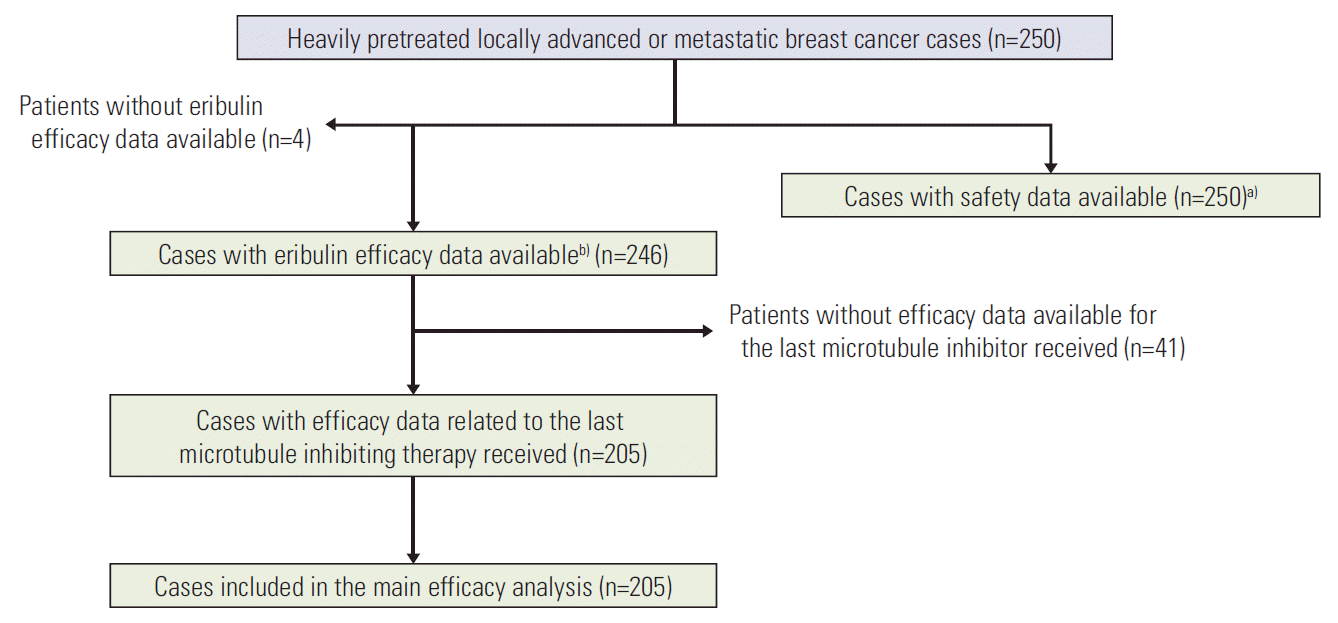
Fig. 2.Kaplan-Meier curves for the whole population (n=246). (A) Progression-free survival. (B) Overall survival. The efficacy population was defined as the group of subjects for whom at least one radiological assessment was available, even if they did not complete one cycle of treatment. One-year and 5-year progression-free survival were 14% and 0%, respectively. One-year overall survival was 48% and 5-year overall survival was 5%. 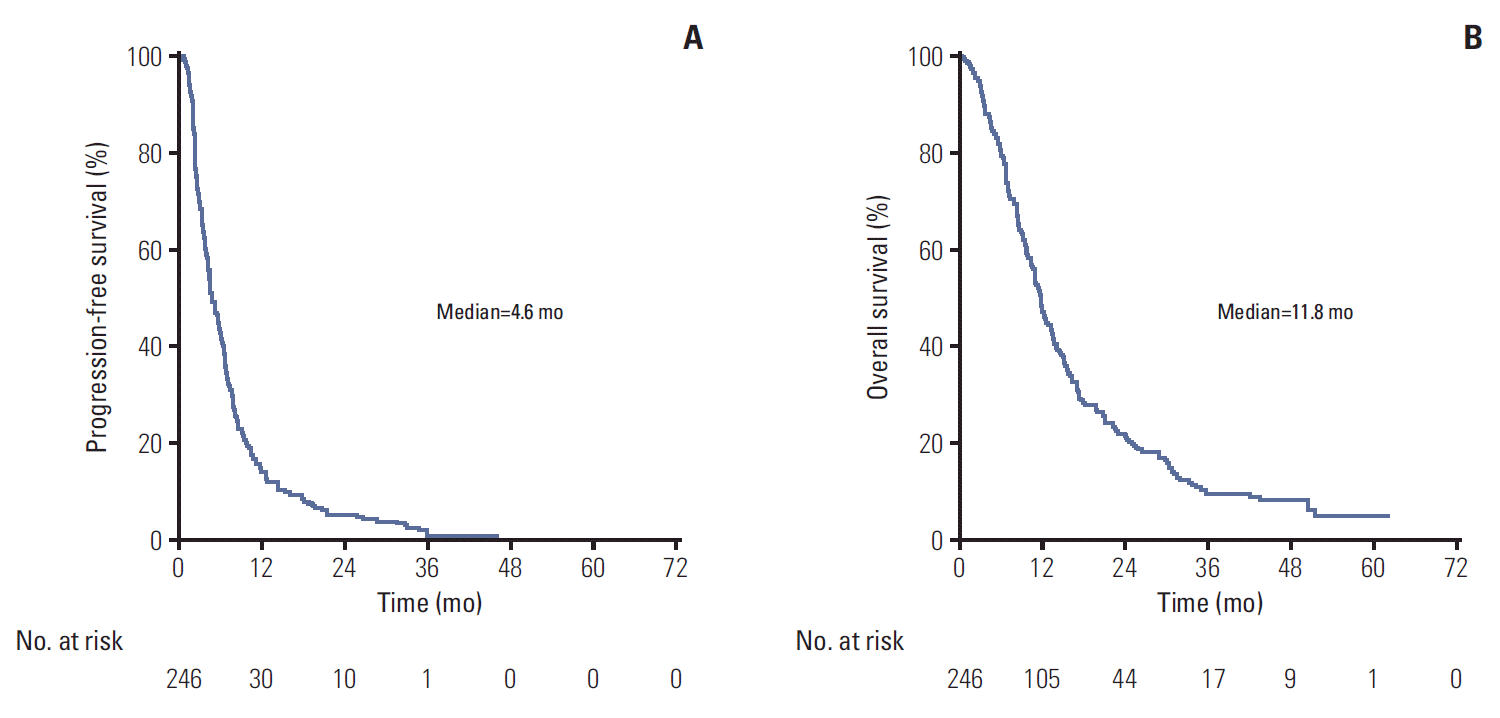
Fig. 3.Kaplan-Meier curves according to response to the last microtubule-inhibiting agent. (A) Progression-free survival. (B) Overall survival. The efficacy population was defined as the group of subjects for whom at least one radiological assessment was available, even if they did not complete one cycle of treatment. 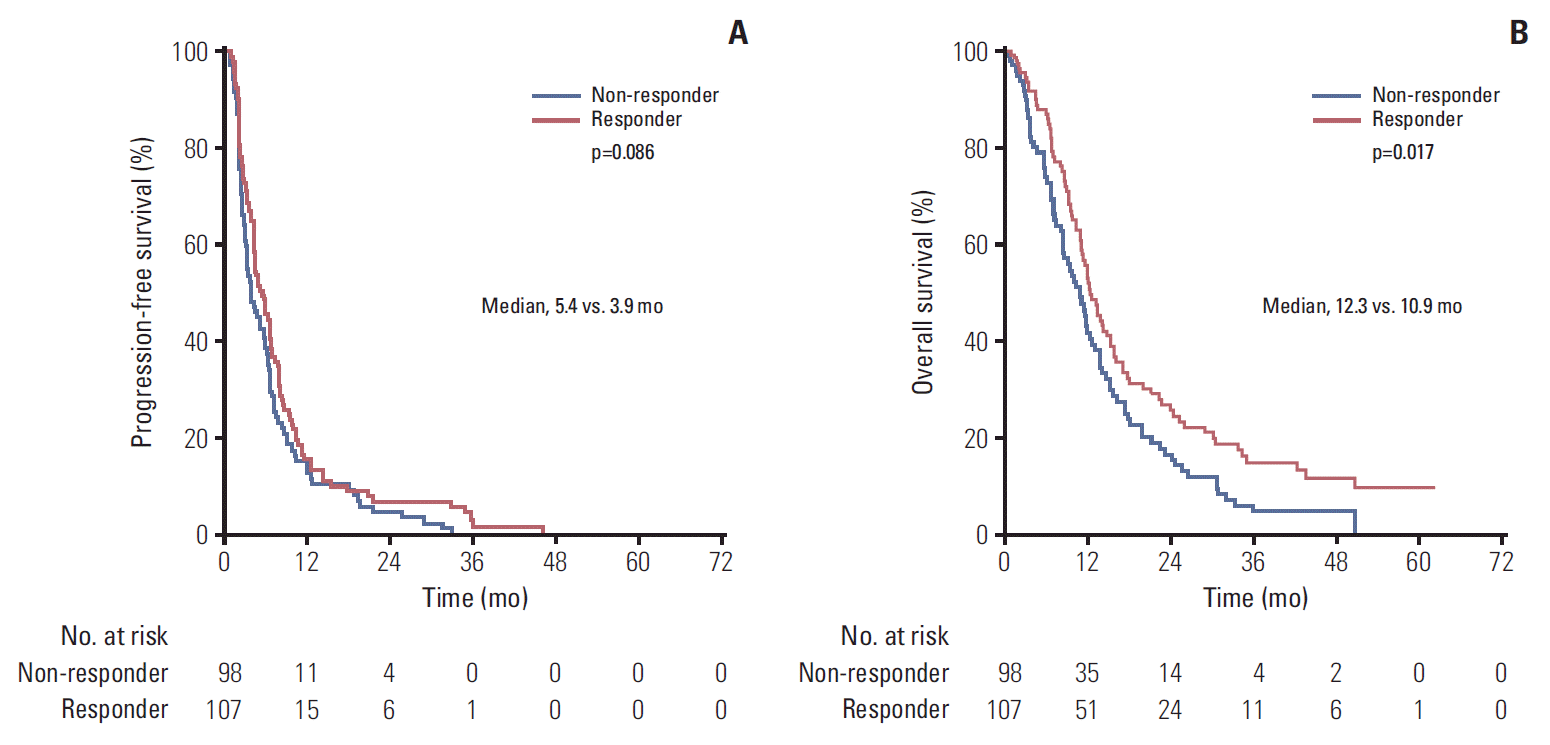
Table 1.Clinical and pathological features Table 2.Most common adverse events (Common Terminology Criteria for Adverse Events ver. 4.0) Table 3.Response to eribulin according to RECIST 1.1 criteria, with a dichotomization on sensitivity to the latest previous microtubule-targeting therapy Table 4.Comparison of clinicopathological features according to response to the latest previous microtubule-inhibiting therapy Table 5.Univariate Cox regression analysis of progression-free survival Table 6.Cox regression analysis of overall survival References1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
2. Cardoso F, Costa A, Senkus E, Aapro M, Andre F, Barrios CH, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol. 2017;28:3111.
3. Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19.
4. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43.
5. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
6. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
7. Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
8. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–39.
9. Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35.
10. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48.
11. Twelves C, Jove M, Gombos A, Awada A. Cytotoxic chemotherapy: still the mainstay of clinical practice for all subtypes metastatic breast cancer. Crit Rev Oncol Hematol. 2016;100:74–87.
12. Kok VC. Eribulin in the management of advanced breast cancer: implications of current research findings. Breast Cancer (Auckl). 2015;9:109–15.
13. Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–23.
14. Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33:594–601.
15. Pivot X, Marme F, Koenigsberg R, Guo M, Berrak E, Wolfer A. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann Oncol. 2016;27:1525–31.
16. Aogi K, Iwata H, Masuda N, Mukai H, Yoshida M, Rai Y, et al. A phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancer. Ann Oncol. 2012;23:1441–8.
17. Inoue K, Saito T, Okubo K, Kimizuka K, Yamada H, Sakurai T, et al. Phase II clinical study of eribulin monotherapy in Japanese patients with metastatic breast cancer who had well-defined taxane resistance. Breast Cancer Res Treat. 2016;157:295–305.
18. Vahdat LT, Pruitt B, Fabian CJ, Rivera RR, Smith DA, Tan-Chiu E, et al. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27:2954–61.
19. Dell'Ova M, De Maio E, Guiu S, Roca L, Dalenc F, Durigova A, et al. Tumour biology, metastatic sites and taxanes sensitivity as determinants of eribulin mesylate efficacy in breast cancer: results from the ERIBEX retrospective, international, multicenter study. BMC Cancer. 2015;15:659.
20. Aftimos P, Polastro L, Ameye L, Jungels C, Vakili J, Paesmans M, et al. Results of the Belgian expanded access program of eribulin in the treatment of metastatic breast cancer closely mirror those of the pivotal phase III trial. Eur J Cancer. 2016;60:117–24.
21. Jimeno A. Eribulin: rediscovering tubulin as an anticancer target. Clin Cancer Res. 2009;15:3903–5.
22. McBride A, Butler SK. Eribulin mesylate: a novel halichondrin B analogue for the treatment of metastatic breast cancer. Am J Health Syst Pharm. 2012;69:745–55.
23. Twelves C, Cortes J, Vahdat L, Olivo M, He Y, Kaufman PA, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014;148:553–61.
24. Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7.
25. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50S.
26. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med. 2007;45:247–51.
27. Dufresne A, Pivot X, Tournigand C, Facchini T, Altweegg T, Chaigneau L, et al. Impact of chemotherapy beyond the first line in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008;107:275–9.
|
|
|||||||||||||||||||||||||||||||||||||||||||||