INTRODUCTION
Metastasis to the female genital tract from a distant primary malignancy is rare, and especially for the genital track tumors that are hepatocellular carcinoma (HCC) (1~3). Furthermore, uterine metastasis of HCC is extremely rare. Since the first report of uterine metastasis involving HCC in 1941 (4), only one additional case has been described in 2006 (5). We report here on a case of uterine metastasis that originated from a primary HCC, and this metastasis did not involve the ovaries. This is the first report of metastatic HCC to the uterus with the absence of ovarian involvement.
CASE REPORT
A 78-year-old postmenopausal woman with a pelvic mass was admitted to Hwa-sun Chonnam University Hospital. On the manual examination, the uterus was enlarged, but no other discrete masses were noted. The CA125, CEA, CA72-4 and CA19-9 levels were within normal limits; however, the serum alpha-fetoprotein (AFP) level was elevated to >50,000 ng/ml (normal level <5.8 ng/ml).
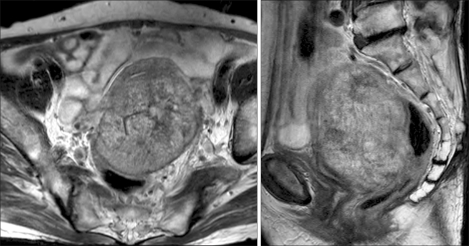
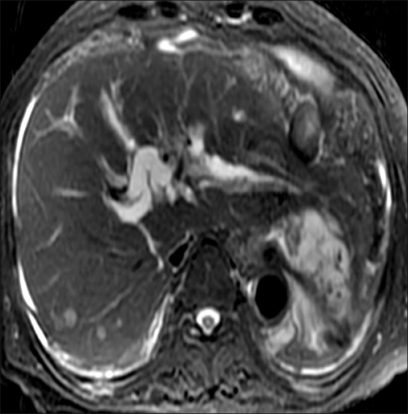
Magnetic resonance imaging (MRI) of the pelvis showed a huge uterine mass that measured 10×7×7 cm. This mass had heterogeneous signal intensity on the T2-weighted image; the mass extended from the myometrium to the subserosa. The mass did not appear to have invaded the sigmoid colon (Fig. 1). A MRI of the abdomen showed a solitary hepatic mass that measured 2×2 cm in the left lateral segment of the left liver lobe. This lesion showed high signal intensity with subtle enhancement on the T2-weighted image (Fig. 2).
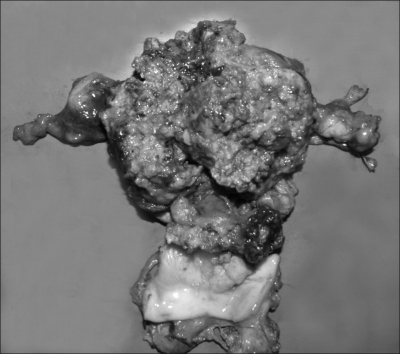
The patient underwent surgery under the tentative diagnosis of an AFP-producing uterine neoplasia with hepatic metastasis. The tumor originating from the uterus had in fact invaded the adjacent sigmoid colon. No other tumor was found in the abdominal cavity. Total abdominal hysterectomy, bilateral salpingo-oophorectomy and a Hartmann's operation were performed. The resected uterus was markedly distorted with a huge gray-whitish tumor mass. The cut surface of the uterus showed multiple subserosal nodules with accompanying hemorrhage and necrosis (Fig. 3).
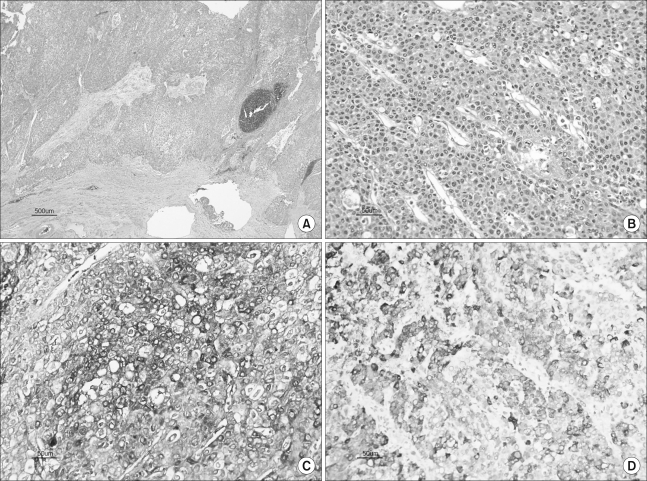
Histopathologic examination of the nodules in the subserosal area revealed large polygonal cancer cells that were separated by thin vessels, and this caused a trabecular arrangement (Fig. 4A, B). These cancer cells had infiltrated the myometrium and the cervix. In addition, there were multiple foci of lymphovascular invasion. The endometrium and both ovaries were free of tumor. Immunohistochemically, the cancer cells were strongly positive for AFP, hepatocyte-specific antigen and cytokeratin, but they were negative for vimentin and estrogen receptor (Fig. 4C, D). The resected sigmoid colon had a transmural tumor that measured 3×2 cm. The histopathologic findings of the colon tumor were similar to those of the uterine wall. Although a liver biopsy was not done, a diagnosis of metastatic HCC to the uterus and the sigmoid colon was confirmed.
The staging CT scan showed a 2.5 cm peripheral enhancing exophytic mass in the left lung base, and this was consistent with pulmonary metastasis. The patient and her family declined further treatment for the HCC. Repeat imaging showed progressive disease and the patient expired with disseminated HCC three months later.
DISCUSSION
HCC has a very aggressive clinical course, with a mean survival of less than 1 year if it is left untreated (6). Extrahepatic spread of HCC is uncommon because of the rapid clinical course. Extrahepatic metastases have recently been detected more frequently due to the improved diagnostic methods and prolonged survival.
There are three different routes for the spread of HCC: hematogenous spread, lymphatic embolization and direct extension to adjacent structures (7,8). The most frequent location for metastatic HCC is the lung. Hematogenous dissemination to the pulmonary capillary network is the presumed mechanism of spread. The other sites of hematogenous metastatic HCC include the bone, adrenal gland, heart, kidneys, pancreas and central nervous system. The regional lymph nodes are the second most common site of metastatic HCC following the lung. Direct infiltrating metastasis is relatively infrequent.
The uterus is an organ that's rarely affected by metastatic carcinoma from extragenital sites (1~3). The first case of metastatic involvement of the uterus from extragenital tumors was reported by Legg in 1878 in a patient who had malignant melanoma with widespread metastases (9). Since that time, there have been several reported series that involved metastatic carcinomas from extragenital sites to the uterus (2,3), including 60 cases reported by Charache in 1941 (4) and a review of 63 cases by Kumar and Hart in 1982 (1). In these series, metastatic neoplasia that involved the uterus primarily originated from the breast, stomach and colon. When metastasic spread to the uterus occurs, the myometrium is more often involved than the endometrium (1).
The ovaries are generally affected when metastasis to the female genital tract from a distant primary tumor occurs (1,2). The metabolic environmental factors of the ovarian stroma, such as the pH and oxygen tension, provide a more hospitable site for implantation and the development of metastases (1). Metastases to the uterus are rare, accounting for less than 10% of all cases of metastases to the female genital tract from extragenital cancers (2). Some authors have suggested that most uterine metastases may be secondary to local retrograde lymphatic spread from preceding ovarian metastases, while hematogenous spread occurs when the ovaries are not affected (3). In our case, metastatic involvement of the ovaries was not detected despite careful examination. We suggest that hematologic spread played a major role in this case because of the presence of uterine metastasis and the absence of ovarian involvement.
There are only two reported cases of uterine metastasis from HCC in the English literature (4,5). Charache (4) did not describe the clinical course and Ryo et al. (5) reported hematologic metastasis from the liver to the ovary, and this was followed by a lymphatic spread from the ovary to the uterus. We report here on a case of uterine metastasis that revealed a primary HCC, and it did not involve the ovaries. This is the first described report of metastatic HCC to the uterus with the absence of ovarian involvement.
Because the level of AFP was abnormally high in our case, the possible existence of an AFP-producing malignancy of the uterus was considered. A malignant mixed Mullerian tumor, hepatoid carcinoma, yolk sac tumor and uterine AFP-producing papillary serous carcinoma are the AFP-producing tumors that involve the uterus (10~13). Because AFP is a representative tumor marker of HCC, the liver should be examined when the AFG level is abnormally high in the serum.
Since the size of the uterine mass was much larger than the size of the hepatic mass, and with uterine metastasis from HCC being very rare, our case was misdiagnosed as a primary uterine mass. Uterine metastasis from HCC can be difficult to diagnosis because it can mimic a primary uterine carcinoma, and especially when the primary tumor is not clinically apparent. When the AFP level is abnormally high during the evaluation of uterine neoplasia, the clinician should consider the possibility of metastatic HCC, as well AFP-producing uterine neoplasia.