Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study
Article information
Abstract
Purpose
As a result of various independently proposed nomenclatures and classifications, there is confusion in the diagnosis and prediction of biological behavior of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). A comprehensive nationwide study is needed in order to understand the biological characteristics of GEP-NETs in Korea.
Materials and Methods
We collected 4,951 pathology reports from 29 hospitals in Korea between 2000 and 2009. Kaplan-Meier survival analysis was used to determine the prognostic significance of clinicopathological parameters.
Results
Although the GEP-NET is a relatively rare tumor in Korea, its incidence has increased during the last decade, with the most significant increase found in the rectum. The 10-year survival rate for well-differentiated endocrine tumor was 92.89%, in contrast to 85.74% in well differentiated neuroendocrine carcinoma and 34.59% in poorly differentiated neuroendocrine carcinoma. Disease related death was most common in the biliary tract (62.2%) and very rare in the rectum (5.2%). In Kaplan-Meier survival analysis, tumor location, histological classification, extent, size, mitosis, Ki-67 labeling index, synaptophysin expression, lymphovascular invasion, perineural invasion, and lymph node metastasis showed prognostic significance (p<0.05), however, chromogranin expression did not (p=0.148). The 2000 and 2010 World Health Organization (WHO) classification proposals were useful for prediction of the prognosis of GEP-NET.
Conclusion
The incidence of GEP-NET in Korea has shown a remarkable increase during the last decade, however, the distribution of tumors in the digestive system differs from that of western reports. Assessment of pathological parameters, including immunostaining, is crucial in understanding biological behavior of the tumor as well as predicting prognosis of patients with GEP-NET.
Introduction
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are a heterogeneous group of tumors having variable clinical findings and biological behavior according to the origin of neuroendocrine cell as well as anatomic site. The term "karcinoid" (carcinoma-like) was first described by Oberndorfer in 1907; since then, remarkable advances in understanding and management of GEP-NETs have been achieved [1-3]. However, as a result of a complicated site-specific grading, staging and classification system proposed by many investigators, there is confusion in the nomenclature for diagnosis and prediction of biological behavior of GEP-NETs [3].
In regard to the nomenclature, in 1980, the World Health Organization (WHO) proposed use of the term "carcinoid tumor" for most neuroendocrine tumors (NETs) of the gastrointestinal tract, except for small cell carcinoma and pancreatic islet cell tumor. However, the term "carcinoid tumor" does not convey the malignant potential of tumors and can be confused with "carcinoid syndrome." Therefore, the term "neuroendocrine neoplasm" has been accepted for use instead of "carcinoid tumor" [3,4]; however, "carcinoid tumor" remains in use for daily practice.
GEP-NETs are known to have diverse biological behavior according to origin of the organ and pathological features [1-3,5-7]. Accurate classification and prognostication are very important for proper treatment of patients [8]. Several classification systems and prognostic markers have been proposed for stratifying the malignant potential of GEP-NETs [2,6, 9-11]. International consensus has been partially achieved, however, due to the various changes in the concept of disease and classification in the last decade, there is still confusion between pathologists and clinicians in use of nomenclature and classification of GEP-NETs, especially small rectal NET. WHO updated the classification of GEP-NET in 2010 and all GEP-NET were categorized as malignant tumors [4]; on the other hand, according to the International Classfication of Disease for Oncology, 3rd edition (ICD-O3), some GEP-NETs belong to the benign or uncertain malignancy [12]. Therefore, for more accurate classification of GEP-NET, a greater understanding of their biological behavior is required.
Improved diagnostic tools and awareness of gastrointestinal NETs has resulted in increased incidence of GEP-NETs worldwide during the last decade. Maggard et al. [13] reported a 271% overall increase in incidence from 1973 to 1997 in the United States (US). Incidence of gastric and rectal carcinoid has shown a remarkable increase, with the most common site being the small intestine, as opposed to the appendix in the past. Similar changes in incidence of GEP-NETs have been reported in other populations outside the US [14,15]. However, Ito et al. [16] described significant differences in location, symptomatic status, and prevalence of malignancy of GEP-NET between Japan and the western population. Several individual studies of GEP-NETs in Korea have described a similar increase in overall incidence of GEP-NET as that of western reports but the low incidence of the small intestinal tumor is similar to the Japanese report [17]. However it is very difficult to determine the population based incidence of GEP-NET in Korea, because in the past some carcinoids had been regarded as benign which were not included in the National Cancer Registration. In addition, there are differences of opinion in regard to the coding of NET, especially the small rectal NET, which was formerly diagnosed as carcinoid without any description of risk factors.
Knowledge of epidemiological and pathological characteristics of GEP-NETs in Korea as well as finding significant prognostic parameters are critical to making informative and standard pathological report protocols for GEP-NETs. In this nationwide comprehensive study, we would like to describe changes in incidence of GEP-NET in Korea during the last decade and the prognostic significance of pathological parameters.
Materials and Methods
1. Participating institutions
We collected and reviewed the pathology reports diagnosed as NET of not only the gastroenteropancreatic tract, but also hepatobiliary tract from the university and general hospitals in Korea from 2000 to 2009; Wonju Christian Hospital, Inha University Hospital, Kangbuk Samsung Medical Center, Asan Medical Center, Samsung Medical Center, Seoul National University Hospital, Severance Hospital, National Cancer Center, Pusan National University Hospital, Chonnam National University Hospital, Kosin University Goespel Hospital, Seoul St. Mary's Hospital, Bucheon-Soonchunhyang Hospital, Soonchunhyang Hospital,Yeungnam University Hospital, Daegu Fatima Hospital, Dong-A University Hospital, Busan Paik Hospital, Daegu Catholic Hospital, Konyang University Hospital, Seoul Paik Hospital, Kyunghee University Hospital, Kangnam Severance Hospital, Kyungpook University Hospital, Ilsan Paik Hospital, Chonbuk University Hospital, Chungnam National University Hospital, Hanyang University Hospital, Jeju University Hospital.
2. Data collection
We collected data of patient's age, sex, operation title, primary tumor site, and diagnosis (according to 2000 WHO classification [1]), tumor size, multiplicity, extent, mitotic count (/10 high power field [HPF]), Ki-67 labeling index [4,11], immunohistochemical expression of synaptophysin and chromogranin, lymphovascular invasion, perineural invasion, lymph node metastasis, and resection margin status [2,9,10,18]. A tumor was described as a multiple tumor if it showed metachronous development within one year after its initial diagnosis. The maximum size of the tumor was measured in mm and was divided into four groups (G1, G2, G3, G4). The tumor extension was evaluated according to the 2010 American Joint Committee on Cancer (AJCC) tumor staging manual for each site where the tumor initially originated from [19]. Mitosis was recorded as G1 (<2/10 HPF), G2 (2-20/10 HPF), and G3 (>20/10 HPF). The Ki67-labeling index was G1 (≤2%), G2 (3-20%), and G3 (>20%). The 2010 WHO classification was performed based on the grading of mitosis or Ki-67 labeling index.The results of immunohistochemical stain, lymphovascular invasion, perineural invasion, and lymph node metastasis were presented as either positive or negative.
1) Inclusion criteria
All pathologically confirmed NET of the gastroenteropancreatic and hepatobiliary tract from 2000 January 1 to 2009 December 31, regardless of the quality of pathology reports and operation title, were included in the first step of analysis.
2) Exclusion criteria
All adenocarcinomas with focal neuroendocrine differentiation without mention of the amount of NET component were excluded. If the NETs had a pathology report of excision or surgical resection, the pathology report of biopsy was also excluded from the same patient. The NET in liver developed after diagnosis of NET in gastrointestinal or the hepatobiliary tract was categorized as metastatic NET. Data regarding size of biopsy specimens were not included for the analysis.
3) Survival data
We collected follow-up data from the National Cancer Registration.
4) Statistical analysis
Data were presented as numbers (%) for categorical variables. To estimate the association between eligible variables and mean survival time, the Kaplan-Meier test was applied together with the log-rank test for comparison of various groups. Due to missing values for the eligible variables, multivariate analysis (e.g., Cox Proportional Hazard Regression) was not performed. PASW ver. 18.0 (SPSS Inc., Chicago, IL) was used for statistical analyses. A p-value of <0.05 was considered statistically significant.
Results
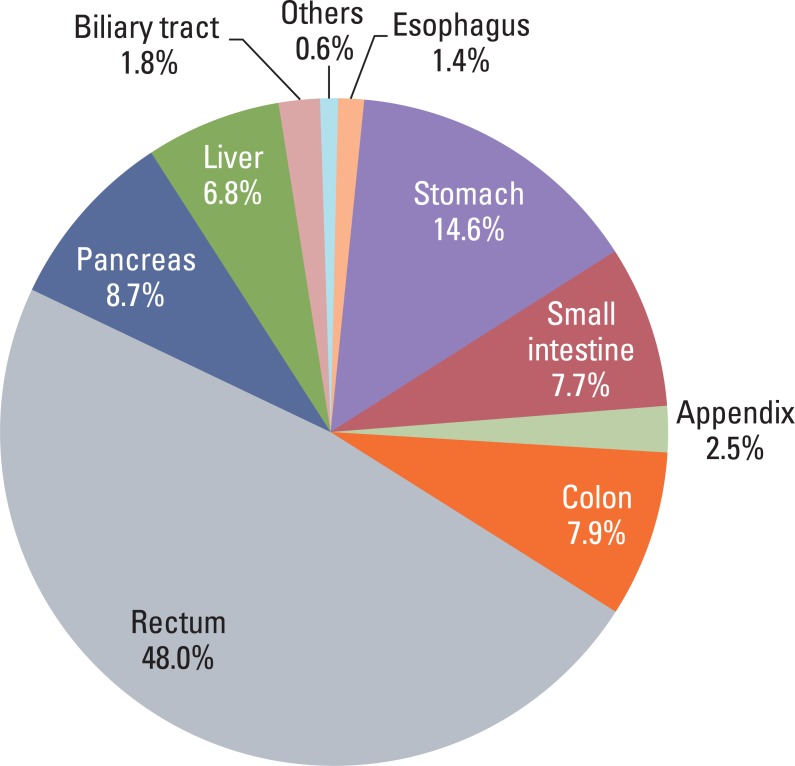
1. Patient population and data in pathology reports
We collected 4,951 pathology reports of patients with GEP-NET. The mean age of patients was 54.66 years old and the sex ratio (M : F) was 1.5 : 1. The highest incidence of GEP-NET was observed in the rectum (48%), while the lowest incidence, 1.4%, was observed in the esophagus. A summary of data identified in the collected pathology reports is shown in Table 1. There was no description of the extension or size if the tumor was obtained by biopsy or partial excision. The rate of lymph node evaluation was low because most tumors were well-differentiated endocrine tumor (WDET) and were treated by simple tumor resection without lymph node dissection. Because some tumors were reported as carcinoid according to the 1980 WHO classification, we reclassified tumors according to the criteria of 2000 WHO classification if data of tumor size and extension were available; WDET, well-differentiated endocrine carcinoma (WDEC), poorly differentiated endocrine carcinoma/small cell carcinoma (PDEC), mixed exocrine-endocrine carcinoma (MEEC), and metastatic endocrine carcinoma (MEC). We also classified tumors according to the 2010 WHO classification if information on mitosis or Ki-67 labeling index were available.
2. Distribution of 4951 GEP-NET according to the organ system
Most GEP-NET were found in the rectum or the stomach (Fig. 1) and the most common site was the rectum. Multiplicity was found in 55 cases (1.3%) and was common in the rectum and stomach. Seventy three cases (1.5%) showed other concurrent pathology in either the same organ or another site: 56 adenocarcinomas in the same organ (stomach [n=34], rectum [n=7], colon [n=5], pancreas [n=4], appendix [n=2], small intestine [n=2], and biliary tree [n=2]), 7 adenomas in the same organ (colon [n=4], rectum [n=2], and stomach [n=1]), 4 lung cancer, 2 cervical cancer, 1 neurofibromatosis type I, 1 neurofibroma in the same organ, 1 retroperitoneal schwannoma, and 1 renal cell carcinoma.
3. Distribution of GEP-NET according to the 2000 and 2010 WHO classification
The distribution of tumors according to diagnosis is shown in Fig. 2. In the reclassification of the tumors according to the 2000 WHO classification, 601 WDET tumors were reclassified as WDEC. The proportion of GEP-NET according to the 2000 WHO classification in each site is shown in Fig. 3. Most rectal tumors were WDET, and most esophageal and biliary tumors were carcinoma.

Distribution of gastroenteropancreatic neuroendocrine tumors according to diagnosis by 2000 and 2010 classification.WDET, well-differntiated endocrine tumor; WDEC, well-differentiated endocrine carcinoma; PDEC, poorly differentiated endocrine carcinoma/small cell carcinoma; MEEC, mixed exocrine-endocrine carcinoma; MEC, metastatic endocrine carcinoma; NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma.
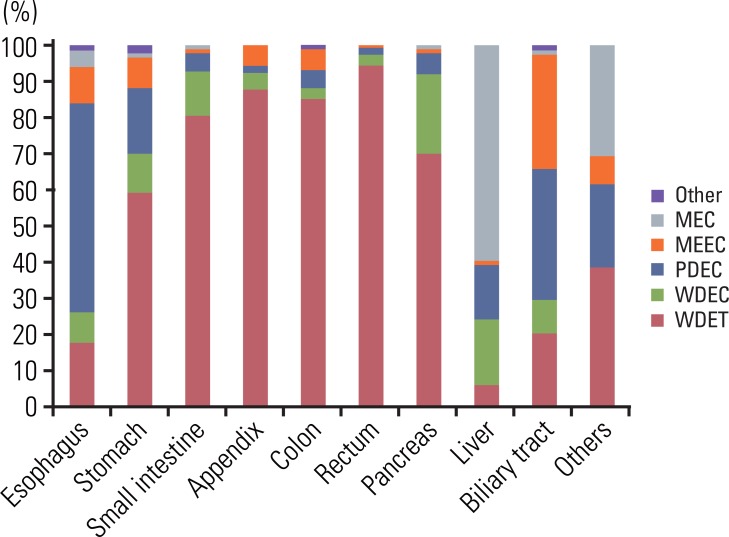
Site distribution of gastroenteropancreatic neuroendocrine tumors in Korea according to the 2000 WHO classification. The most common tumor is WDET in rectum. WHO, World Health Organization; WDET, well-differntiated endocrine tumor; MEC, metastatic endocrine carcinoma, MEEC, mixed exocrine-endocrine carcinoma; PDEC, poorly differentiated endocrine carcinoma/small cell carcinoma; WDEC, well-differentiated endocrine carcinoma.
4. Changes in the incidence of GEP-NET in Korea during the last decade
A continuous increase has been observed in the incidence of GEP-NET, with the incidence in 2009 being nine times that reported in 2000 (Fig. 4). During the last decade, the incidence of GEP-NET has been on the rise, particularly in the rectum (Fig. 5). Although changes in the incidence of GEP-NET according to the 2000 WHO classification was most significant in WDET, the incidence of WDEC and PDEC has also increased since 2003, though the incidence is still low in comparison with other malignancies of the digestive tract (Fig. 6).
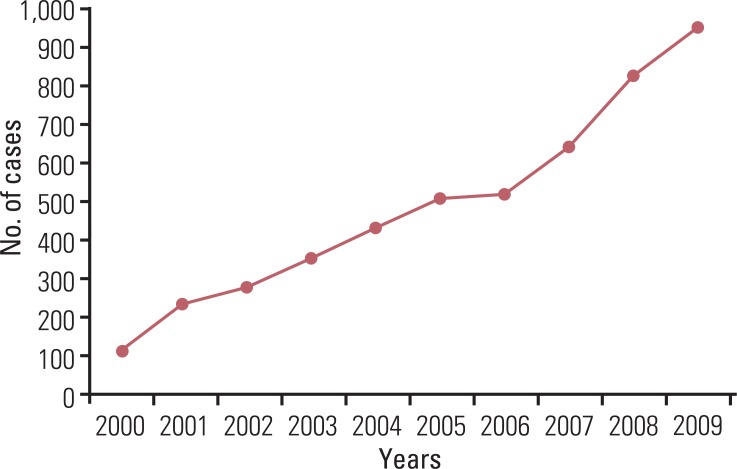
Changes in incidence of gastroenteropancreatic neuroendocrine tumors in Korea during the last decade according to the number of annual diagnosis.
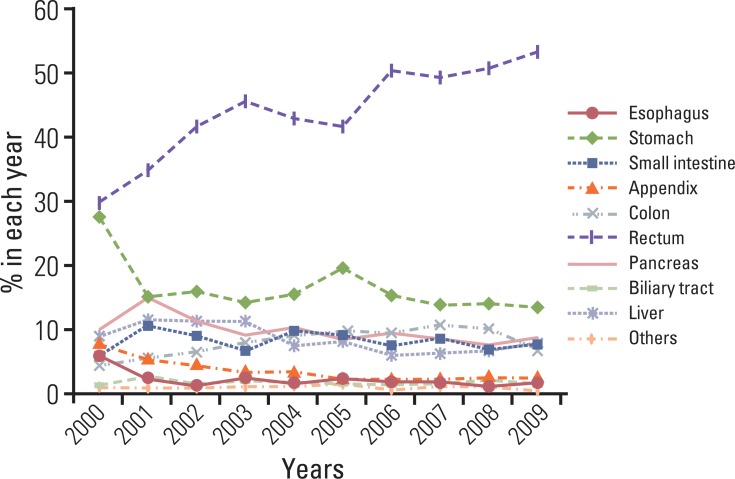
Changes in annual incidence of gastroenteropancreatic neuroendocrine tumors in each site during the last decade.
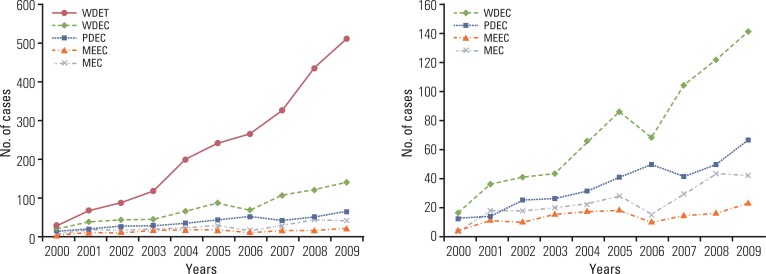
Changes in incidence of gastroenteropancreatic neuroendocrine tumors in Korea during the last decade according to the 2000 WHO classification of tumors. WHO, World Health Organization; WDET, well-differntiated endocrine tumor;WDEC, well-differentiated endocrine carcinoma; PDEC, poorly differentiated endocrine carcinoma/small cell carcinoma; MEEC, mixed exocrine-endocrine carcinoma; MEC, metastatic endocrine carcinoma.
5. Proportion of disease related death (DRD) in GEP-NETs according to the tumor site
Survival rate according to site is shown in Fig. 7. DRD was most common in the hepatobiliary system (62.2%), and least common in the rectum (5.1%). Most patients with rectal NET survived.

Site distribution of gastroenteropancreatic neuroendocrine tumors according to the survival. Mean survival time is the longest in stomach neuroendocrine tumor (154.42±7.74 months) and the shortest in biliary tract neuroendocrine tumors (44.32±6.02 months). Disease related death is most common in hepatobiliary tract.
6. Five- and 10-year survival rate according to the 2000 and 2010 WHO classification of NET
The five- and 10-year survival rates of well differentiated tumors were distinctly different from those of poorly differentiated and metastatic tumors (Fig. 8). Five-year survival rates were similar to 10-year survival rates in each diagnosis group.
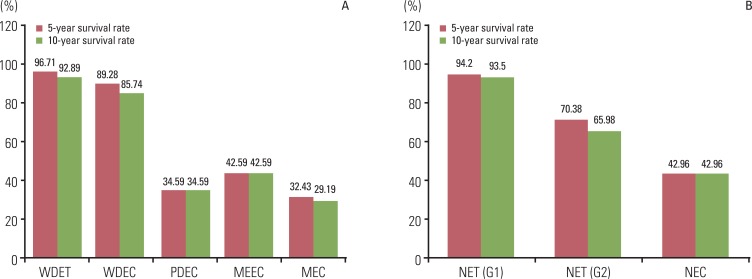
Five- and 10-year survival rate according to the 2000 (A) and 2010 WHO classification of NET (B). According to the 2000 WHO classification, the difference of 10 year survival rate between NET G1 and NET G2 is more distinct than the difference between WDET and WDEC. WHO, World Health Organization; NET, neuroendocrine tumor; WDET, well-differntiated endocrine tumor; WDEC, well-differentiated endocrine carcinoma; PDEC, poorly differentiated endocrine carcinoma/small cell carcinoma; MEEC, mixed exocrine-endocrine carcinoma; MEC, metastatic endocrine carcinoma; NEC, neuroendocrine carcinoma.
7. Prognostic significance of pathological parameters
For all cases examined, the site (p=0.000), diagnosis by 2000 WHO classification (p=0.000), extent (p=0.000), size (p=0.000), mitosis (p=0.000), Ki-67 labeling index (p=0.000), synaptophysin expression (p=0.003), lymphovascular invasion (p=0.000), perineural invasion (p=0.000), and lymph node metastasis (p=0.000) showed prognostic significance, however, chromogranin expression did not (p=0.148).
In the gastrointestinal tract, except for the appendix, all parameters, except synaptophysin (p=0.476) and chromogranin (p=0.089), showed prognostic significance. The lack of chromogranin expression appeared to be related to better survival, but it was not statistically significant. However, in the appendix, synaptophysin expression was associated with better prognosis, compared to synaptophysin negative tumors.
In analysis of WDET in all sites, chromogranin expression (p=0.018), lymphovascular invasion (p=0.024), lymph node metastasis (p=0.040) and size (p=0.024) showed prognostic significance, but mitosis (p=0.395), Ki-67 (p=0.817), synaptophysin (p=0.083), and perineural invasion (p=0.083) did not. However, in rectal WDET, synaptophysin expression and perineural invasion were significant prognostic factors. WDET in colon and rectal tumors showed the best prognosis, in contrast to the worst prognosis in biliary tract tumors.
In Kaplan-Meier survival analysis with Log-Rank test in WDEC, mitosis (p=0.002), Ki-67 (p=0.041), lymphovascular invasion (p=0.010), lymph node metastasis (p=0.033) & size (p=0.002) showed prognostic significance. The small intestinal tumor showed the best prognosis, in contrast to the worst prognosis in biliary tract tumors.
However, in the poorly differentiated neuroendocrine carcinoma, perineural invasion (p=0.011), tumor extension (p=0.000), and sites (p=0.007) showed prognostic significance. Gastric tumors showed the best prognosis, in contrast to the worst in biliary tract tumors. In the mixed exo- and endocrine tumor, lymph node metastasis (p=0.013), tumor extension (p=0.024), and sites (p=0.015) showed prognostic significance. Small intestinal and pancreatic tumors showed the best prognosis, incontrast to the worst prognosis in esophagus.
Discussion
In this study, we described a significant increase in incidence of GEP-NET in Korea during the last decade. During the last decade, the incidence of GEP-NET in Korea has shown a significant increase. The rectal well differentiated NET was the most common and showed the most significant increase during the last decade, compared to the western report, which described the small intestinal NET as the most common. This change could be attributed to improved diagnostic tools, such as endoscopy. The incidence of small intestinal NET was 7.7%, which is similar to that reported in the Japanese study (9.6%) [16]. Incidence of WDEC, PDEC, MEEC, and MEC has shown a 5- to 9-fold increase in the last five years, although they are still relatively rare, compared to other malignant tumors of the digestive system. These changes are in concordance with the western report; however, the reasons are not yet clear.
Gosset and Masson first described carcinoids as endocrine-related tumors in 1914 [20]. Later, in 1963, Williams and Sandler classified them according to their embryologic site of origin as foregut, mid gut, and hind gut tumor [20]. Although it was the first description of morphological and biological differences between tumors, this classification did not reflect the diverse spectrum of GEP-NET. In the first WHO classification of GEP-NETS in 1980, the term carcinoid was used to describe all gastrointestinal neuroendocrine tumor regardless of biologic behavior and pathological characteristics [21]. In 2000 and 2004, the WHO reclassified NET into well-differentiated and poorly differentiated tumors [1]. Well-differentiated tumors were subdivided into benign, uncertain malignancy, or carcinoma. They recommended that the term carcinoid be used only for benign well-differentiated tumors, however, some pathologists are still using "carcinoid tumor" for all well-differentiated NET. The WHO recently updated the classification and proposed the term neuroendocrine neoplasms as the appropriate term for describing tumors originating from the diffuse endocrine system in the human body. According to the 2010 WHO classification, neuroendocrine neoplasms were devided into NET G1, NET G2, and neuroendocrine carcinoma NEC based on cell proliferation. In this retrospective study, the 2000 WHO classification system was used for diagnosis of GEP-NET. This is because all of the pathology reports we used were made before the 2010 WHO classification and mitotic counts were recorded in only 32.7% of collected pathology reports. Some tumors were diagnosed as carcinoid regardless of the size and extent according to the 1980 WHO classification. Therefore, all carcinoids were reclassified according to the 2000 WHO classification based on tumor size and extent, if the information was available, and 17.68% of carcinoids were then reclassified as well differentiated neuroendocrine carcinoma. This means that the classification system used for diagnosis should be mentioned in the pathology report.
In regard to the biologic behavior of GEP-NET, ICD-O3 classified the L-cell type carcinoid in rectum or appendix as uncertain malignancy coded as /1. This has recently caused some confusion in the category of malignancy of GEP-NET, particularly the small rectal WDET. According to the 2000 WHO classification, the rectal WDET, the most common tumor in Korea, belongs to the benign or uncertain malignancy. However, the WHO classification updated in 2010 recommended classification of all GEP-NET as malignancy, although the L-cell type rectal NET remains as uncertain malignancy. However, there is no definitive description of diagnostic criteria of L-cell type tumor. In 2011, a Colonoscopy Study Group of the Korean Society of Coloproctology described the metastatic potential of colorectal carcinoid [22]. In colorectal NET, lymph node metastasis was found in 5.1% (21 out of 414 cases) and was related to tumor size. The rectal NET measuring less than 10 mm had a 1.99% lymph node metastatic rate (7 out of 359 cases). However, they did not describe the association with patient's survival. Jernman et al. [23] found that none of the NET G1 had metastatic disease. In addition, tumor recurrence was found only in incomplete resection of NET G1 tumors. In the Japanese report, metastasis showed significant correlation with tumor size. Tumors less than 10 mm and confined to the submucosa without vessel invasion did not show any metastasis [24]. However, few cases of small rectal carcinoid (<10 mm) accompanied by multiple liver metastasis have been reported in the literature [25]. All reported cases showed poor prognosis if the patient had liver metastasis, even though the tumor size was very small. However, the pathological factors that can predict the metastasis in small sized NET remains to be clarified. In our data, the 10-year survival rate of WDET was 92.89%, in contrast to 85.74% in WDEC, and 34.59% in PDEC. Rectal WDET showed a 10-year survival rate of 98% and DRD was found in nine cases among 1,045 WDET with available follow-up data. Only four of these patients were treated by excision or surgical resection with a clear margin, therefore, DRD was actually 0.38%. Tumor size was less than 10 mm (range, 2.5 to 9 mm) for all four cases, and survival duration ranged from 3-23 months. From these results, the rectal WDET should not be classified as benign even if the size is less than 10 mm. On the other hand, because most tumors showed a benign clinical course, it is also difficult to classify as malignancy. Further study of pathological parameters associated with patient survival, especially in the small rectal WDET is required.
To predict the biological behavior of GEP-NET, several pathological features have been proposed by many investigators. For proper evaluation, the minimum data set for the pathology report has recently been proposed [3,9,10]. Tumor extension, histological differentiation, lymph node metastasis, lymphatic invasion, and perineural invasion of the tumor are well known as the default features for pathological evaluation of GEP-NET as well as other malignant tumors of the gastrointestinal tract. Unlike other gastrointestinal tract epithelial malignancy, site and size are known to be very important as prognostic markers in NET. As neuroendocrine markers, synaptophysin (a small vesicle associated marker) and chromogranin (a large secretory granule associated marker) were the mainstay. In addition, proliferation activity, assessed by mitotic count or Ki-67 immunostain, was described as a significant prognostic factor by a European group. In this study, we performed survival analysis according to the pathological parameters described above, however, multivariate analysis (e.g., Cox Proportional Hazard Regression) was not available due to missing values for the eligible variables in the pathology reports. All pathological parameters examined in this study, except for chromogranin expression, showed prognostic significance in all cases. However, the lack of chromogranin expression showed a significant association with better prognosis in WDET. In WDET, mitosis (p=0.395) and Ki-67 labeling index (p=0.817) were not prognostic factors. This was attributed to the lack of variation between cases in each group. The size of the tumor, particularly those greater than 1.5 cm, and proliferative activity showed an association with poor survival in WDEC. Therefore, for proper evaluation of GEP-NET, all pathological parameters examined in this study, including the immunostain for neuroendocrine markers as well as Ki-67 labeling index, should be mentioned in the pathology report. Further study of the correlation between pathological parameters and multivariate analysis is needed.
Conclusion
The incidence of GEP-NET in Korea has shown a remarkable increase during the last decade, however, the distribution of tumors in the digestive system differs from that of western reports. Although tumor size and extent are well known prognostic factors, DRD was rarely found in the very small rectal WDET. Further study and meticulous assessment of pathological parameters, including immunostain, are critical to understanding the biological behavior of tumors as well as predicting prognosis of patients with GEP-NET.
Acknowledgments
The research was supported in part by a Korean Cancer Association Novartis Research Grant.
Notes
Conflict of interest relevant to this article was not reported.