The Oncologic Implications of Tumor Multiplicity in Intrahepatic Cholangiocarcinoma: Its Prognostic Value Might Be Underestimated
Article information
Abstract
Purpose
In the latest staging system of the American Joint Committee on Cancer for intrahepatic cholangiocarcinoma (IHCCC), solitary tumors with vascular invasion and multiple tumors are grouped together as T2. However, recent studies report that multifocal IHCCC has a worse prognosis than a single lesion. This study aimed to investigate the risk factors for IHCCC and explore the prognostic significance of multiplicity after surgical resection.
Materials and Methods
A total of 257 patients underwent surgery for IHCCC from 2010 to 2019 and the clinicopathological data were retrospectively reviewed. Risk factor analysis was performed to identify variables associated with survival after resection. Survival outcomes were compared between patients with solitary and multiple tumors.
Results
In multivariable analysis, the presence of preoperative symptoms, tumor size, lymph node ratio, multiplicity, and tumor differentiation were identified as risk factors for survival. Among 82 patients with T2, overall survival was significantly longer in patients with solitary tumors (sT2) than in those with multiple tumors (mT2) (p=0.017). Survival was compared among patients with stage II-sT2, stage II-mT2, and stage III. The stage II-sT2 group showed prolonged survival when compared with stage II-mT2 or stage III. Survivals of stage II-mT2 and stage III patients were not statistically different.
Conclusion
Tumor multiplicity was an independent risk factor for overall survival of IHCCC after surgical resection. Patients with multiple tumors showed poorer survival than patients with a single tumor. The oncologic significance of multiplicity in IHCCC should be reappraised and reflected in the next staging system update.
Introduction
Intrahepatic cholangiocarcinoma (IHCCC) arises from the second-order bile ducts or more peripheral ducts, and is the second most common primary liver malignancy. The incidence is higher in Eastern countries, including Korea than in Western countries, showing geographical variation. Since it was reported that the worldwide incidence has increased over the last decade [1], the need for proper diagnosis and management of IHCCC is gaining significance.
The only curative treatment for IHCCC is surgical resection. To assess resectability and to classify stage groups after surgical resection, the American Joint Committee on Cancer (AJCC) staging system is widely used as a standard. In the 7th AJCC system, IHCCC had its own staging system, which was independent from hepatocellular carcinoma. However, in the latest 8th system, some major changes have been applied [2,3]. One of the changes is presented in the T2 category. In the 7th system, T2 was subdivided into T2a and T2b according to the number of tumors (multiplicity) and vascular invasion whereas in the 8th system, T2 is no longer sub-classified. Now, the prognostic value of multiplicity is regarded as equivalent to that of vascular invasion [4].
However, some recent studies have shown that tumor multiplicity is associated with a poor prognosis of IHCCC. A recent study using the National Cancer Database of the United States suggested that patients with multifocal T2N0 IHCCC had poorer overall survival (OS) than those with solitary T2N0 diseases [5]. Another multi-institutional study identified that IHCCC with satellite nodules in the same liver segment is associated with a worse prognosis compared to a single IHCCC [6]. In the present study, we aimed to explore the prognostic impact of tumor multiplicity in patients with surgically resected IHCCC.
Materials and Methods
1. Patient database
From January 2010 to December 2019, a total of 257 patients underwent surgical resection for IHCCC in Samsung Medical Center, Seoul, South Korea. The demographic and clinicopathological data of the patients were retrospectively reviewed. In the patient’s medical history, preoperative disease-related symptoms included abdominal pain, jaundice, and unintentional weight loss. Preoperative laboratory data included serum total bilirubin level, carbohydrate antigen 19-9 (CA 19-9), and inflammatory markers (neutrophil-to-lymphocyte ratio [NLR] and platelet-to-lymphocyte ratio [PLR]). Pathology reports included the size and differentiation of the tumor, tumor multiplicity, numbers of harvested and metastatic lymph nodes (LNs), lymphovascular invasion, or perineural invasion (PNI), and the residual tumor status (R status). Pathological staging was based on the 8th edition of the AJCC staging manual [3]. Tumor multiplicity was defined when there were more than two distinct lesions found in a surgical specimen, regardless of Couinaud liver segments.
2. Survival outcomes
After hospital discharge, patients underwent surveillance using contrast-enhanced abdomino-pelvic computed tomography (CT) and a CA 19-9 level check, every 3 or 6 months. Recurrence was diagnosed when suspicious lesions were detected by CT scans and patients exhibited elevated CA 19-9 levels.
Recurrence-free survival (RFS) was defined as the time interval from the date of operation to the date when recurrence was diagnosed. OS was calculated as the time from the date of operation to death from any causes. The last update of follow-up data was performed in August 2022.
3. Statistical analysis
To identify risk factors for recurrence and survival, uni- and multivariable logistic regression analyses were performed. Variables with a p-value of < 0.1 from univariable analysis were included in a multivariable analysis. Hazard ratios (HRs) were reported with 95% confidence intervals (CIs). Kaplan-Meier survival curves with log rank tests were used to compare survival among patients, according to tumor multiplicity and stage. All statistical analyses were performed using IBM SPSS ver. 26 (IBM Corp., Armonk, NY).
Results
1. Patient characteristics
The demographic and clinicopathological data of the patients are shown in Table 1. The mean age was 63.7 years, and 58 patients (22.6%) had chronic liver disease. Hemihepatectomy or extended hemihepatectomy was performed in 190 patients (73.9%), and sectionectomy or segmentectomy was performed in the other 67 patients (26.1%). Bile duct resection was additionally performed in 17 patients (6.6%). Among all patients, 34 patients (13.2%) presented with tumor multiplicity. R0 resection was achieved in 241 patients (93.8%). The median RFS was 29 months, and median OS was 52 months.
2. Risk factors for survival
Risk factor analysis was performed to identify variables associated with RFS and OS after surgical resection of IHCCC (Table 2). In the multivariable analysis for RFS, elevated preoperative CA 19-9 (HR, 2.691; 95% CI, 1.437 to 5.030; p=0.002), preoperative NLR (HR, 1.558; 95% CI, 1.210 to 2.007; p=0.001), tumor size (HR, 1.157; 95% CI, 1.026 to 1.305; p=0.018), and PNI (HR, 2.111; 95% CI, 1.162 to 3.837; p=0.014) were independent risk factors. In terms of OS, preoperative symptoms (HR, 1.912; 95% CI, 1.129 to 3.246; p=0.016), tumor size (HR, 1.137; 95% CI, 1.020 to 1.267; p=0.021), LN ratio (HR, 8.594; 95% CI, 2.837 to 26.031; p < 0.001), tumor multiplicity (HR, 1.948; 95% CI, 1.062 to 3.572; p=0.031), and tumor differentiation (HR, 1.615; 95% CI, 1.110 to 2.352; p=0.012) were significantly related factors.
3. Survival outcomes according to tumor multiplicity
As shown in Fig. 1, RFS was compared in patients with T2 IHCCC according to tumor multiplicity. In all T2 patients (n=82), RFS was significantly longer in patients with single tumor than those with multiple tumors (median survival, 28.1 vs. 8.0 months; p=0.007) (Fig. 1A). To eliminate the prognostic effect of LN metastasis, another survival curve was drawn after excluding patients with N1 disease (n=66) (Fig. 1B). Patients in whom LN status was not assessed during surgery (Nx) were regarded as N0. Patients with tumor multiplicity showed significantly poorer RFS than those without tumor multiplicity (median survival, 37.0 vs. 7.8 months; p=0.004).
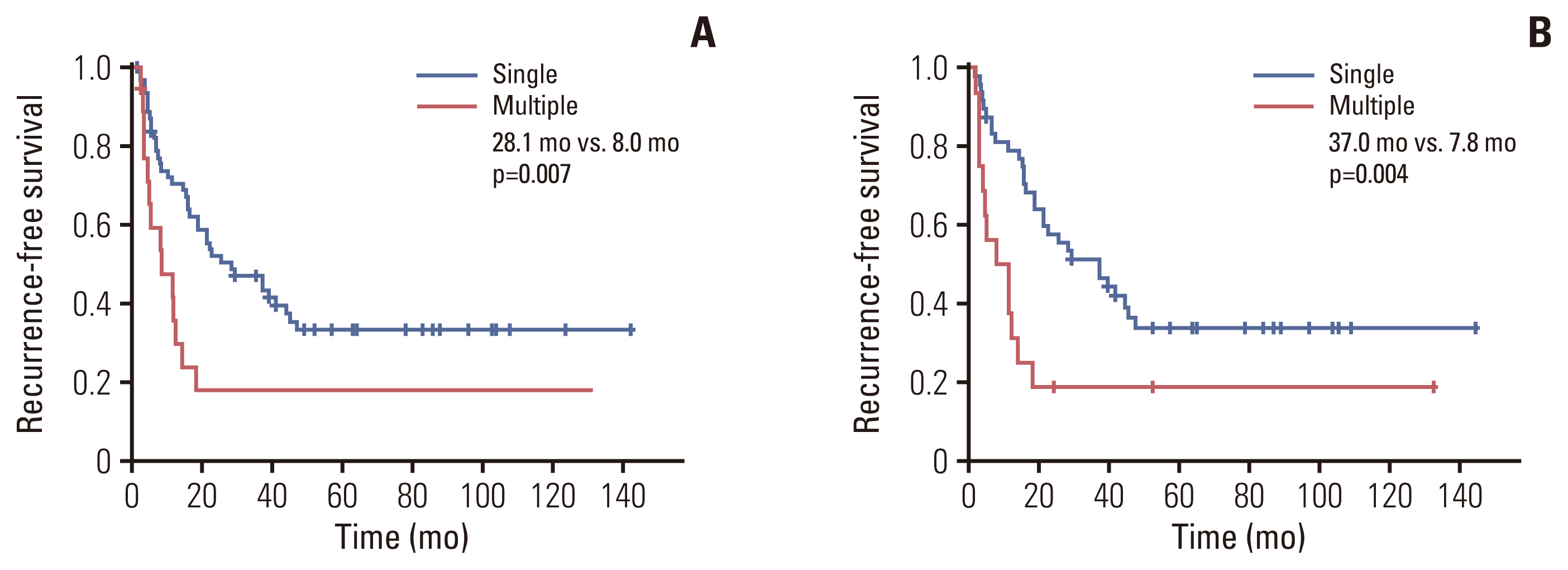
Comparisons of recurrence-free survival between patients with single-T2 and multiple-T2 lesions. (A) In all patients (n=82). (B) In T2/N0 or T2/Nx patients (n=66).
In the analysis for OS, patients with a T2-single tumor showed prolonged survival than those with T2-multiple tumors (median survival, 45.0 vs. 29.0 months; p=0.017) (Fig. 2A). After adjustment for LN status, OS in patients with a T2-single tumor was significantly longer than in those with T2-multiple tumors (median survival, 52.0 vs. 29.0 months; p=0.010) (Fig. 2B).
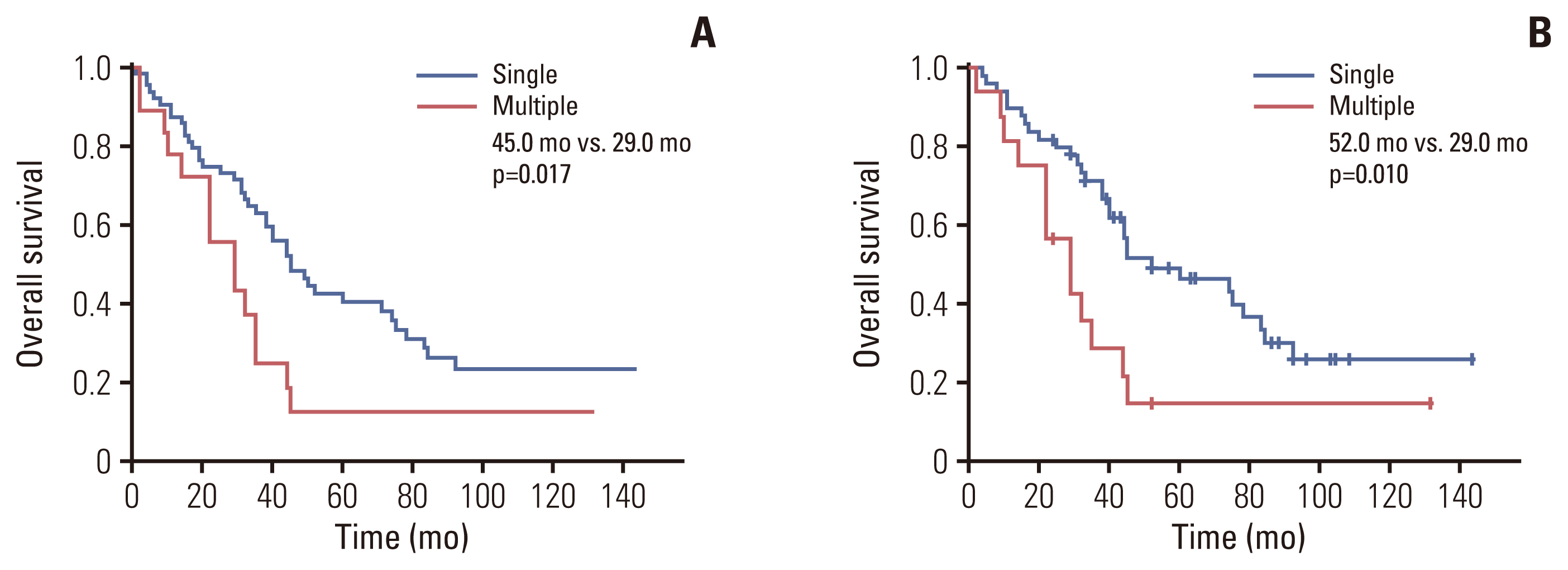
Comparisons of overall survival between patients with single-T2 and multiple-T2 lesions. (A) In all patients (n=82). (B) In T2/N0 or T2/Nx patients (n=66).
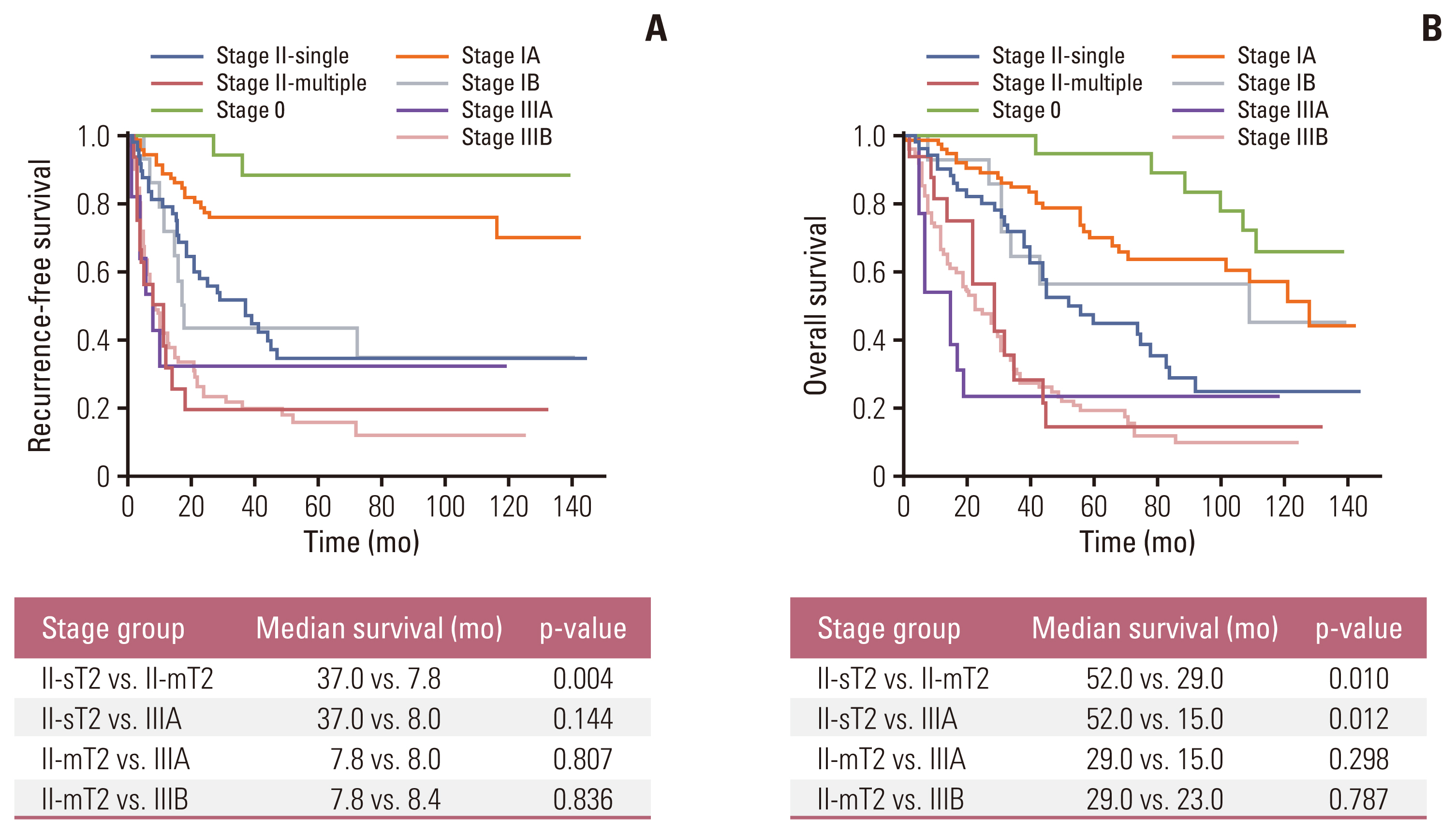
To explore the prognostic effect of tumor multiplicity, we compared RFS and OS according to the AJCC 8th staging system (Fig. 3). Patients with stage II were divided into two groups: stage II with a single tumor (stage II-sT2) and stage II with multiple tumors (stage II-mT2). The RFS of the stage II-sT2 group was superior to that of the stage II-mT2 group (median survival, 37.0 vs. 7.8 months; p=0.004) (Fig. 3A). Also, the stage II-sT2 group demonstrated longer OS than the stage II-mT2 group (median survival, 52.0 vs. 29.0 months; p=0.010) and stage IIIA group (median survival, 52.0 vs. 15.0 months; p=0.012) (Fig. 3B). Both the RFS and OS of the stage II-mT2 group did not significantly differ from those of the stage IIIA or IIIB group.
Discussion
The recently updated AJCC staging system for IHCCC suggests that multiple tumors would have prognostic impacts equivalent to that of a solitary tumor with vascular invasion. The present study aimed to explore the clinical implications of tumor multiplicity in surgically resected IHCCC and identified that multiplicity is an independent risk factor for survival. In the survival analysis, patients with T2-multiple tumors showed worse survival than those with a T2-solitary tumor, regardless of nodal status. There was no statistically significant difference in OS between patients with stage II-multiple T2 and those with stage IIIA (T3N0).
The issue that first needs to be addressed is that no consensus has been reached regarding terminology that indicates the existence of multiple tumors in IHCCC. Other than multiplicity, ‘multifocality’, ‘satellite nodules’, ‘daughter nodules’, and ‘intrahepatic metastasis’ have been used in previous studies. Even the recent AJCC staging system does not state further details on ‘multiple tumors’, such as the number, location, or patterns of distribution. This lack of clarity in the definition of multiple tumors might be attributed to the former version of the staging system, in which the staging of IHCCC was according to that of primary liver tumors such as hepatocellular carcinoma (HCC). The distinct system for IHCCC, independent from HCC, was first provided in the 7th edition [7]. Contrary to HCC, in which multiple primary tumors may arise from the background hepatic pathology such as liver cirrhosis, multiple lesions in IHCCC could be regarded as hematogenous dissemination from one primary focus [8]. Despite the possibility of disseminated intrahepatic disease, surgery with curative intent is tried when there is no extrahepatic involvement. In the present study, which included patients with curative resection, tumor multiplicity was defined as the presence of multiple lesions confined in the same geographic hepatic area, accompanying the potential for curative surgery. The issues on terminology regarding multiple tumors should be further discussed in the next update of the staging system.
The prognostic significance of multiplicity in IHCCC has been demonstrated in several recent studies. In 2008, Conci et al. [6] compared the survival of patients with resected IHCCC according to the patterns of tumor distribution. The authors defined ‘satellite nodules’ when additional lesions were located in the same Couinaud liver segment, and ‘multifocality’ when multiple lesions were scattered through different liver segments. These two patterns were independent risk factors for survival and associated with a poor OS compared to patients with a single IHCCC lesion. Patients with multifocality had significantly worse survival outcomes than those with satellites, but still had a better prognosis than those with non-curative (R2) resection. Other studies also proposed the necessity for the upstaging of multiple lesions by comparing survival outcomes with other advanced stage disease [9,10]. The results are somewhat consistent with the present study in that multiple lesions were related with a worse OS. In another study which utilized a Surveillance, Epidemiology, and End Results database, the authors used the term “liver metastasis” to refer to T2-multiple IHCCC and analyzed the prognosis by comparing it to tumors of other stages [8]. The authors suggested that stage II with multiplicity should be regarded as M1 disease, considering the poorer outcomes of patients with multiple lesions compared to those with other early-stage tumors. In this regard, ‘resectability’ of IHCCC is an issue which needs a consensus of opinion. Despite the technological advances in radiology, not all multiple lesions could be identified preoperatively in imaging studies, especially when they are small peritumoral lesions [6]. Even in cases of preoperatively perceptible multiplicity, some surgeons proceed with surgery while other surgeons may introduce palliative chemotherapy [11,12]. Since the appropriate candidates for curative-intent surgery in multiple IHCCC, in the absence of extrahepatic metastasis, have yet to be determined [10,12–14], a multidisciplinary approach should be considered in order to assess the risk and benefit of surgical resection and to devise other liver-directed treatment options.
Besides tumor multiplicity, several other factors were identified as risk factors for survival after resection of IHCCC. Among several preoperative variables, NLR was associated with an increased risk of recurrence. Preoperative inflammatory markers, such as NLR, can be readily obtained from by the results of basic blood or body fluid tests and have been extensively researched as prognostic factors in biliary tract cancers [15–17]. It is supposed that inflammatory markers might reflect the peritumoral inflammatory microenvironment, which triggers the release of several growth factors and leads to cancer progression [18,19]. In previous studies, PLR, systemic immune-inflammation index, and lymphocyte-to-C-reactive protein have also been investigated as potential immunonutritional markers related to IHCCC [20–22]. In a recent study which suggested predictive nomograms for IHCCC, the authors developed a laboratory risk score using a combination of preoperative CA 19-9, NLR, platelet counts, and albumin to predict long-term survival [23]. Further investigation is required to explore other potential biomarkers, or combinations of them, and their prognostic values in IHCCC.
In risk factor analysis for OS, lymph node ratio (LNR) was an independent risk factor, rather than LN metastasis itself. Since LNR consists of the numbers of both yielded and metastatic LNs, it is suggested that harvesting a certain number of LNs during an operation might be inevitable for accurate tumor staging. However, despite the association between LN status and survival outcomes of IHCCC [24,25], much controversy surrounds the role and extent of LN dissection and its survival benefit [26–31]. In previous studies, some authors argued that lymphadenectomy is confined to a procedure for staging since it has little effect on survival [26–28]. Others regarded extensive node dissection as a part of curative resection of IHCCC, which is associated with prolonged survival [29–31]. Considering that accurate staging enables the estimation of prognosis and provision of appropriate adjuvant treatment for an individual patient, a well-designed prospective study regarding the implication of LN dissection in IHCCC should be performed.
The present study has several limitations. The results of the study might be influenced by a significant bias largely due to its retrospective nature. Most of all, there was substantial heterogeneity between surgeons in the selection of surgical candidates, owing to the absence of a consensus regarding resectability and the extent of surgery required in patients with IHCCC. In the same context, data on preoperative imaging for assessing multiplicity and vascular invasion were not included. Another point is that detail was not provided about the number and location of multiple lesions in the pathology reports. Despite all the potential limitations, it is apparent that definition and classification of multiplicity in IHCCC should be introduced, and the prognostic significance of multiplicity should be adjusted for in the next staging system update. Furthermore, comprehensive research using large-scale prospective data needs to be forthcoming to investigate the relevance of surgical treatment in patients with multiple IHCCC lesions.
Notes
Ethical Statement
This study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2022-08-113). Our Institutional Review Board of Samsung Medical Center waived the need for written informed consent from the participants because the research involved no more than minimal risk to subjects, and there was no reason to assume rejection of agreement.
Author Contributions
Conceived and designed the analysis: Yoon SJ, Park S, Kim H, Shin SH, Heo JS, Han IW.
Collected the data: Yoon SJ, Park S, Han IW.
Contributed data or analysis tools: Yoon SJ, Park S, Kim H, Shin SH, Heo JS, Han IW.
Performed the analysis: Yoon SJ, Park S, Han IW.
Wrote the paper: Yoon SJ, Park S, Kim H, Shin SH, Rhu J, Choi GS, Kim JM, Joh JW, Han IW.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
The authors would like to thank Hyemin Kim (Data Manager, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine) for help with data collection.