Development of the Korean Association for Lung Cancer Clinical Practice Guidelines: Recommendations on Radial Probe Endobronchial Ultrasound for Diagnosing Lung Cancer - An Updated Meta-Analysis
Article information
Abstract
Purpose
Radial probe endobronchial ultrasound (RP-EBUS) accurately locates peripheral lung lesions (PLLs) during transbronchial biopsy (TBB). We performed an updated meta-analysis of the diagnostic yield of TBB for PLLs using RP-EBUS to generate recommendations for the development of the Korean Association of Lung Cancer guidelines.
Materials and Methods
We systematically searched MEDLINE and EMBASE (from January 2013 to December 2022), and performed a meta-analysis using R software. The diagnostic yield was evaluated by dividing the number of successful diagnoses by the total lesion number. Subgroup analysis was performed to identify related factors.
Results
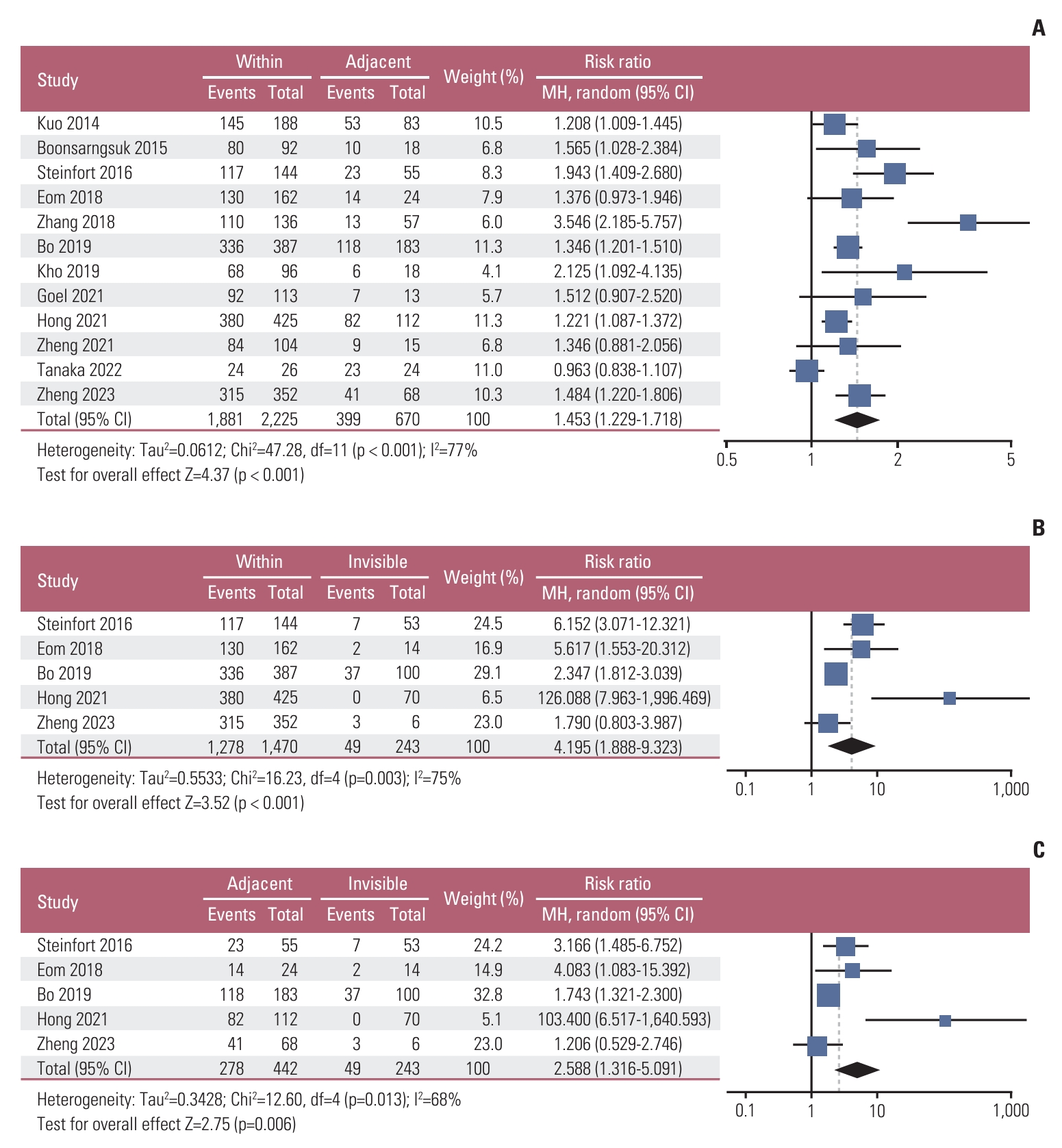
Forty-one studies with a total of 13,133 PLLs were included. The pooled diagnostic yield of RP-EBUS was 0.72 (95% confidence interval [CI], 0.70 to 0.75). Significant heterogeneity was observed among studies (χ2=292.38, p < 0.01, I2=86.4%). In a subgroup analysis, there was a significant difference in diagnostic yield based on RP-EBUS findings (within, adjacent to, invisible), with a risk ratio of 1.45 (95% CI, 1.23 to 1.72) between within and adjacent to, 4.20 (95% CI, 1.89 to 9.32) between within and invisible, and 2.59 (95% CI, 1.32 to 5.01) between adjacent to and invisible. There was a significant difference in diagnostic yield based on lesion size, histologic diagnosis, computed tomography (CT) bronchus sign, lesion character, and location from the hilum. The overall complication rate of TBB with RP-EBUS was 6.8% (bleeding, 4.5%; pneumothorax, 1.4%).
Conclusion
Our study showed that TBB with RP-EBUS is an accurate diagnostic tool for PLLs with good safety profiles, especially for PLLs with within orientation on RP-EBUS or positive CT bronchus sign.
Introduction
The National Lung Cancer Screening Trial (NLST) demonstrated a 20% reduction of mortality in lung cancer by low-dose computed tomography, which led to an increased detection rate of peripheral lung lesions (PLLs) (1.5 million PLLs per 5 million U.S. population annually) [1,2]. However, only 5.2% of PLLs were finally diagnosed with lung cancer, implying that most PLLs were benign [1]. Because most of them are benign, it is necessary to select the target patients who need invasive examination carefully. To this end, positron emission tomography–computed tomography can be considered in the intermediate risk group, and even if biopsy is performed, non-surgical biopsy is recommended [3].
The most common non-surgical procedures to diagnose PLLs are bronchoscopic transbronchial biopsy (TBB) and transthoracic needle biopsy (TTNB) [3,4]. TBB using the conventional bronchoscope showed a suboptimal diagnostic yield for malignancy ranging from 0.34-0.63 in the diagnosis of PLLs [3]. To overcome this issue, radial probe endobronchial ultrasound (RP-EBUS) has been introduced, providing a circumferential ultrasound image of the surrounding lung, confirming the accurate location of PLLs [5]. RP-EBUS with a guide sheath (GS) is a commonly performed TBB procedure enabling access to and detection of PLLs through the bronchoscope’s working channel [6]. After the detection of PLLs by RP-EBUS with GS, RP-EBUS is withdrawn, leaving the GS near PLLs as an extended working channel. Then, biopsy instruments are inserted through GS for tissue acquisition.
Although safe, a previous meta-analysis by Wang Memoli et al. [7] reported that the pooled diagnostic yield of TBB using RP-EBUS for PLLs is 71%, which is lower than that of TTNB (90%). To increase the diagnostic yield of TBB, newer technologies, such as virtual bronchoscopic navigation (VBN) [8], electromagnetic navigation bronchoscopy (ENB) [9], and ultrathin bronchoscopes [10], have been introduced with increasing frequency over the last decade, in addition to previous technology such as fluoroscopy (Flu) [3]. However, there is a lack of systematic research reflecting the current status of TBB performance using RP-EBUS.
Thus, we aimed to evaluate the updated meta-analysis of the diagnostic yield of TBB for PLLs using RP-EBUS based on recently published articles in the last 10 years and to generate recommendations for the development of the Korean Association of Lung Cancer guidelines on RP-EBUS. Furthermore, we evaluated the factors affecting the diagnostic yield and associated complications.
Materials and Methods
1. Literature search
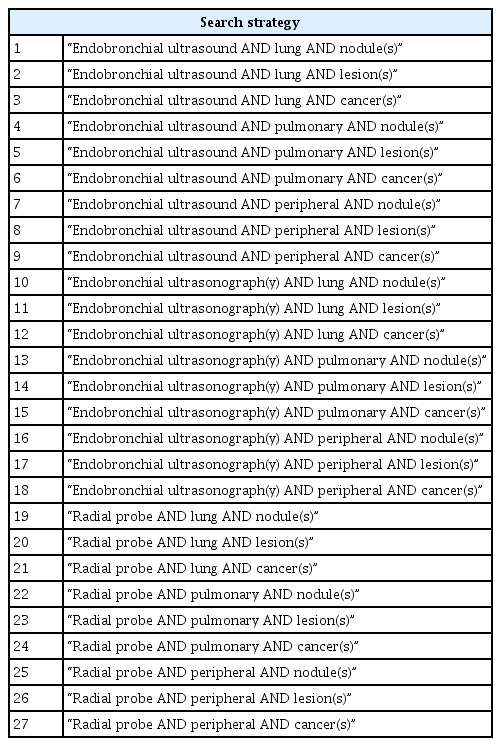
We performed a meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. The study protocol is registered with the PROSPERO database (Identifier: CRD42022378949). We searched MEDLINE and EMBASE (from January 2013 to December 2022) to identify all studies that employed RP-EBUS to evaluate PLLs using a predetermined protocol (Table 1). A manual search of references cited in original and review papers was done for relevant studies, which might have been missed by the electronic search.
2. Selection of studies
All articles identified by the search strategy were independently assessed by four authors (S.H.K., H.S.C., I.K., and J.S.E.). Discordance was resolved by consensus. Abstracts were initially examined, and studies were selected for inclusion only after all reviewers assessed the full-text articles. Criteria for inclusion were as follows: (1) RP-EBUS for diagnosis of PLL providing a diagnostic yield, (2) diagnosis confirmed histologically or by close clinical follow-up, and (3) studies where at least 50 patients were enrolled.
We excluded review articles, meta-analysis articles, letters, case reports with fewer than 50 patients, articles not available in English, articles focusing on modalities other than RP-EBUS, or articles only limited to PLLs with a narrow spectrum (e.g., malignant lesion, ground-glass opacity lesion [GGO]). Furthermore, articles focusing on cone-beam computed tomography (CT) or robotic bronchoscopy were also excluded due to the high risk of selection bias. When two or more studies were published by the same author(s), the methods sections were reviewed to check for overlapping study periods. If so, we included only one publication with the greatest number of patients to prevent duplication of the study cohorts.
3. Data extraction
All data were independently extracted by S.H.K., H.S.C., and I.K., followed by a comparison of extracted data. Disagreements were resolved by further discussion with the other investigator (J.S.E.). The following were retrieved: author, year of publication, study design (randomized controlled trial, prospective, retrospective, or unknown), total number of lesions, number of successful diagnoses, type of bronchoscope (standard bronchoscope, thin bronchoscope, ultrathin bronchoscope), use of guidance modalities (e.g., GS, VBN, Flu, ENB), biopsy methods (e.g., forceps biopsy, cryobiopsy), mean lesion size, prevalence of malignancy, RP-EBUS findings, histologic diagnosis, presence of CT bronchus sign, lesion character (solid, part-solid, GGO), distance from the hilum (central, inner third; intermediate, middle third; peripheral, outer third in the lung field on CT scan) [12], complications, and reference standard.
The quality of selected studies was evaluated by S.H.K., H.S.C., and I.K., using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool [13]. This validated tool contains 14 signaling questions to evaluate four main components (patient selection, index test, reference standard, and flow and timing) in two categories (risk of bias and applicability concerns). Disagreements were resolved by further discussion with the other investigator (J.S.E.).
4. Statistical analysis
Meta-analysis was performed using the meta package of R statistical software (ver. 4.0.5, http://www.R-project.org). A p-value of < 0.05 was considered statistically significant. The primary outcome was the diagnostic yield with a 95% confidence interval (CI), calculated by dividing the number of successful diagnoses by the total number of lesions. Inverse variance weighting across selected studies was applied to evaluate the pooled diagnostic yield, where the weight of each study was based on the number of lesions.
Subgroup analysis was performed to identify the factors associated with diagnostic yield. Stratified analysis on diagnostic yield was based on lesion size (≤ 20 mm vs. > 20 mm), histologic diagnosis (malignant vs. benign), RP-EBUS findings (within vs. adjacent to vs. invisible), CT bronchus sign (present vs. absent), lesion character (solid vs. partsolid vs. GGO), and distance from the hilum (peripheral vs. non-peripheral [central and/or intermediate]). In addition, subgroup analysis based on adjunctive modalities (ENB, GS, Flu, VBN), bronchoscope types (standard, thin, ultrathin bronchoscope), and use of cryobiopsy were evaluated. A subgroup meta-analysis was performed using the Mantel-Haenszel risk ratio (RR) [14,15]. RR > 1 was in favor of the former variable for the diagnostic yield, while RR < 1 was in favor of the latter variable.
Study heterogeneity was assessed using the Cochran Q test (χ2 test) and quantified by the I2 index [16]. Statistical heterogeneity was indicated in cases of p < 0.01 in the χ2 test [16], and I2 index values of > 50% indicated significant heterogeneity [17]. Random-effect models with the inverse variance method were applied to reflect the variability of effect sizes among included studies with diversity in adjunctive modalities [18]. Publication bias was evaluated using funnel plot asymmetry [19] based on the Egger and Begg tests [20,21]. We used funnel plots of standard error or diagnostic yield (logit transformed).
Results
1. Literature search and study selection
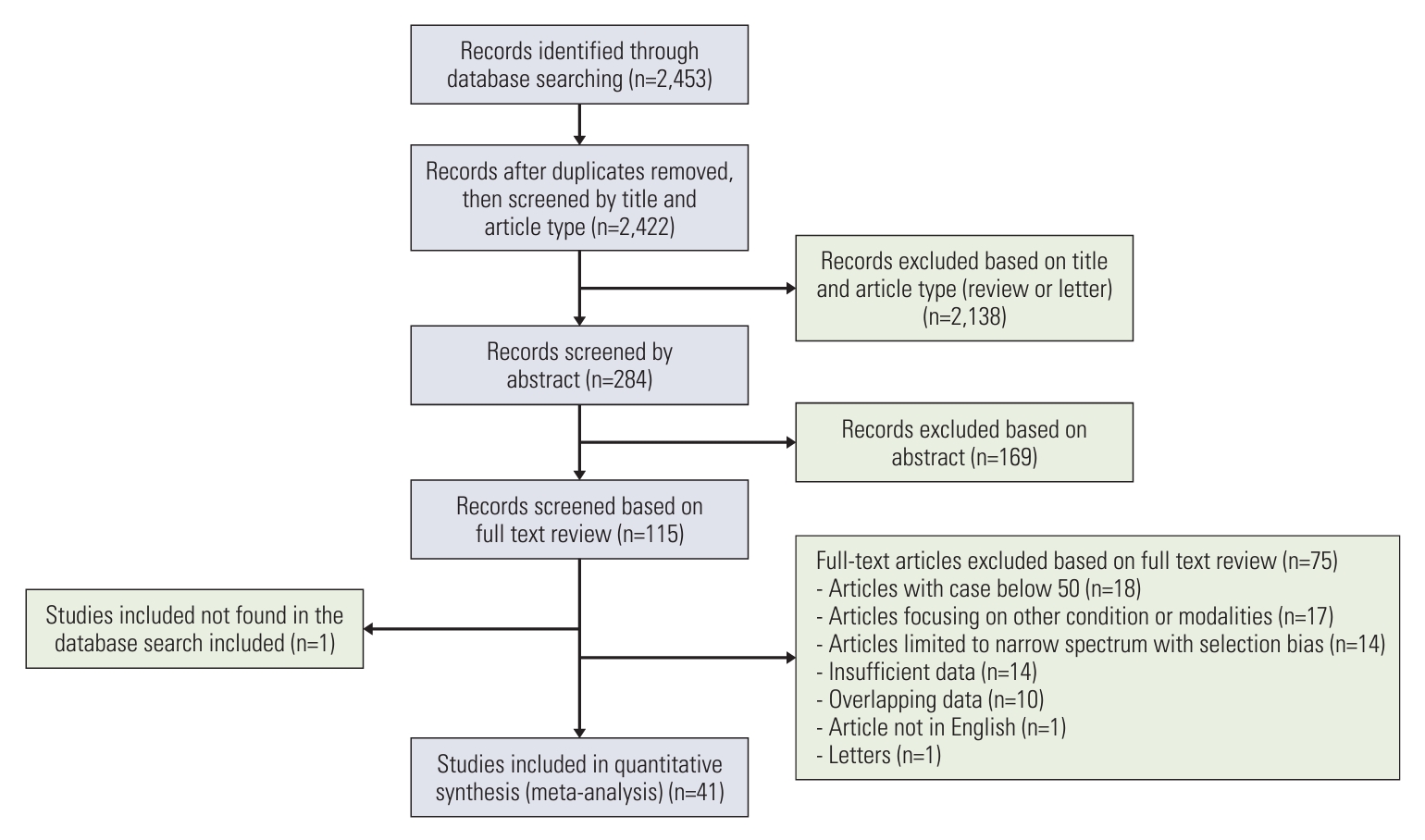
After removing duplicates, the search algorithm revealed 2,422 potentially relevant papers (Fig. 1). Following the abstract review, 115 articles were selected for full-text review. Of these, 75 articles were excluded according to the exclusion criteria, and one study missed in the database search was added [22]. Therefore, 41 studies formed the basis of our systematic review [22-62].
2. Study description
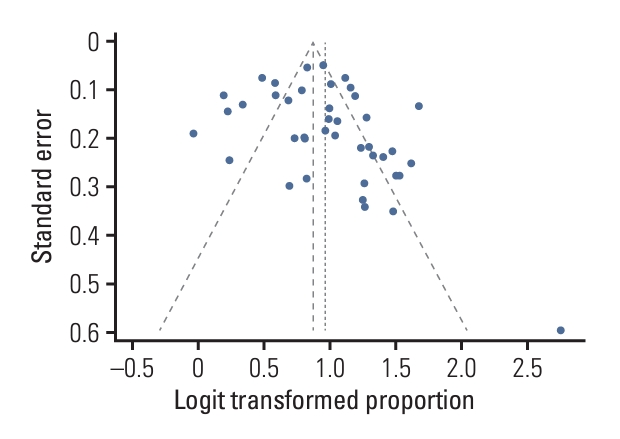
A total of 13,133 PLLs were included. Table 2 lists the study characteristics and summarizes their features [22-62]. Overall, 13 studies were randomized controlled trials, seven were prospective, and 21 were retrospective studies. The prevalence of malignancy was reported in 35 studies (median, 78%; interquartile range, 68 to 83). Among them, 15 studies showed a prevalence of malignancy ≤ 75%, whereas 20 studies showed > 75%. There was variation in additional guidance devices used among included studies, such as GS (31 studies), Flu (26 studies), VBN (16 studies), ultrathin bronchoscopy (5 studies), and ENB (1 study). S1 Table provides a quality assessment of all included studies based on QUADAS-2. The overall analysis showed good performance in the patient selection and index test criteria. However, it showed poor performance in the reference standard in addition to flow and timing criteria, which indicates the potential for significant bias. The funnel plot (Fig. 2) was not asymmetric, with both Egger’s (p=0.156) and Begg’s tests (p=0.103) showing insignificant p-values, indicating the absence of publication bias.
3. Test performance: meta-analysis
The inverse variance weighted overall diagnostic yield was 0.72 (95% CI, 0.70 to 0.75) (Fig. 3) [22-62]. The diagnostic yield among studies ranged from 0.49 to 0.94. The χ2 value of 293.38 (p < 0.01) and I² index of 86.4% indicated substantial heterogeneity across studies. The pooled sensitivity and specificity for malignant PLLs in 30 studies were 0.76 (95% CI, 0.72 to 0.79) and 0.98 (95% CI, 0.96 to 0.99), respectively [22-25,29,31-35,37-46,48,49,51,55-57,59-62].
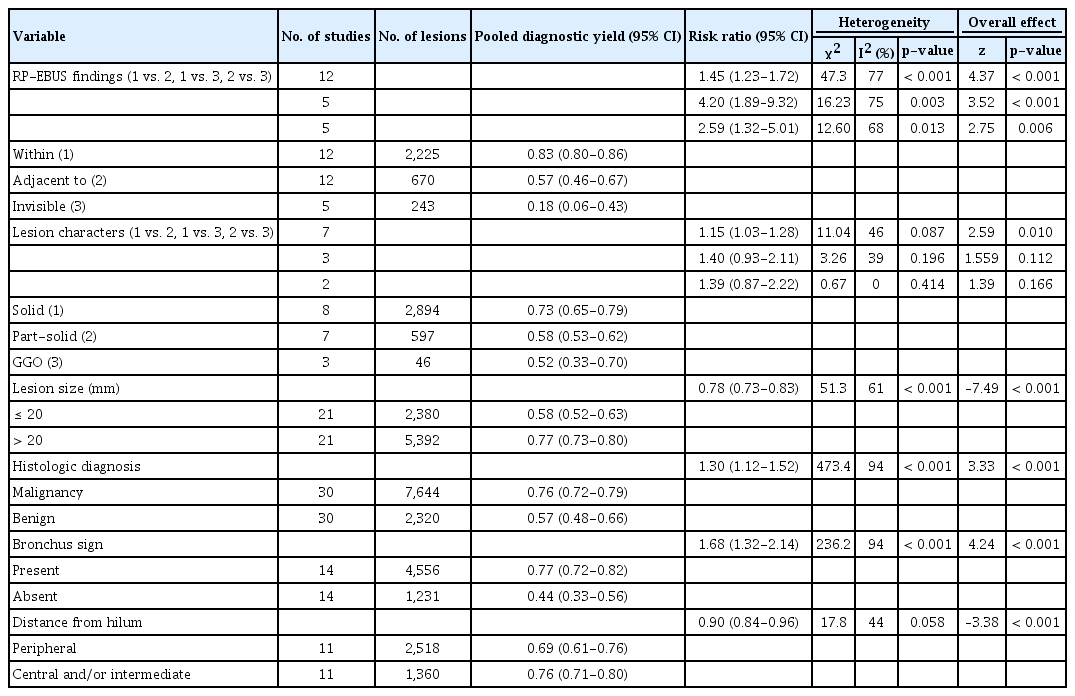
The factors related to diagnostic yield were further evaluated by subgroup meta-analysis. Regarding trichotomous variables (Table 3), there were significant differences in the pooled diagnostic yield based on RP-EBUS findings, where RR was 1.45 (95% CI, 1.23 to 1.72) between within and adjacent to in 12 studies, 4.20 (95% CI, 1.89 to 9.32) between within and invisible in five studies, and 2.59 (95% CI, 1.32 to 5.01) between adjacent to and invisible in five studies (Fig. 4A-C) [28,31,38,42,45-47,52,53,55,61,62]. As for lesion characters (Table 3), there was a significant difference in the pooled diagnostic yield between solid and part-solid, where RR was 1.15 (95% CI, 1.03 to 1.28) in seven studies. However, there was a difference in the pooled diagnostic yield without significance in the other variables, where RR was 1.40 (95% CI, 0.93 to 2.11) between solid and GGO in three studies, and 1.39 (95% CI, 0.93 to 2.11) between part-solid and GGO in two studies (S2A-S2C Fig.) [22,34,42,53,55,56,60,61].
Regarding dichotomous variables (Table 3), the pooled diagnostic yield was significantly different based on lesion size in 21 studies (≤ 20 vs. > 20 mm: RR, 0.78; 95% CI, 0.73 to 0.83) (S3A Fig.) [22,24,26,27,31-35,37,41,42,45,46,48,53,55,59-62], histologic diagnosis in 30 studies (malignancy vs. benign: RR, 1.30; 95% CI, 1.12 to 1.52) (S3B Fig.) [22-25,29,31-35,37-46,48,49,51,55-57,59-62], CT bronchus sign in 14 studies (present vs. absent: RR, 1.683; 95% CI, 1.32 to 2.14) (S3C Fig.) [22,27,28,30,33-35,40,45,53,56,60-62], and distance from the hilum in 11 studies (peripheral vs. non-peripheral: RR, 0.90; 95% CI, 0.84 to 0.96) (S3D Fig.) [22,30,35,37,40,45,55,56,60-62].
4. Test performance based on technologies
Regarding frequently used adjunctive modalities (Table 4), Flu+GS was the most commonly used combination during TBB using RP-EBUS (13 studies) [25,29-32,37,39,42,44,47,50,51,58], followed by Flu+GS+VBN (10 studies) [22,24,30,35,40,54,56,57,60,62], GS only (seven studies) [26,27,34,43,46,49,53], and no adjunctive modalities (six studies) [23,28,33,41,45,52]. The pooled diagnostic yields of adjunctive modalities were 0.73 (95% CI, 0.67 to 0.79) for Flu+GS+VBN, 0.73 (95% CI, 0.67 to 0.78) for Flu+GS, 0.72 (95% CI, 0.68 to 0.76) for GS only, and 0.70 (95% CI, 0.62 to 0.76) for no adjunctive modalities (p=0.903) (S4 Fig.). Regarding a single adjunctive modality, the pooled diagnostic yield was higher without statistical significance for GS (0.72; 95% CI, 0.69 to 0.75) compared with non-GS (0.70; 95% CI, 0.62 to 0.78) (p=0.658), VBN (0.74; 95% CI, 0.69 to 0.79) compared with non-VBN (0.71; 95% CI, 0.68 to 0.74) (p=0.356), but not for Flu (0.73; 95% CI, 0.69 to 0.77) compared with non-Flu (0.73; 95% CI, 0.69 to 0.76) (p=0.929) (S5A-S5D Fig.). Regarding bronchoscope type, the pooled diagnostic yields of thin (22 studies) [22,24,30,34,35,37-42,44-46,48,50,53,57,58,60-62], standard (12 studies) [23,27-29,32,33,36,44,50,52,54,59], and ultrathin (four studies) [22,35,55,57] bronchoscopes were 0.73 (95% CI, 0.68 to 0.78), 0.71 (95% CI, 0.66 to 0.75), and 0.73 (95% CI, 0.69 to 0.77), respectively (p=0.715) (S6 Fig.). Regarding the biopsy method, the pooled diagnostic yield of cryobiopsy (six studies) [47,52,54,57,59,61] was 0.78 (95% CI, 0.70 to 0.85) (one study, not evaluable due to insufficient data) [60].
5. Complication rates
Complication rates were 6.8% (726 out of 10,700 lesions) across 34 studies [22,23,26-36,39-43,45-49,51-55,57-62], whereas seven studies [24,25,37,38,44,50,56] did not report complication rates (Table 2). Among 34 studies, no complication was noted in one study [55]. The most common complication was bleeding, with a pooled rate of 4.5% (482 out of 10,700). The pooled rate of pneumothorax was 1.4% (148 out of 10,700), whereas that of chest tube insertion was 0.3% (33 out of 10,700). The pooled infection rate (including pneumonia) was 0.4% (45 out of 10,700). There were two cases of lifethreatening complications without death (one myocardial infarction and one severe bleeding). No death was reported in any study.
Discussion
To our knowledge, this is the first updated meta-analysis investigating the performance of RP-EBUS for diagnosing PLLs, including 13,133 PLLs from recent publications in 10 years. Our study showed the overall diagnostic yield of RP-EBUS as 0.72. Moreover, subgroup analysis showed that lesion size, histologic diagnosis, RP-EBUS findings, CT bronchus sign, lesion character, and distance from the hilum were associated with the performance of TBB using RP-EBUS. Lastly, our study showed a good safety profile during TBB using RP-EBUS, where the overall complication rate was 6.8% (bleeding, 4.5%; pneumothorax, 1.4%).
RP-EBUS finding was previously reported as an important factor for the diagnostic yield, which correlates with our study results. Tay et al. [25] reported that visualized PLLs on RP-EBUS were associated with significantly higher diagnostic yield than invisible PLLs on RP-EBUS (0.66 vs. 0.20; p=0.001). Among visualized PLLs on RP-EBUS, Ali et al. [63] reported significantly higher diagnostic yield in within oriented lesions compared to that of adjacently oriented lesions on RP-EBUS (0.79 vs. 0.52; p < 0.001). In line with this, the presence of CT bronchus sign was also known to be associated with the diagnostic yield, which correlates with our study finding. The same group also reported significantly higher diagnostic yield in PLLs with bronchus sign compared to that of PLLs without bronchus sign on CT (0.77 vs. 0.52, p=0.008) [63]. This might be attributed to the fact that the presence of CT bronchus sign is significantly associated with the visualization of the lesion on RP-EBUS (odds ratio [OR], 31.1; p < 0.001) and within the orientation of RP-EBUS to the lesion (OR, 44.8; p < 0.001) [27]. Therefore, TBB with RP-EBUS is highly recommended in within oriented or CT bronchus sign present PLLs.
Our study also showed that smaller size (≤ 2 cm), benign, and peripheral location were associated with significantly lower diagnostic yield during TBB using RP-EBUS. A previous meta-analysis showed that the diagnostic yield of PLLs ≤ 2 cm and > 2 cm were 0.61 and 0.76, respectively (p < 0.001) [63]. This might be attributed to lower visualization of PLLs ≤ 2 cm compared to PLLs > 2 cm on RP-EBUS (0.49 vs. 0.90, p < 0.001) [64]. Furthermore, the previous meta-analysis also showed that the diagnostic yields of malignant PLLs and benign PLLs were 0.72 and 0.60, respectively (p=0.018) [63]. This might be associated with a higher visualization rate in malignant PLLs compared to benign PLLs on RP-EBUS (0.85 vs. 0.66, p=0.025), which could be attributed to distinct features of bronchial invasion and airway distortion in malignant PLLs compared to ill-defined borders with subtle changes in benign PLLs, such as inflammation [25,32]. Lastly, Huang et al. [64] showed that the diagnostic yield of peripheral and central PLLs were 0.50 and 0.94, respectively (p < 0.001), which might be attributed to differences in accessibility regarding RP-EBUS scanning and sampling. Therefore, to improve the diagnostic yield in PLLs with the above factors, our study highlights the need for newer technologies in the field of bronchoscopic TBB.
Regarding subgroup analysis of the frequently used adjunctive modalities, our study showed that the diagnostic yield of RP-EBUS with GS (0.72) was slightly higher than that of RP-EBUS alone (0.70), as well as that of the GS group (0.72) compared with that of the non-GS group (0.70). GS enables accurate, repeated sampling at the same lesion after fixation at PLLs, and also prevents excessive bleeding by wedging GS after TBB [6]. In a recent study by Oki et al. [60], GS showed significantly higher diagnostic yield compared to non-GS (0.55 vs. 0.74, p=0.033), where interaction was especially evident based on lobar locations for GS (upper, 0.63 vs. lower, 0.46; p=0.004) and non-GS (upper, 0.43 vs. others, 0.50; p=0.197) (p-interaction=0.003). However, due to the small diameter of GS (SG-200C; Olympus; external diameter, 1.95 mm) for thin bronchoscopes, the use of biopsy tools for larger samples such as standard forceps (1.8 or 1.9 mm) is limited. This can be overcome by additional TBB by the non-GS method following the GS method, which is beneficial, especially in part-solid or GGO nodules [37,50]. Furthermore, there is a technical issue such as kinking of GS, which interrupts the introduction of biopsy tools, which can be overcome by parallel alignment of the bronchoscope to GS and advancement of the bronchoscope to PLLs as close as possible [42].
For the diagnosis of PLLs, transthoracic needle aspiration/biopsy (TTNA/B) is another alternative method that shows a high diagnostic yield (> 90%) [4]. However, it is associated with a higher risk of complications, especially pneumothorax (any pneumothorax, 15%; pneumothorax requiring a chest tube, 6.6%) [4]. Therefore, TTNA/B could be recommended for PLLs, especially those with pleural contact, but should be performed with caution considering the high complication rates, especially in patients with chronic obstructive pulmonary disease, pleural effusion, and interstitial lung disease [4]. The diagnostic yield of TBB with RP-EBUS may be relatively low, but recent advances in the field of bronchoscopy have improved it. Ultrathin bronchoscopy showed improved RP-EBUS positioning for PLLs, as well as an improved diagnostic yield of PLLs, even in peripheral locations from the hilum and upper lobes with an acute angle [15]. In a recent meta-analysis by Folch et al. [65] on ENB, RP-EBUS combined with ENB showed a pooled sensitivity of 0.80 (95% CI, 0.74 to 0.83) in PLLs suspected of lung cancer, which was higher than that with ENB alone (0.72; 95% CI, 0.66 to 0.76). Cryobiopsy is characterized by large tissue samples with good quality, which is associated with improved diagnostic yield, even in PLLs ≤ 2 cm and adjacent to findings on RP-EBUS [66,67]. Thus, TBB could be a reasonable modality in patients, especially those with a positive bronchus sign and a high risk of complications during TTNA/B.
Although our study includes recent publications from the past 10 years, we reported the diagnostic yield of PLLs as 0.72, which does not appear to be significantly different from the 0.71 reported in the subgroup analysis of RP-EBUS in a previous meta-analysis [7]. Possible explanations are as follows. First, our study included a higher proportion of studies with a low risk of bias (n=16/41, 0.39) compared to the previous meta-analysis (n=5/19 [one study not reported], 0.26) [7]. Studies with a lower risk of bias are known to be associated with a significantly lower diagnostic yield compared to those with a high risk of bias (0.66 vs. 0.71, p=0.018) [68]. Thus, the diagnostic yield in the previous meta-analysis might have been overestimated, thereby hampering the accurate evaluation of the diagnostic yield of RP-EBUS between the recent and past publications. Second, the included studies evaluating new modalities, such as cryobiopsy (seven studies), ENB (one study), and ultrathin bronchoscope (five studies), were relatively limited in number. A relatively small number of included studies on these factors might have led to the underestimation of the accurate diagnostic yield of PLLs.
Our study has some limitations. First, the quality of the included studies was inconsistent, which might be attributed to different designs, patient selection, and reference standards, including the radiological follow-up period. This might have led to heterogeneity in the diagnostic yield among the included studies. Second, the number of studies on GGO of lesion character was also relatively low, which could hamper accurate evaluation of overall diagnostic yield and RR compared with other variables of lesion character. Third, our study excluded articles not available in English, systematic reviews, and conference abstracts due to the possibility of low study quality. However, this could have an influence on publication bias. Fourth, our study could not collect data on the grade of bleeding due to the heterogeneity of the included studies, which limited analysis of clinically meaningful bleeding.
In conclusion, our study showed that TBB with RP-EBUS is an accurate diagnostic tool for diagnosing PLLs with good safety profiles, especially in PLLs with within orientation on RP-EBUS or the CT bronchus sign.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
An ethics statement is not applicable because this study is based exclusively on the published literature.
Author Contributions
Conceived and designed the analysis: Eom JS.
Collected the data: Kim SH, Chung HS, Kim I, Eom JS.
Contributed data or analysis tools: Kim SH, Kim J, Kim MH, Lee MK, Kim I, Eom JS.
Performed the analysis: Kim SH, Kim J, Kim MH, Lee MK, Kim I, Eom JS.
Wrote the paper: Kim SH, Eom JS.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
This work was supported by a clinical research grant from Pusan National University Hospital in 2024, the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF-2022R1F1A1074117), and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR20C0026).