Incidence and Features of Lymphoid Proliferation and Lymphomas after Solid Organ or Hematopoietic Stem Cell Transplantation in a National Database Cohort
Article information
Abstract
Purpose
Post-transplantation lymphoproliferative disorders (PTLDs) after hematopoietic stem transplantation (HCT) or solid organ transplantation (SOT) result in poorer outcomes, including death. There are limited large cohort data on the incidence and natural course of PTLD in Asians.
Materials and Methods
We investigated PTLD using Korean national health insurance claims data of 47,518 patients who underwent HCT or SOT in 2008-2020. Patient demographics, time and type of PTLD diagnosis, type of PTLD treatment, and death data were collected. We used Fine and Gray subdistribution hazard models to calculate the cumulative incidence and risk factors for PTLD.
Results
During median follow-up of 5.32 years, PTLD occurred in 294 of 36,945 SOT patients (0.79%) and 235 of 10,573 HCT patients (2.22%). Cumulative incidence of PTLD were 0.49% at 1 year, 1.02% at 5 years, and 1.50% at 10 years post-transplantation. Age < 20 years (subdistribution hazard ratio [SHR] of 1.67 in age 10-19, SHR 1.51 in age 0-9), HCT (SHR 3.02), heart transplantation (SHR 2.27), and liver transplantation (SHR 1.47) were significant risk factors for PTLD. The presence of PTLD was associated with an increased risk of death (hazard ratio of 2.84). Overall, 5-year survival of PTLD patients was 68.9% (95% confidence interval, 64.9 to 73.2).
Conclusion
We observed a steady increase in PTLD over 10 years after HCT or SOT in this large cohort study. Pediatric age group, HCT, liver transplantation, and heart transplantation were suggested to be risk factors for PTLD, and PTLD was associated with a higher risk of death.
Introduction
Post-transplantation lymphoproliferative disorders (PTLDs) are defined as abnormal lymphoid proliferations associated with immunosuppression after hematopoietic stem cell transplantation (HCT) or solid organ transplantation (SOT) [1,2]. The nomenclature for PTLD has undergone major changes in the recent edition of the World Health Organization Classification of Haematolymphoid Tumors [3]. Histologic features of lymphoid proliferation are noted first, regardless of etiology, followed by categorization based on background immune deficiency/dysregulation and viral associations. As noted in the classification, PTLD includes lymphoid proliferations with heterogeneous morphologic features, including hyperplasias, polymorphic disorders, and other lymphoproliferative subtypes.
Epstein-Barr virus (EBV) is a major factor contributing to the oncogenic process of PTLD, and the high incidence of primary EBV infection after transplantation leads to generally higher rates of PTLD in children than in adults. Adults with EBV reactivation also exhibit an increased incidence of lymphoid proliferation, although 20%-50% of PTLD patients are EBV-negative, with their tumors caused by other viruses or unknown factors [2,4,5].
Initial treatment of early lesions and polymorphic PTLD consists of reducing immune suppression. Rituximab, administered with or after multi-agent chemotherapy, has shown success in treating CD20-positive B cell PTLD [6,7], and conventional chemotherapy is recommended for CD20-negative B cell PTLD, T cell PTLD, and Hodgkin lymphoma [1,2,7]. Moreover, EBV surveillance and preemptive interventions are widely recommended for patients at high risk of developing PTLD [2,7].
Data regarding PTLD in Asian populations are limited to only case series and a few large cohort studies [8-11]. Although it has been suggested that there is a predominance of late-onset PTLD in Asians because of differences in EBV seroprevalence, features of PTLD are mostly undefined [8]. No large-scale study of PTLD has included patients who have undergone either HCT or SOT. Therefore, we used the Korean National Health Insurance Review Assessment (HIRA) database to describe the incidence and characteristics of PTLD in a large national cohort of Asians. Risk factors for PTLD and prognosis of patients with PTLD were also investigated in transplanted organ–based groups.
Materials and Methods
1. Data source
This nationwide, retrospective, cohort study was based on inpatient and outpatient claims data from the HIRA database between 2008 and 2020. HIRA is a health insurance claims database covering approximately 98% of the Korean population. It contains information on patient demographics, diagnoses, procedures, and treatments (healthcare services and inpatient and outpatient prescriptions) [12]. Diagnoses are based on the International Classification of Diseases, 10th revision (ICD-10) diagnostic codes. All patient and healthcare provider identifying information is encrypted in HIRA to protect personal information.
2. Study population
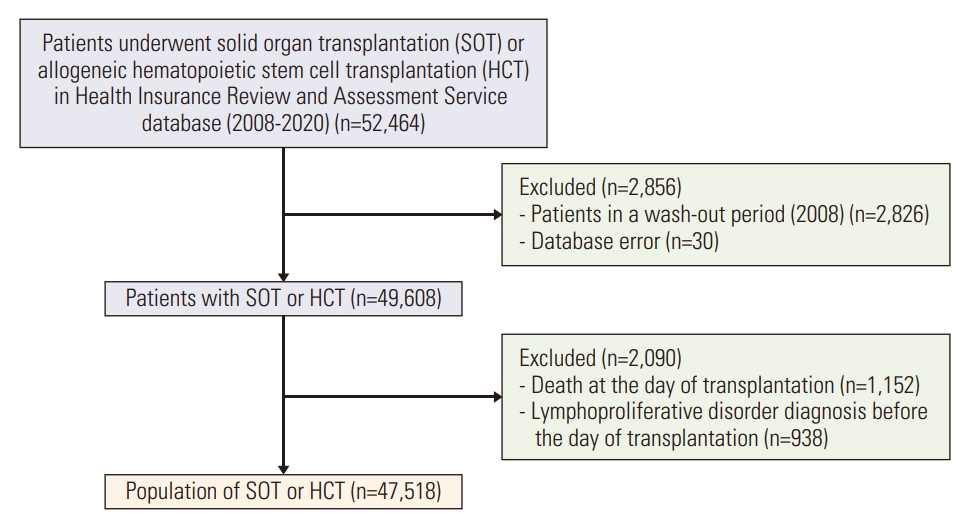
All individuals who underwent allogeneic HCT or SOT during 2008-2020 were considered for inclusion (n=52,464) (Fig. 1). For patients who underwent ≥ 2 transplantations, only the first procedure (HCT or SOT) was considered in the analysis. We excluded patients who underwent ≥ 2 SOTs on the same day. We used a 1-year washout period to exclude patients with a preexisting transplanted organ (n=2,826). Other exclusion criteria were death on the day of transplantation and lymphoproliferative disorder diagnosed before transplantation. S1 Table shows the ICD-10 codes used to identify transplantation type and PTLD diagnosis. The final study population included 47,518 patients who underwent HCT or SOT.
3. Covariates
The patients were divided into age groups based on 10-year intervals for pediatric patients and 20-year intervals for adults aged < 60 years. The SOT group was divided into kidney, liver, heart, lung, and “other” (intestine or pancreas) transplantation. PTLD was divided into five groups: Hodgkin lymphoma, mature B cell lymphoma, mature T/natural killer (NK) cell lymphoma, myeloma, and plasmacytomalike PTLD, and unspecified type of non-Hodgkin lymphoma or lymphoproliferative disorder. We extracted records of rituximab and chemotherapeutic agents (drugs approved for lymphoma by the Korea Ministry of Food and Drug Safety) prescribed after PTLD diagnosis. S1 Table shows the medication ICD-10 codes.
4. Statistical analysis
Baseline patient characteristics were summarized using median (first quartile, third quartile) for continuous variables and frequency (%) for categorical variables. Patients with or without PTLD were compared using the Mann-Whitney U test for continuous variables and chi-squared test for categorical variables. We followed all patients from the transplantation date to the date of PTLD diagnosis, date of death, or study end date (December 31, 2020), whichever occurred first.
The cumulative incidence of PTLD was estimated using Fine and Gray subdistribution hazard models, with death considered a competing event [13]. We compared cumulative PTLD incidences across subgroups (based on sex, age at transplantation, transplantation type [SOT vs. HCT], and SOT type) using Gray’s test. We examined risk factors associated with PTLD after HCT or SOT using Fine and Gray subdistribution hazard models, with sex, age at transplantation, and transplantation type considered potential risk factors. We performed univariable analysis for each variable and multivariable analysis to examine the effect of each factor after adjusting for the other factors. We included sex, age group, and transplantation type in the multivariable analysis. We used Cox proportional hazards models to investigate the association between PTLD and death, with PTLD diagnosis considered a time-varying covariate. We performed univariable and multivariable analyses to identify factors associated with mortality for all patients and each transplantation group. In multivariable analysis, PTLD, sex, and age group were included as variables. We further plotted the Kaplan-Meier curves to probe the prognosis of the PTLD patients, overall and by the type of PTLD. Two-sided p-values < 0.05 were considered statistically significant. All analyses were conducted using SAS Enterprise Guide ver. 7.1 (SAS Institute Inc., Cary, NC).
Results
1. Patient characteristics
Table 1 shows the baseline characteristics of the 47,518 patients included in the study. The majority of patients were male (n=29,528, 62.1%) and in the 40-59 years age group (n=26,985, 56.8%). A total of 36,945 patients (77.8%) underwent SOT and 10,573 patients (22.3%) underwent HCT (S2 Table). HCT was more common than SOT in patients < 20 years of age, whereas SOT was more common than HCT in adults. Kidney transplantation was the most common transplantation (n=21,145, 44.5%), followed by liver transplantation (n=13,649, 28.7%) and HCT (n=10,573, 22.3%).
Median follow-up duration of all subjects was 5.32 years and was longer in patients without PTLD than in those with PTLD (5.36 vs. 1.37 years). Among the 47,518 individuals who underwent SOT or HCT, 529 (1.1%) developed PTLD during the study period. There was a higher proportion of mortality cases in the PTLD group (30.1%) than in those without PTLD (14.4%) (p < 0.001).
2. Cumulative incidence of PTLD
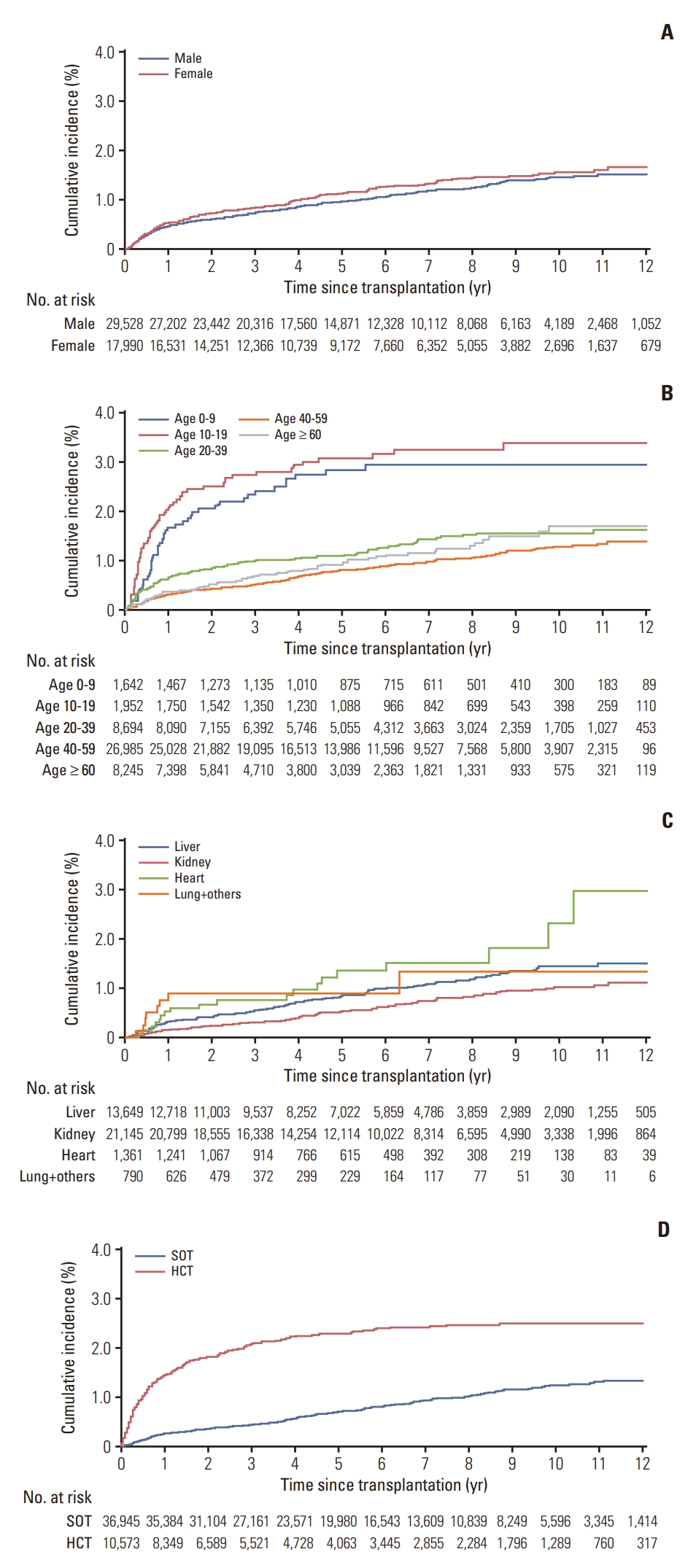
The cumulative incidence of PTLD was 0.49% at 1 year and 1.02% at 5 years among all patients. The cumulative incidence of PTLD was slightly lower in males than in females, but the difference was not statistically significant (p=0.184) (Fig. 2). The pediatric population had a higher cumulative incidence of PTLD than adults (p < 0.001). The highest incidences were found in the 10-19 years age group (2.0% at 1 year and 3.03% at 5 years after transplantation). The < 10 years age group had the second highest cumulative incidences of PTLD (1.64% and 2.79% at 1 and 5 years, respectively). Cumulative incidences of PTLD were higher in the HCT group (1.39% at 1 year and 2.27% at 5 years) than in the SOT group (0.23% at 1 year and 0.67% at 5 years) (p < 0.001). Among types of SOT, kidney transplantation was associated with the lowest incidences of PTLD (0.14% at 1 year and 0.52% at 5 years), whereas heart and lung plus other transplantations were associated with the highest cumulative incidences of PTLD (p < 0.001). S3 Table shows the cumulative incidences of PTLD among all patients, as well as in sex, age, type of transplantation, and type of SOT groups, at 1, 5, and 10 years posttransplantation.
3. Risk factors for PTLD and death
In univariable analysis, the age group with the highest risk for PTLD was the 10-19 years group, with a subdistribution hazard ratio (SHR) of 2.32 (95% confidence interval [CI], 1.69 to 3.18). Among types of transplantation, HCT (SHR, 3.46; 95% CI, 2.80 to 4.28), other transplantation (SHR, 3.28; 95% CI, 1.04 to 10.33), heart transplantation (SHR, 2.47; 95% CI, 1.53 to 3.97), and liver transplantation (SHR, 1.47; 95% CI, 1.16 to 1.87) were significant risk factors for PTLD (compared to kidney transplantation) (Table 2). In multivariable analysis, the following were risk factors for PTLD: 10-19 years age group (SHR, 1.67; 95% CI, 1.21 to 2.31), < 10 years age group (SHR, 1.51; 95% CI, 1.05 to 2.17), HCT (SHR, 3.02; 95% CI, 2.40 to 3.79), heart transplantation (SHR, 2.27; 95% CI, 1.41 to 3.66), and liver transplantation (SHR, 1.47; 95% CI, 1.15 to 1.88).
In multivariable analysis, the presence of PTLD was associated with a significantly increased risk of death (hazard ratio [HR], 2.84; 95% CI, 2.40 to 3.36; p < 0.001) (S4 Table). Although sex was not a risk factor of PTLD, males had significantly worse survival than females after transplantation (p < 0.001). Older age was also a risk factor for death, with pediatric patients having better survival, although they had a higher incidence of PTLD. Kidney transplantation patients had better survival than other transplant recipients, except for those who underwent “other” (intestine or pancreas) transplantation.
We also examined risk factors for PTLD and death according to transplantation type (S5-S8 Tables) Although pediatric patients exhibited an overall higher risk of PTLD, this increased risk was not statistically significant after kidney transplantation. However, patients aged < 10 years who underwent liver transplantation and patients aged 10-19 years who underwent HCT did have significantly increased risks of PTLD. Kidney transplantation was associated with a higher risk for death in patients ≥ 40 years of age. In patients who underwent liver transplantation, the 20-39 years age group had a higher risk for death compared with the pediatric age groups but a lower risk for death than the ≥ 60 years age group. Among subjects who underwent HCT, the 20-39 years age group also had a higher risk for death compared with pediatric patients and a lower risk for death compared with both the 40-59 years and ≥ 60 years groups.
4. PTLD types, treatment, and survival
Among the 529 subjects diagnosed with PTLD, mature B cell lymphoma was the most common type of PTLD (n=260, 49.2%), followed by unspecified type of non-Hodgkin lymphoma or lymphoproliferative disease (n=142, 26.8%). Myeloma and plasmacytoma-like PTLD developed in 86 patients (16.3%), mature T/NK cell lymphoma in 24 patients (4.5%), and Hodgkin lymphoma in 17 patients (3.2%). PTLD was treated with chemotherapy alone in 132 patients (25.0%), rituximab alone in 46 patients (8.7%), and chemotherapy plus rituximab in 149 patients (28.2%) (S9 Table). Overall survival of the PTLD population was 76.9% (95% CI, 73.4 to 80.6) at 1 year and 68.9% (95% CI, 64.9 to 73.2) at 5 years, with no differences in survival between types of PTLD (S10 Fig.).
Discussion
In this large cohort of 47,518 individuals who underwent HCT or SOT, the 5-year cumulative risk of PTLD was 2.27% after HCT and 0.67% after SOT. Patient age and type of transplanted organ affected the incidence of PTLD. Furthermore, the presence of PTLD increased the risk of death after HCT or SOT.
The incidence of PTLD has been reported in several large cohort studies. With HCT, the incidence ranges widely, according to different settings. Among 18,014 patients in the International Bone Marrow Transplant Registry and Fred Hutchinson Cancer Research Center (FHCRC) registry, the cumulative incidence of PTLD was 1.0% at 10 years [14]. A Japanese group reported a 0.79% incidence of PTLD at 2 years among 40,195 patients > 16 years of age [11]. The cumulative incidence among 26,901 patients in the data from the Center for International Blood and Marrow Transplant Research registry and FHCRC was 0.4% overall, although it varied from 0.2% to 8.1% according to risk factors [15]. The European Group for Blood and Marrow Transplantation reported a wide range of PTLD incidences, according to the type of HCT donor [16]. Our 1-year, 5-year, and 10-year cumulative incidence rates of PTLD after HCT were slightly higher or similar to the rates in previous reports. Our slighter higher rate may be attributed to our inclusion of all possible diagnoses of PTLD, including not only non-Hodgkin lymphoma (the most common type of PTLD) but also other diseases such as Hodgkin lymphoma and plasma cell disorders. Moreover, in long-term studies of Western populations, the cumulative incidence of PTLD after HCT increased steeply within approximately 1 year after transplantation and then plateaued, whereas in our study, we observed a steady gradual increase in incidence of PTLD over a 12-year period. This suggests that later-onset PTLD may be more common in our Asian population.
We also observed an increased risk of PTLD after HCT in the 10-19 years age group, which is another unique result of this study. Little is known about age at transplantation as a risk factor for PTLD, but age < 18 years was suggested as a risk factor (odds ratio, 3.76) in one single-center study, and age > 50 years was found to be a risk factor (relative risk, 5.1) in another large cohort data [15,17]. Although we were unable to examine differences in EBV infection according to age group or other risk factors of PTLD in this large database study, primary EBV infection may have influenced the higher risk of PTLD in our pediatric population [5,18].
In this study, we included a large number of individuals (1,322 pediatric patients and 35,623 adults) who underwent various types of SOT. Our cumulative incidence rates of PTLD after each type of organ transplantation were comparable to previously reported rates. However, as with HCT recipients, the cumulative incidence of PTLD after SOT continuously increased over the study period, in contrast to data from Western cohorts [19].
Various registry studies of kidney transplantation (which was the most common SOT in our study population) have shown PTLD incidence rates ranging from < 1% to 3.6% [19-21]. These rates are similar to our rates of 0.14%, 0.52%, and 1.01% at 1 year, 5 years, and 10 years after kidney transplantation. The incidence of PTLD after kidney transplantation has a bimodal age distribution, with peaks in pediatric patients and older individuals [21-23]. In this report, patients < 10 years of age had a higher risk of PTLD after kidney transplantation compared with adults, although the difference did not reach statistical significance (SHR, 3.16; p=0.062).
For liver transplantation, registry data of pediatric patients who underwent transplantation in the United States and Canada showed 1.3% to 4.2% incidence rates of PTLD, with pre-transplantation EBV positive status, younger age at transplantation, and multiple rejection episodes as risk factors for PTLD [24]. PTLD is known to be more common in pediatric patients than in adults after liver transplantation, although few large cohort studies have directly compared different age groups [24]. In our study, the youngest age group (< 10 years) had a significantly higher SHR for PTLD than older ages. This suggests that children, who are more commonly EBV naïve, have a higher likelihood of PTLD than adults after liver transplantation. Although this concept is generally accepted, it is sometimes difficult to detect significant differences according to EBV status in studies with a small sample size [9].
Among SOT patients, heart and lung transplant recipients, who require intensive immune suppression, are known to have a higher incidence of PTLD [19,25-27]. In our cohort, patients who underwent lung or other organ transplantation had the highest cumulative incidence of PTLD in the first year after transplantation, while patients who underwent heart transplantation had the highest incidence at 5 years and 10 years after transplantation. Similar results were found in previous studies, with the type of immunosuppressants or transplantation of lymphoid tissue with the donor lung suggested as possible underlying mechanisms (although further investigations are required) [19,26]. However due to small number of pediatric transplant cases of lung or heart, we could not find the age as a risk factor in our cohort.
Male patients exhibited a higher HR of death compared to females; however, there was no evidence to suggest a sex-related prognosis difference after HCT or SOT. It should be noted that the data collected from the HIRA included not only disease-related deaths but also other causes of mortality. We hypothesized that the higher HR of death for males might be indicative of a lower life expectancy in men.
Patients with PTLD after SOT or HCT had worse outcomes than patients without PTLD. The HR for death in this study was 2.84 in the PTLD group, compared with the non-PTLD group. However, compared to 10-year survival data of lymphoma patients diagnosed during 2006-2014 in Korea (75.6%-77.3% for Hodgkin lymphoma and 54.4%-58.3% for non-Hodgkin lymphoma), the 10-year survival of patients with PTLD in our study was not remarkably low [28]. These survival data suggest that with appropriate treatment, patients with PTLD can achieve outcomes similar to those of lymphoma patients with no previous transplantation.
In our cohort, cumulative incidence of PTLD among SOT continuously increased over 12 years. PTLD that occurs 1 or 2 years after SOT or HCT is defined as late-onset PTLD. It is known that EBV-negative cases and old age is more related to the late-onset than in early-onset type [2,18,22]. There are few reports showing late-onset PTLD incidence in a long period. Our data showed more steady increase in PTLD cumulative incidence when we compare to that of recent British cohort data [19].
This study has limitations. First, use of registry data prevented us from defining EBV serology status before or after transplantation or at the time of PTLD diagnosis. Hence, we could not determine the influence of EBV in our study population, although EBV status is a critical marker for PTLD risk assessment, and preemptive monitoring of serum EBV DNA is required to predict outcomes of PTLD patients [2,5,9,11,18,19,21]. However, seropositivity rates of EBV in the Korean population reach 95% by adolescence and young adulthood [29], so it is likely that most patients in these age groups had prior EBV infection. We were also unable to collect data on types of immunosuppressants or detailed information regarding PTLD diagnosis and treatment, graft rejection or recurrence information that may have influenced patient prognosis. Additionally, the number of patients who underwent heart, lung, or other organ transplantation was relatively small compared to the number who underwent kidney transplantation, liver transplantation, or HCT. Moreover, age distribution of patients who underwent SOT or HCT was different. This may have over-emphasized the contribution of certain types of transplantation when considering all SOT and HCT patients. Finally, our data depended on the reliability of information entered into the HIRA database. Data entry errors may have occurred, and drugs used outside national insurance coverage were not accounted for.
Nevertheless, our study has the main strength of providing an overview of PTLD incidence and prognosis in nationwide data. Few studies have included patients who developed PTLD after SOT and HCT in the same cohort, and although some large-scale SOT studies examined general cancer risk, they did not focus on PTLD. All data in this study were obtained from the same population, the individuals of which share similar ethnic, environmental, and viral backgrounds and transplantation settings, reducing selection bias. Importantly, we observed a steady occurrence of lymphoproliferative disorders years after transplantation, implying that late-onset PTLD is an important issue in our population.
In conclusion, based on large-scale nationwide data, we were able to define the cumulative incidences of PTLD after HCT or SOT according to sex, age, and type of transplantation. Pediatric age group, HCT, liver transplantation, and heart transplantation were significant risk factors for the development of PTLD in the Korean population. Our results reflect the natural course of PTLD and provide valuable information for long-term follow-up in clinical settings, as well as for the design of further studies regarding PTLD.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study was approved by the Care Record Supply Committee of HIRA (M20210115945) and the Institutional Review Board/Ethics Committee of Severance Hospital, Yonsei University Health System. Patient consent was waived because of the study’s retrospective design and use of de-identified data.
Author Contributions
Conceived and designed the analysis: Hahn SM, Lee M, Hyun J, Lim S, Kang JM, Ahn JG, Joo DJ, Jung I, Ihn K.
Contributed data or analysis tools: Hahn SM, Lee M, Kang JM, Jung I, Ihn K.
Performed the analysis: Lee M, Jung I.
Wrote the paper: Hahn SM, Lee M.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This study was supported by a faculty research grant from the Department of Surgery of Yonsei University College of Medicine.