Expanded Access Program Pralsetinib in Advanced Non–Small Cell Lung Cancer with Rearranged during Transfection (RET) Gene Rearrangement
Article information
Abstract
Purpose
Rearranged during transfection (RET) gene rearrangement is a well-known driver event in non–small cell lung cancer (NSCLC). Pralsetinib is a selective inhibitor of RET kinase and has shown efficacy in oncogenic RET-altered tumors. This study evaluated the efficacy and safety of expanded access program (EAP) use of pralsetinib in pretreated, advanced NSCLC patients with RET rearrangement.
Materials and Methods
Patients who received pralsetinib as part of the EAP at Samsung Medical Center were evaluated through a retrospective chart review. The primary endpoint was overall response rate (ORR) per the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 guidelines. Secondary endpoints were duration of response, progression-free survival (PFS), overall survival (OS), and safety profiles.
Results
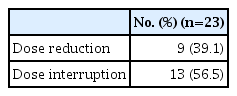
Between April 2020 and September 2021, 23 of 27 patients were enrolled in the EAP study. Two patients who were not analyzed due to brain metastasis and two patients whose expected survival was within 1 month were excluded from the analysis. After a median follow-up period of 15.6 months (95% confidence interval [CI], 10.0 to 21.2), ORR was 56.5%, the median PFS was 12.1 months (95% CI, 3.3 to 20.9), and the 12-month OS rate was 69.6%. The most frequent treatment-related adverse events (TRAEs) were edema (43.5%) and pneumonitis (39.1%). A total of 8.7% of patients experienced extra-pulmonary tuberculosis. TRAEs with a common grade of three or worse were neutropenia (43.5%) and anemia (34.8%). Dose reduction was required in nine patients (39.1%).
Conclusion
Pralsetinib presents a clinical benefit when used in patients with RET-rearranged NSCLC, consistent with a pivotal study.
Introduction
Lung cancer is a leading cause of death worldwide. In 2020, there were 2.21 million new cases of lung cancer, and nearly 1.80 million people died from the disease [1]. Non–small cell lung cancer (NSCLC) represents 85% of all lung cancer cases, and the majority of patients presents with advanced disease. Adenocarcinoma is the most common type of NSCLC and accounts for approximately 40% of lung cancers [2]. The development of therapies targeting oncogenic driver kinases has transformed the management of a variety of solid tumors. In lung adenocarcinomas, specific molecular changes (e.g., alterations in activation of the epidermal growth factor receptor [EGFR], anaplastic lymphoma kinase [ALK], and ROS proto-oncogene 1 receptor tyrosine kinase [ROS1] genes) are often present, and targeted tyrosine kinase inhibitor (TKI) therapy provides significant improvements in survival and quality [3].
Rearranged during transfection (RET) gene rearrangement is a well-known oncogenic driver activated in NSCLC, medullary thyroid cancer, and papillary thyroid cancer. RET rearrangements are present in approximately 1%–2% of NSCLCs, particularly adenocarcinoma, and lead to upregulation of RET genes. Constituent activation of the downstream signaling pathway is related to tumor invasion, migration, and uncontrolled cell proliferation [4]. RET fuses with various heterologous upstream partner genes in NSCLC. The most common fusions are KIF5B-RET, followed by CCDC6-RET and NCOA4-RET, although more than 35 genes have been reported to fuse with RET [5–7]. RET rearrangements tend to be mutually exclusive from other major NSCLC driver alterations such as Kirsten rat sarcoma virus (KRAS) or EGFR mutations and ALK or ROS1 gene rearrangements. Also, RET rearrangements are common in young never-smokers and are generally associated with a low tumor mutation load, low programmed death ligand 1 expression, and poor response to immunotherapies (IOs) [6,8].
Pralsetinib, also known as BLU-667, is a potent and selective inhibitor of RET kinase and has shown efficacy in oncogenic RET-altered tumors. In vitro and in vivo models have shown that pralsetinib was able to inhibit a wide spectrum of RET alterations, including KIF5B-RET, CCDC6-RET, and mutations encoding RET M918T, RET C634W, RET V804E, RET V804L, and RET V804M amino acid changes [6]. In the ARROW study, 87 patients with RET fusion–positive NSCLC previously treated with platinum-based chemotherapy were administered pralsetinib 400 mg once a day (QD). The resulting overall response rate (ORR) was 61% (95% confidence interval [CI], 50 to 71) and the median progression-free survival (PFS) was 17.1 months (95% CI, 8.3 to 22.1) at a median follow-up of 17.1 months (interquartile range [IQR], 14.6 to 20.3). In 27 treatment-naïve patients, the ORR was 19% (95% CI, 50 to 86), and the median PFS was 9.1 months at a median follow-up of 11.6 months (IQR, 11.0 to 17.3). The median OS was not reached in either subgroup [9].
The objective of this retrospective observational study was to assess the real-world efficacy and safety of pralsetinib in pretreated, advanced NSCLC patients with RET rearrangement who participated in the early access program (EAP) at Samsung Medical Center, Korea.
Materials and Methods
1. Patients and study design
The pralsetinib EAP was initiated in April 2020 to enable early access to pralsetinib for patients with RET rearrangement NSCLC who were unable to participate in clinical studies. We performed a retrospective analysis of the efficacy and safety of pralsetinib with RET-rearranged NSCLC patients enrolled in the pralsetinib EAP at Samsung Medical Center, Korea. We included patients aged 18 years or older with metastatic or unresectable pathologically confirmed NSCLC with RET rearrangement who received prior chemotherapy. There were no limitations on the quantity or type of prior systemic treatments. Patients with treated or asymptomatic brain metastases were also enrolled in the study.
Pralsetinib was provided by F. Hoffmann-La Roche Ltd. as a part of Global Expanded access program. Roche has permitted publication of this publication, but has no role in it.
2. Procedures
Patients received an initial oral dose of pralsetinib 400 mg QD. Patients were treated with pralsetinib in 28-day cycles until disease progression, unacceptable toxicity, patient withdrawal of consent, or death. We permitted continued treatment with pralsetinib for patients who developed disease progression if the investigator thought that clinical benefit was maintained. Dose reduction and interruption were permitted according to physician judgment.
Baseline computed tomography of the chest, abdomen, and pelvis was performed before the first dose of pralsetinib. Baseline brain imaging was not required. Disease progression was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1, and central nervous system (CNS) disease was evaluated by the Response Assessment of Neuro-Oncology (RANO) criteria [10]. Treatment-related adverse events were graded using the Common Terminology Criteria for Adverse Events (CTCAE) ver. 5.0.
Baseline characteristics, treatment history, efficacy, and safety outcomes were evaluated through retrospective chart review.
3. Outcomes
The primary endpoint was the investigator-assessed overall response rate (ORR) per RECIST ver. 1.1. ORR was defined as the proportion of patients with a confirmed complete response or partial response according to RECIST ver. 1.1. Secondary endpoints were duration of response (DOR), PFS, overall survival (OS), time to discontinuation (TTD), intracranial ORR (icORR), intracranial disease control rate (icDCR), and safety profiles.
Median DOR was defined as the time from the first response to pralsetinib and disease progression or death due to any cause. Median PFS was defined as the time from the first dose of pralsetinib and the first occurrence of disease progression or death. Median TTD was measured from the date of initiation of pralsetinib until the last dose of pralsetinib or death. Live patients who had not experienced treatment discontinuation were censored at the analysis cutoff date. Median OS is defined as the time between the start of pralsetinib treatment and the occurrence of death from any cause. We censored patients who were alive at the time of the last data cutoff at the time of the last follow-up.
4. Statistical analysis
Statistical analysis was performed using SPSS software, ver. 27.0 (IBM Corp., Armonk, NY). Median PFS, OS, DOR, and TTD were estimated from Kaplan-Meier curves. The log-rank test was used to test comparison between subgroups. The statistical significance level was set at p < 0.05 for all tests.
Results
1. Patients and treatment
Between April 2020 and September 2021, 27 patients with advanced or metastatic RET-fusion-positive NSCLC received pralsetinib at Samsung Medical Center, Korea. Data are presented up to December 2022. Two patients who were not measurable due to brain metastasis and two patients whose expected survival was within 1 month were excluded from the analysis. Overall, 23 patients were included and all had been previously treated. The median number of prior systemic therapy cycles was two (range, 1 to 8) (Table 1).
The median age at pralsetinib start was 51 years (range, 29 to 74 years), and 14 patients were female (60.9%). Most patients were never-smokers (n=17, 73.9%), four patients (17.4%) were ex-smokers, and two patients (8.7%) were current smokers. The majority of patients (n=20, 87.0%) had adenocarcinoma histology.
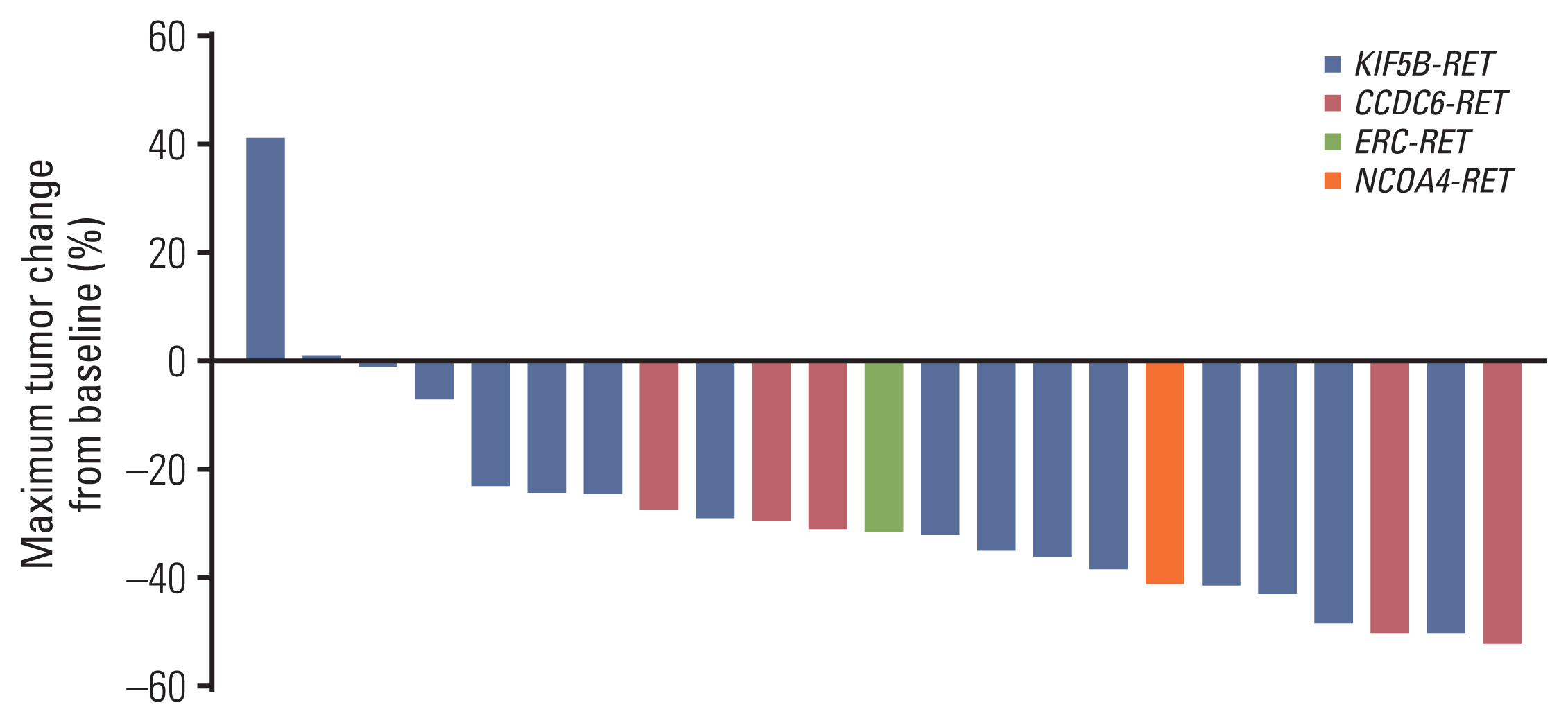
In molecular profiling with next-generation sequencing, concurrent activating alterations including EGFR, ALK, and ROS1 were not revealed. The RET-fusion was confirmed using the commercial next-generation sequencing panels CancerSCAN, Oncomine Focus Assay or Trusight oncology 500, or Guardant 360. The most common RET fusion identified was KIF5B-RET in 16 patients (69.6%) followed by CCDC6-RET in five patients (21.7%). Brain metastases were identified in 11 patients (47.8%) and liver metastases were identified in three patients (13.0%) at the time of pralsetinib treatment.
2. Efficacy
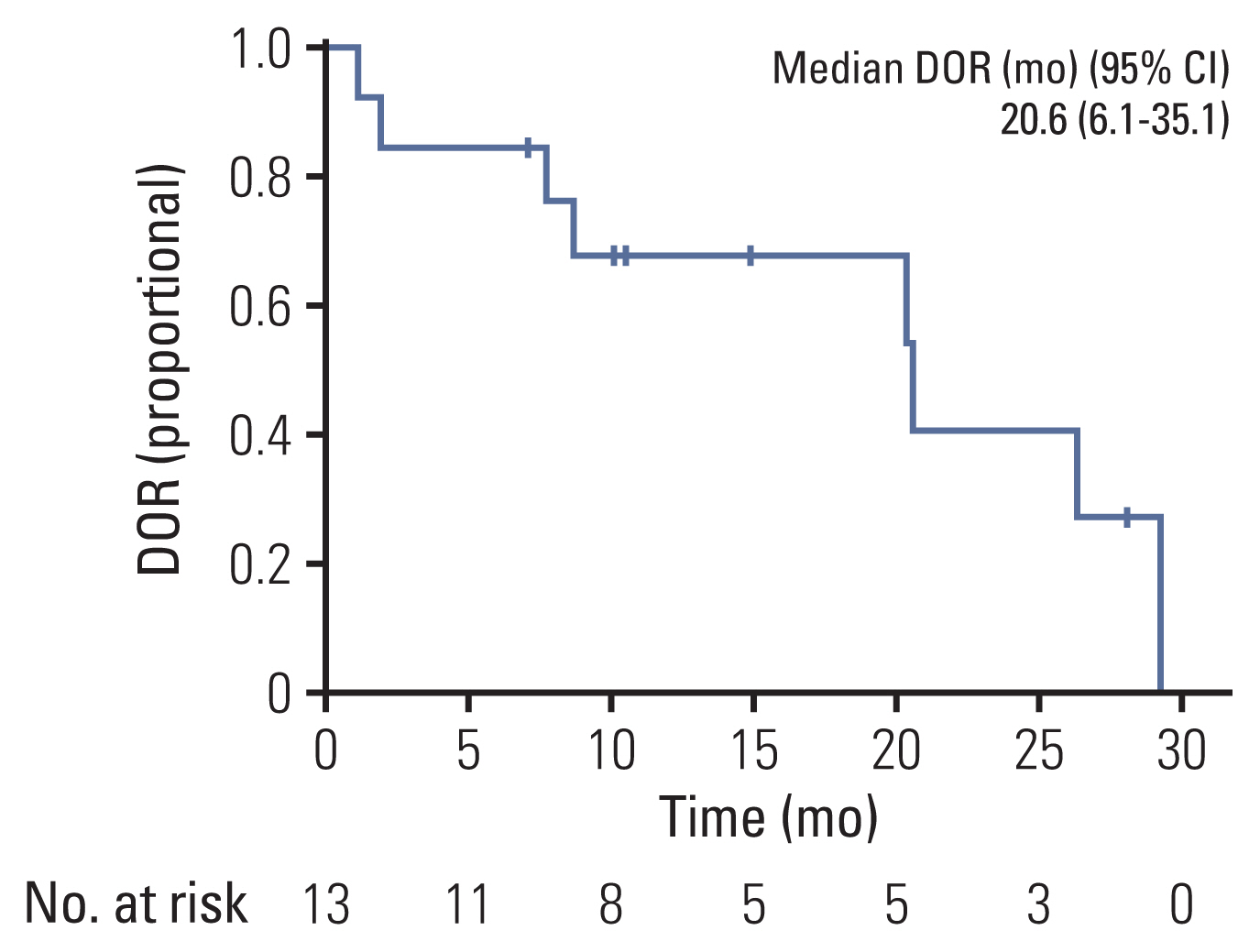
The best overall response recorded at any single time point from the start of treatment until disease progression based on RECIST (ver. 1.1) is included in a waterfall plot (Fig. 1). After the median follow-up time of 15.6 months (95% CI, 10.0 to 21.2), ORR was 56.5%, with no complete responses recorded, and the disease control rate (DCR) was 96.6% (Tables 2 and 3). In eight patients with measurable brain metastases on baseline brain magnetic resonance imaging (MRI), two patients expired before the intracranial response measure. The icORR of the remaining six patients was 33.3%, and the icDCR was 100.0%. The median DOR was 20.6 months (95% CI, 6.1 to 35.1) (Fig. 2). Three patients in the overall cohort had a CNS progression event. Two patients had no measurable brain metastases on the baseline brain MRI but developed measurable brain metastases during treatment. Two patients who had no baseline brain images before treatment were also diagnosed with brain metastasis during treatment.
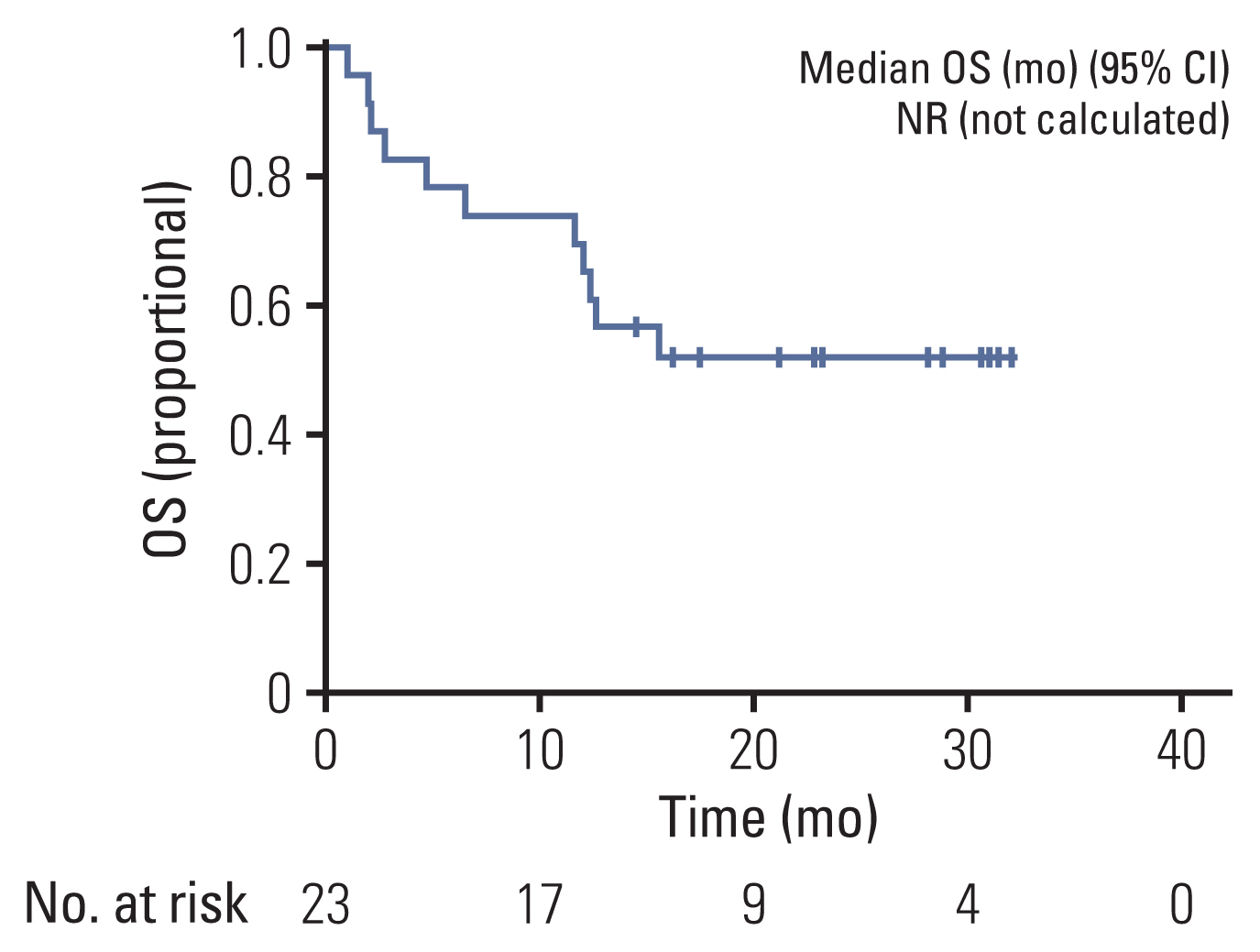
At the data cutoff time, 11 patients were maintained on pralsetinib, and the median time to discontinuation was 21.2 months (95% CI, not calculated). The probability of patients continuing pralsetinib for 12 months was 52.2% (Fig. 3A). The median DOR was 20.6 months (95% CI, 6.1 to 35.1). At data cutoff, 17 patients had disease progression or had died and the median PFS was 12.1 months (95% CI, 3.3 to 20.9) and the PFS rate at 12 months was 52.2% (Fig. 4A). At data cutoff, 11 patients had expired, and the median OS was not estimable due to the limited follow-up. The OS rate at 12 months was 69.6% (Fig. 5).

(A) Time to discontinuation (TTD). (B) TTD by fusion partners. (C) TTD by chemotherapy line. CI, confidence interval.
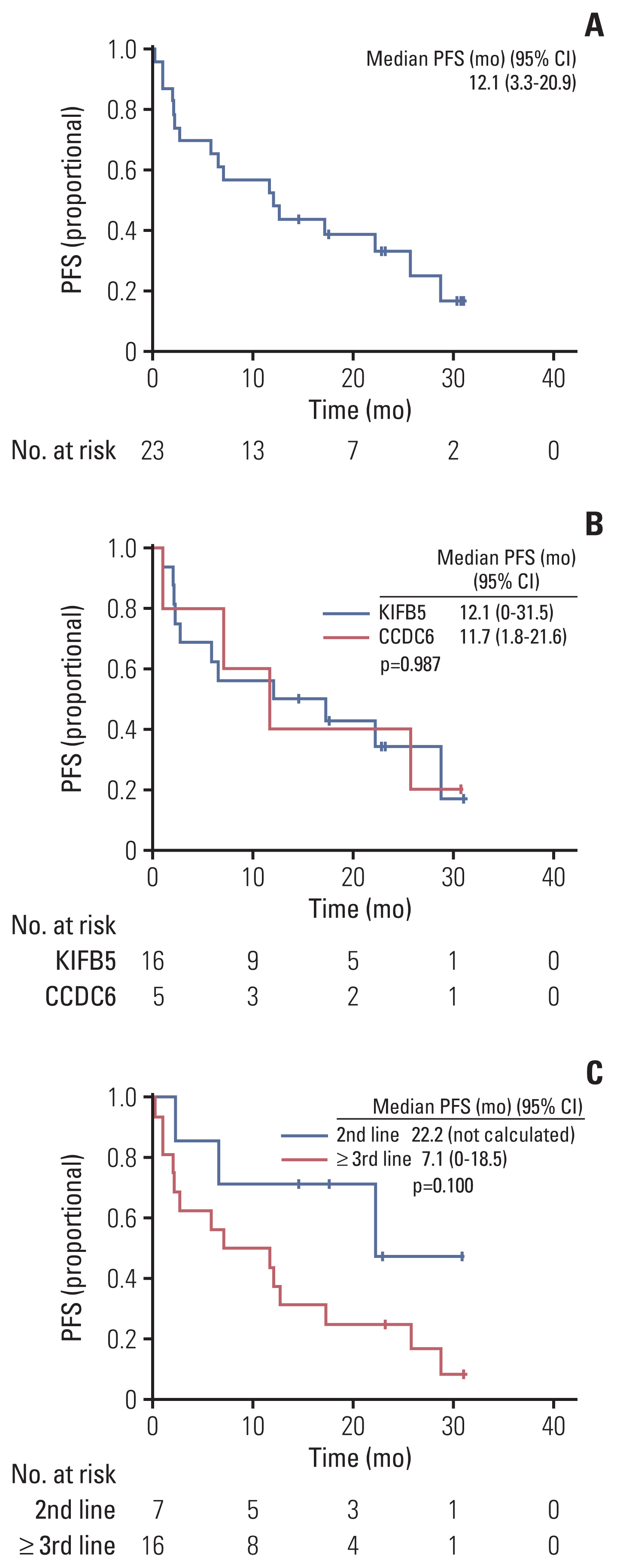
(A) Progression-free survival (PFS). (B) PFS by fusion partners. (C) PFS by chemotherapy lines. CI, confidence interval.
In subgroup analysis, the median TTD was not reached in the KIFB5-RET fusion group and 12.0 months (95% CI, 0 to 28.7) in the CCDC6-RET fusion group (p=0.680) (Fig. 3B). The median PFS was 12.1 months (95% CI, 0.0 to 31.5) in the KIFB5-RET fusion group and 11.7 months (95% CI, 1.8 to 21.6) in the CCDC6-RET fusion group (p=0.987) (Fig. 4B).
The median TTD was not reached in the second-line group and was 11.0 months (95% CI, 8.5 to 13.5) in the third-line or later group (p=0.037) (Fig. 3C). The median PFS was 22.2 months (95% CI, not calculated) in the second-line group and 7.1 months (95% CI, 0.0 to 18.5) in the third-line or later group (p=0.100) (Fig. 4C).
3. Safety
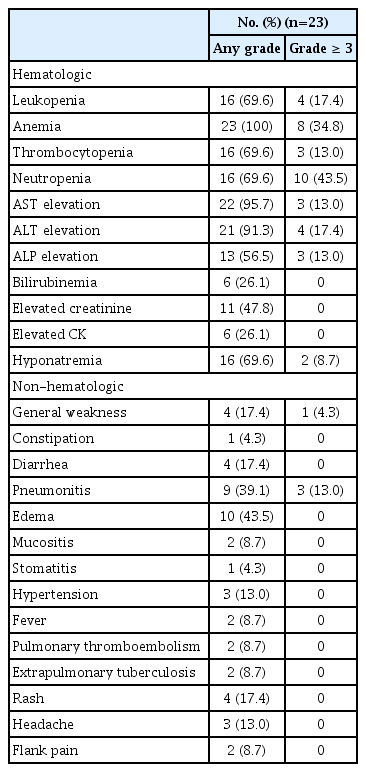
Most patients experienced treatment-related adverse events (TRAEs) of some grade (Table 4). The most common non-hematologic TRAEs were edema (n=10, 43.5%), pneumonitis (n=9, 39.1%), general weakness (n=4, 17.4%), and rash (n=4, 17.4%). The most common hematologic TRAEs were anemia (23, 100.0%), increased liver enzymes (aspartate aminotransferase, n=22, 95.7%; alanine transaminase, n=21, 91.3%), neutropenia (n=16, 69.6%), and thrombocytopenia (n=16, 69.6%). The most common grade 3 or greater TRAEs were neutropenia (n=10, 43.5%) and anemia (n=8, 34.8%).
Interruption of pralsetinib due to TRAEs occurred in 13 patients (56.5%). Nine patients (39.1%) had at least one dose level modification due to TRAEs (Table 5). The most frequent adverse events leading to dose interruption were grade 3 pneumonitis in all three (33.3%) of affected patients, and extrapulmonary tuberculosis in two patients (10.5%) [11]. Out of a total of 23 patients, 14 (60.9%) had previously received IO treatment, and five out of nine patients (55.6%) who experienced pneumonitis had received IO treatment previously; however, more pneumonitis did not occur in those who had previously received IO treatment. Treatment-related death was not observed.
Discussion
RET fusions were first identified in lung cancer in 2012 and found in approximately 1%–2% of NSCLCs [4,7]. It is known that RET-rearrangement is an oncogenic driver activated in NSCLC, but before the development of selective target therapy for RET rearrangement, multiple kinase inhibitors (MKIs) were used as treatment. Despite their therapeutic utility in MKI studies on RET-driven cancers, the ORR and median PFS in RET-rearranged NSCLCs are lower than those reported in patients with oncogene-driven NSCLCs receiving targeted TKIs. In addition, due to the nonselective nature of MKIs, off-target adverse effects such as hypertension and rash occurred, resulting in dose reduction and reduced antitumor effects [6,12–14].
In the LIBRETTO-001 trial (NCT03157128), selpercatinib showed an ORR of 68% and the median DOR and PFS were 20.3 and 18.4 months, respectively, in RET-fusion positive NSCLC. In initial data from the phase I/II ARROW trial (NCT03037385), ORR in previously treated patients was 61% and ORR in treatment-naïve patients was 70%. The median PFS was 17.1 months. Based on two global phase II clinical trials, selective RET-targeting selpercatinib and pralsetinib have received U.S. Food and Drug Administration (FDA) approval, are included in the National Comprehensive Cancer Network (NCCN) guidelines, and have become preferred first-line treatments for RET fusion-positive advanced NSCLC [9,15].
This EAP provided early access to pralsetinib and collective efficacy and safety information. Unlike the ARROW study, treatment-naïve patients were not included in this study, and all patients had received prior systemic chemotherapy [9]. Although the median prior treatment line was 2 (range, 1 to 8), the ORR and DCR were 56.5% and 96.6%, respectively, which showed the effect of pralsetinib. In 13 patients who showed a partial response, the median DOR was 20.6 months. In this EAP, treatment with pralsetinib beyond disease progression was allowed if the investigator judged ongoing clinical benefit. TTD can potentially evaluate the safety and efficacy of chemotherapy in the real world. At the data cutoff, the median follow-up was 15.6 months and the median TTD was 21.2 months, demonstrating that if the patient is tolerable, patients might remain on pralsetinib beyond progression while being clinically stable and without main organ dysfunction in actual clinical practice.
The proportion of patients achieving an icORR was 33.3% and the icDCR was 100.0%. These results demonstrate the ability of pralsetinib to cross the blood-brain barrier and maintain intracranial therapeutic levels and show potential use in patients with intracranial disease or in those who are at risk of brain metastases.
Data from the phase I/II ARROW study confirmed that the highly-selective RET inhibitor pralsetinib is active in patients with RET fusion-positive NSCLC, regardless of fusion partner or prior treatment. Our findings in ARROW and RWD support previous studies suggesting that patients with CCDC6 RET-driven disease may have a better prognosis than those with KIF5B RET-driven disease [9].
In this study, despite most patients being heavily pretreated, the safety profile was consistent with earlier trials of pralsetinib. Pralsetinib was well tolerated with a manageable safety profile. Most adverse events were transient and managed successfully with dose interruption or reduction. Even two patients with extra-pulmonary tuberculosis were well controlled after discontinuation of the drug and treatment with anti-tuberculosis drugs. Prior IO treatment may not be a significant risk factor for developing pneumonitis in pralsetinib. However, all three patients with grade 3 pneumonitis who contributed to treatment discontinuation had previously experienced IO treatment. Further studies should be conducted to investigate the association between IO treatment and pneumonitis during pralsetinib in larger patient cohorts.
This study has several limitations. The retrospective and single-arm nature is a limitation and the number of patients was small. It is necessary to be careful in interpreting the subgroup analysis. Second, it was difficult to obtain accurate results for icORR and CNS PFS because brain imaging was not performed at baseline, and routine follow-up evaluation was not performed.
In conclusion, pralsetinib was found to have clinical benefit, consistent with a pivotal study, including intracranial responses, and a manageable safety profile when used in patients with RET-rearranged NSCLC.
Notes
Ethical Statement
Patients provided written informed consent to participate in this study and for publication. This retrospective study was conducted according to the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The Samsung Medical Center Institutional Review Board approved the protocol (IRB number 2022-12-104-001).
Author Contributions
Conceived and designed the analysis: Jeon Y, Lee SH.
Collected the data: Jeon Y, Park S.
Contributed data or analysis tools: Jeon Y, Jung HA, Sun JM.
Performed the analysis: Jeon Y, Jung HA, Ahn JS, Ahn MJ, Park K.
Wrote the paper: Jeon Y, Lee SH.
Conflicts of Interest
Sehoon Lee has declares research funding to his institution from Merck, AstraZeneca, and Lunit. He serves on the advisory board of AstraZeneca, Roche, Merck, Pfizer, Lilly, BMS/Ono, Takeda, Janssen, IMBdx and also reports travel support from Novartis.
Acknowledgments
This study was supported by Future Medicine 20*30 Project of the Samsung Medical Center [SMX1220091].