Individualized Concurrent Chemotherapy for Patients with Stage III–IVa Nasopharyngeal Carcinoma Receiving Neoadjuvant Chemotherapy Combined with Definitive Intensity-Modulated Radiotherapy
Article information
Abstract
Purpose
This retrospective study aimed to re-evaluate the effect of concurrent chemotherapy in patients with locally advanced nasopharyngeal carcinoma (NPC) in the era of intensity-modulated radiotherapy (IMRT).
Materials and Methods
A total of 498 patients who received neoadjuvant chemotherapy (NCT) combined with concurrent chemoradiotherapy (CCRT) or IMRT were retrospectively reviewed. The distribution of baseline characteristics was balanced using propensity score matching. Additionally, the results of NCT+IMRT and NCT+CCRT were compared using Kaplan-Meier survival analysis, and differences in survival rates were analyzed using the log rank test.
Results
There were no significant differences in overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), and local progression-free survival (LRFS) between the two groups. Patients were further categorized into risk subgroups based on pretreatment Epstein-Barr virus (EBV) DNA cutoff values using receiver operating characteristic curve analysis. There were no statistically significant differences in OS, PFS, DMFS, and LRFS between patients who received NCT+CCRT and NCT+IMRT in the high-risk group. In the low-risk group, although there were no differences between NCT+CCRT and NCT+IMRT in OS, PFS, and LRFS, patients who received NCT+CCRT had better DMFS than those who received NCT+IMRT.
Conclusion
Pretreatment EBV DNA level can be used to individualize concurrent chemotherapy for patients with locally advanced NPC. Patients with low pretreatment EBV DNA levels may benefit from concurrent chemotherapy, whereas those with high levels may not. Other treatment modalities need to be explored for high-risk patients to improve their prognosis.
Introduction
Nasopharyngeal carcinoma (NPC) is endemic to the Fujian and Guangdong provinces of southern China, with the non-keratinized undifferentiated type being the most prevalent pathological type there [1,2]. Radiotherapy is the primary treatment for NPC because of its high sensitivity to radiation [3]. Concurrent chemoradiotherapy (CCRT) has shown survival benefits compared with radiotherapy alone, thus becoming the mainstay treatment for locally advanced NPC [4]. With the widespread use of intensity-modulated radiotherapy (IMRT), the locoregional control rate of NPC has markedly improved [5]. Studies have proven that neoadjuvant chemotherapy (NCT) combined with CCRT further improves the prognosis of NPC [6,7]. A multicenter prospective study reported that after treatment with three cycles of NCT, followed by CCRT, the 3-year overall survival (OS) rate and recurrence-free survival rate of patients with stage III–IVa NPC can reach 96.6% and 85.3%, respectively [8]. According to the National Comprehensive Cancer Network guidelines, NCT combined with CCRT is the standard treatment for patients with stage III–IVa NPC. Recent phase 3 multicenter trial published in JAMA showing no difference in clinical outcomes with IMRT alone vs. CCRT for stage II NPC patients [9], which motivating this follow-up study and hypothesis. Given that IMRT and NCT have significantly improved treatment outcomes for NPC [5], concurrent chemotherapy (CCT) may provide only limited survival benefits while leading to excessive toxicity, reducing the quality of life [10]. Particularly, there is no consensus regarding the role of CCT in patients with stage III–IVa NPC undergoing standard treatment.
Epstein-Barr virus (EBV) infects approximately 90% of individuals worldwide and is closely related to the onset of NPC [11,12]. Plasma EBV DNA load is a significant factor in predicting the prognosis of NPC [13]. Moreover, Sun et al. [14] demonstrated that pretreatment EBV DNA level is a powerful indicator that can help in guiding CCT in patients with stage II–III NPC, thus suggesting that EBV DNA could be further adapted for guiding individualized treatment and risk stratification.
Based on these premises, we conducted this retrospective study to compare survival between patients who received neoadjuvant chemotherapy combined with concurrent chemoradiotherapy (NCT+CCRT) and those who received neoadjuvant chemotherapy combined with radiotherapy alone (NAC+IMRT). In addition, patients were further classified based on risk stratification with the plasma EBV DNA load to explore which group of patients would benefit most from receiving CCT in the era of IMRT.
Materials and Methods
1. Patients
From January 2016 to December 2017, 498 patients with histologically proven stage III–IVa NPC at Fujian Hospital were included in the study. The inclusion criteria were as follows: (1) 18 years of age or older; (2) no presence of secondary malignancy, pregnancy, or lactation; (3) Eastern Cooperative Oncology Group performance score ≤ 1; (4) complete EBV DNA data before treatment; (5) receipt of two to four cycles of NCT. This study was approved by the appropriate Research Ethics Committee at the Fujian Medical University Cancer Hospital (approval No. K2022-071-01).
2. Treatment and follow-up
NCT regimens included docetaxel plus platinum (TP) and gemcitabine plus platinum (GP). All patients with stage III–IVa NPC received NCT followed by definitive IMRT. Cisplatin-based CCT is routinely recommended to patients with this type of tumor; however, 82 patients refused to receive CCT and underwent treatment by IMRT alone. The delivery and techniques of IMRT have been previously described [15]. Note that adjuvant chemotherapy (AC) is not a routine treatment and only a few patients who are considered to have poor tumor response to radiotherapy receive AC. The chemoradiotherapy-related toxicity was graded using the National Cancer Institute Common Toxicity Criteria (NCI CTC v4.0). Follow-ups were conducted every 3 months for the first 2 years after treatment, every 6 months thereafter for 5 years, and annually after 5 years. The primary endpoints were OS, progression-free survival (PFS), distant metastasis-free survival (DMFS), and local progression-free survival (LRFS). OS was calculated as the time from diagnosis to the date of death or last follow-up. PFS was defined as the period from the date of diagnosis to the date of death or disease progression, LRFS was defined as the period from the date of diagnosis to the date of death or local recurrence, DMFS was defined as the period from the date of diagnosis to the date of death or distant metastasis.
3. Quantitative detection of the plasma EBV DNA level
Plasma EBV-DNA samples were collected before NCT, and the number of copies of plasma EBV-DNA per milliliter was quantified by reverse transcription–quantitative polymerase chain reaction. The detailed detection method has been explained in the Supplementary Material. The cutoff value of EBV DNA before treatment was calculated based on the receiver operating characteristic (ROC) curve analysis.
4. Statistical analysis
The sample size calculation was carried out by PASS ver. 15 (NCSS, Kaysville, UT; http://www.ncss.com/software/pass) software, and the specific calculation method has been explained in the Supplementary Material. Univariate and multivariate analyses were performed using Cox proportional hazards models and included the following variables: T category, N category, EBV DNA, sex, age, NCT cycle, CCT, and AC. Kaplan-Meier survival curves and log-rank tests were used to analyze and assess the survival difference between subgroups. The 1:2 nearest-neighbor match method was used to balance the distribution of baseline characteristics based on the T category, N category, EBV DNA level, sex, age, NCT cycle, and AC. Statistical analyses were performed using R Project, ver. 4.1.2 (https://www.R-project.org) and SPSS Statistics for Windows, ver. 26.0 (IBM Corp., Armonk, NY) software. All tests were two-sided, and a p-value < 0.05 was considered statistically significant.
Results
Overall, 498 patients with stage III–IVa NPC were included in this study. The median (range) age at baseline was 47 years (range, 19 to 73 years), and the median follow-up time was 45 months (range, 6 to 65 months). Overall, 416 patients received NCT+CCRT, and 82 patients received NCT+IMRT. All patients completed two to four cycles of NCT, of which 256 patients received two cycles of NCT and 242 received more than two cycles (Table 1).
1. Cutoff value for EBV DNA load
The median EBV DNA concentration in the 498 patients was 3,885 copies/mL (range, 0 to 985,000 copies/mL). ROC analysis revealed that the cutoff value of positive EBV DNA for OS was 5,955 copies/mL (sensitivity, 0.730; specificity, 0.419; area under the curve, 0.662; 95% confidence interval, 0.572 to 0.752) (Fig. 1). Therefore, our study used 6,000 copies/mL for classifying patients into two different risk subgroups. The 3-year OS, PFS, DMFS, and LRFS of patients in the low-risk group were 96.8%, 88.1%, 95.4%, and 93.8%, respectively. For patients in the high-risk group, the 3-year OS, PFS, DMFS, and LRFS were 89.1%, 74.2%, 83.1%, and 93.9%, respectively. Patients with a low EBV DNA load had better OS, PFS, and DMFS than those with a high EBV DNA load (p=0.001, p < 0.001, and p < 0.001); however, no significant difference was observed in the LRFS between these patients subgroups (p=0.852).
2. Univariate and multivariate analysis
In the entire cohort, the 3-year OS, PFS, DMFS, and LRFS were 93.5%, 82.0%, 90.0%, and 93.8%, respectively. Univariate analysis showed that there were no significant differences in OS, PFS, DMFS, and LRFS between patients who received NCT+CCRT and those who received NCT+IMRT (p=0.591, p=0.967, p=0.343, and p=0.683, respectively). The multivariate Cox analysis showed that EBV DNA and N category were significant independent predictors of OS and DMFS (p=0.015 and p=0.025; p=0.001 and p=0.006, respectively) and that EBV DNA and age were significant independent predictors of PFS (p < 0.001 and p=0.036, respectively) (Table 2).
3. Propensity score matching
Table 1 shows that the NCT cycle is unbalanced between the two groups; therefore, we performed 1:2 nearest-neighbor matching to balance prognostic factors. After propensity score matching (PSM), 129 patients received NCT+CCRT and 79 patients received NCT+IMRT (Fig. 2). All patients received at least three cycles of NCT, and none of the patients received AC in the matched cohort. The baseline characteristics of patients were well-balanced between the two groups (Table 3). The 3-year OS, PFS, DMFS, and LRFS of the matched patients were 96.5%, 82.2%, 91.1%, and 92.0%, respectively. Survival analysis revealed that there were no significant differences between different treatment groups in OS, PFS, DMFS, and LRFS (p=0.265, p=0.618, p=0.155, and p=0.978, respectively). The Cox regression model identified no independent predictors after PSM (Table 4).
Treatment schedule of study patient inclusion. CCRT, concurrent chemoradiotherapy; EBV, Epstein-Barr virus; IMRT, intensity-modulated radiotherapy; NCT, neoadjuvant chemotherapy; NPC, nasopharyngeal carcinoma.
4. Adverse events
Table 5 summarizes the chemoradiotherapy-related adverse event after PSM. During the treatment of locally advanced NPC, nausea, anorexia, dry mouth, and mucositis are the most common non-hematological adverse events. The incidence of nausea (75.0% vs. 48.1%, p=0.001) and anorexia (34.9% vs. 16.5%, p=0.004) was significantly higher in the NCT+CCRT group, and the NCT+CCRT group also showed a tendency of higher hepatoxicity (alanine transaminase, 16.3% vs. 3.8%, p=0.006; aspartate aminotransferase, 9.3% vs. 3.8%, p=0.136). In addition, although there was no statistical difference, leukopenia, neutropenia, anemia, and thrombocytopenia are more common in the NCT+CCRT group.
In terms of late adverse events, NCT+CCRT group showed more skin/neck tissue fibrosis (37.2% vs. 31.6%, p=0.415), hearing impairment (23.3% vs. 16.5%, p=0.240) and dry mouth (31.0% vs. 19.0%, p=0.056), but the difference between two groups did not reach statistically significant. Rare severe late toxic reactions were observed except for two cases of grade 3–4 skin/neck tissue fibrosis and one case of severe hearing loss.
5. Survival analysis based on EBV DNA risk stratification
We further categorized the patients into a high-risk group (EBV DNA level ≥ 6,000 copies/mL) and a low-risk group (EBV DNA level < 6,000 copies/mL) and analyzed the survival outcome between NCT+IMRT and NCT+CCRT in the different risk subgroups. After PSM, 36 patients of those who received NCT+IMRT and 66 of those who received NCT+CCRT were included in the high-risk group. Survival analysis of the high-risk group showed that there were no statistically significant differences between patients who received NCT+CCRT and those who received NCT+IMRT in terms of OS, PFS, DMFS, and LRFS (p=0.417, p=0.945, p=0.931, and p=0.691) (Fig. 3). Therefore, in high-risk patients, NCT+CCRT does not bring survival benefits compared with NCT+IMRT.

Kaplan-Meier survival curves for high-risk patients after propensity score matching: (A) overall survival, (B) progression-free survival, (C) distant metastasis-free survival, and (D) locoregional relapse-free survival. NCT+CCRT, neoadjuvant chemotherapy combined with concurrent chemoradiotherapy; NCT+IMRT, neoadjuvant chemotherapy combined with concurrent chemoradiotherapy.
In addition, we evaluated the survival differences bet-ween two groups of patients who underwent the two different treatment modes in the low-risk group. After matching, 43 patients who received NCT+IMRT and 63 patients who received NCT+CCRT were included. Survival analysis revealed that there was no significant difference in the survival outcomes between patients who received NCT+CCRT and those who received NCT+IMRT in terms of OS, PFS, and LRFS (p=0.320, p=0.309, and p=0.628, respectively). However, patients who received NCT+CCRT had better DMFS than those who received NCT+IMRT (p=0.017) (Fig. 4). Therefore, NCT+CCRT can effectively reduce distant metastasis and improve the DMFS in low-risk patients compared with NCT+IMRT.
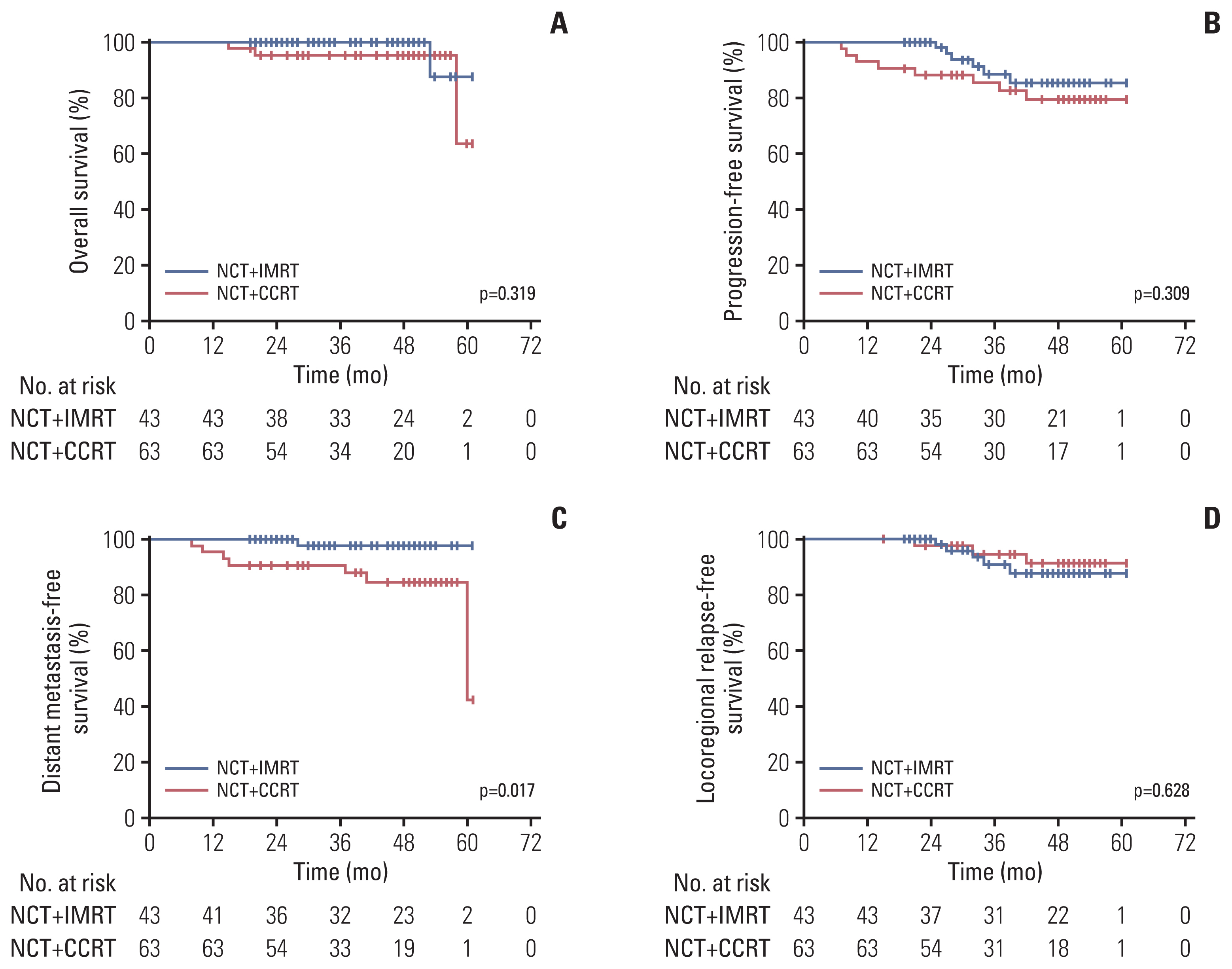
Kaplan-Meier survival curves for low-risk patients after propensity score matching: (A) overall survival, (B) progression-free survival, (C) distant metastasis-free survival, and (D) locoregional relapse-free survival. NCT+CCRT, neoadjuvant chemotherapy combined with concurrent chemoradiotherapy; NCT+IMRT, neoadjuvant chemotherapy combined with concurrent chemoradiotherapy.
Discussion
In the era of conventional radiotherapy, CCRT is the main treatment mode for locally advanced NPC. Compared with radiotherapy alone, CCRT can effectively reduce local recurrence and distant metastasis, thus improving the survival outcome of patients with NPC [4]. However, despite improving the patients’ prognoses, CCT can also induce toxicity and reduce their quality of life [16]. The locoregional control rate of NPC has markedly improved since the widespread use of IMRT [5], challenging the role of CCT as part of the treatment. Several studies revealed that the combination of NCT plus IMRT was as effective as NCT plus CCRT for managing locally advanced NPC [17–19]. However, most studies are limited by inconsistent chemotherapy regimens and NCT cycles, making it difficult to draw convincing conclusions. In our study, the NCT regimen included TP and GP, conforming with the recommendation of the American Society of Clinical Oncology (ASCO) and Chinese Society of Clinical Oncology (CSCO) guidelines [4]. More importantly, all patients received at least three cycles of NCT after matching, eliminating the impact of insufficient NCT intensity on survival outcomes. Survival analysis after PSM showed that there were no significant differences in OS, PFS, DMFS, and LRFS between the NCT+IMRT group and the NCT+CCRT group. Therefore, the efficacy in both groups was comparable after receiving sufficient NCT. Since CCRT has more side effects than radiotherapy alone [10], these results also indicated that CCRT might not be necessary after adequate NCT.
Furthermore, we used pretreatment EBV DNA levels for risk stratification and further assessed whether they could be used to individualize treatment in patients with NPC. Based on the ROC analysis results, we used 6,000 copies/mL as the cutoff value [20,21]. The Kaplan-Meier survival curve confirmed that this cutoff value could accurately divide patients into two different subgroups based on the risk level, reflecting a good prognostic value. However, it has been shown that there is considerable variation in the pretreatment cutoff values for EBV DNA among different laboratories [13,22]. Considering that this is the result of a single-center study, it is necessary to further verify the cutoff values in multicenter studies in the future.
Survival analysis in the low-risk group showed that CCT reduced distant metastasis and improved DMFS but had no statistical effects on the OS, PFS, and LRFS. A possible explanation could be that the local control rate of NPC had reached a ceiling, making the main goal of CCT in the era of IMRT to reduce distant metastasis rather than avoid local recurrence. Based on this result, CCT can bring benefits for patients in the low-risk group and thus should be routinely recommended. Surprisingly, there were no significant differences in the survival outcome between NCT+CCRT and NCT+IMRT groups in the high-risk group, indicating that aggressive chemotherapy could not further improve the prognosis of patients with high-risk NPC. This is in contrast to the results of Sun et al. [14], which indicate that high-risk patients can continue to benefit from CCRT. However, the study mainly focused on NPC patients with stages II–III, who did not receive NCT. Studies have shown that with the increasing cumulative cisplatin dose, the overall toxicity became heavier, increasing the risk of treatment-related death and incidences of severe acute toxicity [23,24]. Those may explain the differences in our research results compared to previous studies. Additionally, EBV DNA is closely related to the burden of the tumor, and thus may be associated with a greater intensity of treatment. Scine radiotherapy is still the main treatment for NPC, a higher tumor burden usually means higher doses of radiotherapy. Further addition of CCT may lead to more serious toxicity and adverse reaction, thus decrease the therapeutic gain of these patients. Consequently, high-risk patients must explore other treatment methods to improve their prognosis. Maintenance chemotherapy in nasopharyngeal carcinoma has been a research hotspot in recent years. Capecitabine maintenance therapy significantly improved PFS for patients with newly diagnosed metastatic NPC [25], and it also exhibited manageable toxic effects. It may become an effective therapy for patients who do not benefit from CCT, thereby further improving their prognosis. We are also conducting a phase II, multicenter clinical trial (NCT04223024) to evaluate the efficacy and safety of oral capecitabine/teggio in high-risk locally advanced patients (stage N3) after radical chemoradiotherapy, with highly anticipated that the results will provide new solutions for patients who do not benefit from CCT. To reduce the toxicity caused by cumulative cisplatin dose, radiotherapy integration with targeted therapy or immunotherapy may be an alternative [26–28], but high-level clinical trials are needed to verify the role of these treatments in nasopharyngeal carcinoma.
There are some limitations in our study. In our center, AC is not a routine treatment and only a few patients who are considered to have poor tumor response to radiotherapy receive AC. Although statistical methods, including multivariate analysis and PSM, were used to reduce bias, prospective studies are still needed in the future. Our paper questions the role of CCT, and it may serve to initiate a randomized trial to verify our results and provide better answers.
In conclusion, pretreatment EBV DNA level can be used as an effective predictor to individualize CCT for patients with locally advanced NPC. In this study, patients with low pretreatment EBV DNA concentration benefited from CCRT, whereas those with high levels did not. Exploring other treatment modalities for high-risk patients is important to improve their prognosis.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the appropriate Research Ethics Committee at the Fujian Medical University Cancer Hospital (approval no: K2022-071-01), and written informed consent was obtained from all participants.
Author Contributions
Conceived and designed the analysis: Ji P, Lu Q, Lin S, Zong J.
Collected the data: Ji P, Lu Q, Chen X, Chen Z.
Contributed data or analysis tools: Ji P, Lu Q, Chen X, Chen Y, Peng X, Chen Z.
Performed the analysis: Ji P, Lu Q, Chen X, Chen Y, Peng X, Lin C.
Wrote the paper: Ji P, Chen Y, Zong J.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.






