Sublobar Resection versus Stereotactic Body Radiation Therapy for Clinical Stage I Non–Small Cell Lung Cancer: A Study Using Data from the Korean Nationwide Lung Cancer Registry
Article information
Abstract
Purpose
Stereotactic body radiotherapy (SBRT) had been increasingly recognized as a favorable alternative to surgical resection in patients with high risk for surgery. This study compared survival outcomes between sublobar resection (SLR) and SBRT for clinical stage I non–small cell lung cancer (NSCLC).
Materials and Methods
Data were obtained from the Korean Association of Lung Cancer Registry, a sampled nationwide database. This study retrospectively reviewed 382 patients with clinical stage I NSCLC who underwent curative SLR or SBRT from 2014 to 2016.
Results
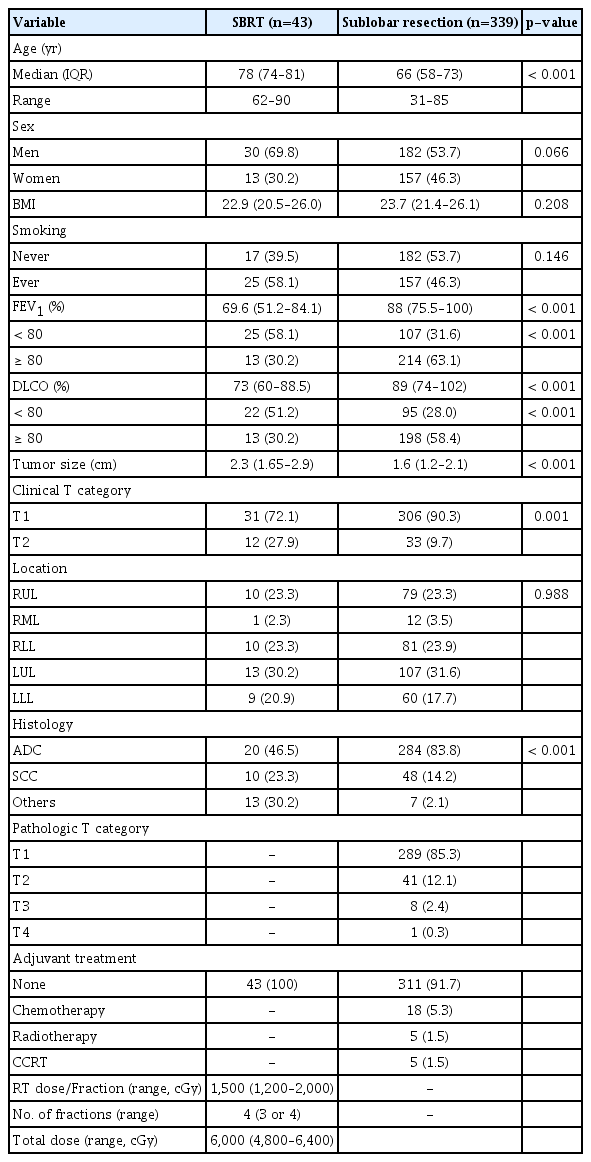
Of the patients, 43 and 339 underwent SBRT and SLR, respectively. Patients in the SBRT group were older and had worse pulmonary function. The 3-year overall survival (OS) rate was significantly better in the SLR group compared with the SBRT group (86.6% vs. 57%, log-rank p < 0.001). However, after adjusting for age, sex, tumor size, pulmonary function, histology, smoking history, and adjuvant therapy, treatment modality was not an independent prognostic factor for survival (hazard ratio, 0.99; 95% confidence interval, 0.43 to 2.77; p=0.974). We performed subgroup analysis in the following high-risk populations: patients who were older than 75 years; patients who were older than 70 years and had diffusing capacity of lung for carbon monoxide ≤ 80%. In each subgroup, there were no differences in OS and recurrence-free survival between patients who underwent SLR and those who received SBRT.
Conclusion
In our study, there were no significant differences in terms of survival or recurrence between SBRT and SLR in medically compromised stage I NSCLC patients. Our findings suggest that SBRT could be considered as a potential treatment option for selected patients.
Introduction
Lobectomy with lymph node dissection or sampling has remained the standard treatment for early-stage non–small cell lung cancer (NSCLC). As life expectancy has increased, the proportion of elderly patients who are diagnosed with resectable NSCLC but not fit for lobectomy due to medical comorbidity accordingly has increased. Traditionally, sublobar resection (SLR) has been recommended to preserve as much functioning lung as possible for these patients. In recent years, stereotactic body radiotherapy (SBRT) or stereotactic ablative radiotherapy has been increasingly recognized as a reasonable alternative to surgical resection for the patients who are at increased risk of postoperative morbidity and mortality [1].
The efficacy and safety of SBRT for treatment of stage I NSCLC has been proved by several retrospective or phase II prospective studies [2–5]. Some retrospective studies reported comparable survival outcomes following either SBRT or surgery [6–9], whereas others found that SLR could lead to better survival outcomes when compared to SBRT [10–14]. A few well-designed prospective randomized trials were started to compare the clinical outcomes following either surgery or SBRT, which, however, were closed early because of the poor patients’ accrual [8,15]. Actual choice of local modality has mainly depended on the preference of the clinician in charge or institution, as there is still no clear guideline on which local treatment modality, between SLR and SBRT, should be chosen in medically compromised early-stage lung cancer patients as of yet. In this context, we comparatively analyzed the real-world practice pattern of treatment modality selection between SBRT and SLR and the subsequent clinical outcomes in treating the stage I NSCLC patients using the Korean nationwide registry database.
Materials and Methods
1. Data source
Data were obtained from the Korean Association for Lung Cancer Registry (KALC-R), which was developed in cooperation with the Korean Central Cancer Registry (KCCR) [16], which has collected the information on the newly diagnosed cancer patients across the country each year [17]. From 2014 to 2016, the KCCR registered 74,636 lung cancer patients [18]. Using a systematic sampling method, about 10% of the total lung cancer patients were extracted from the KCCR database [19]. The sample size of each participating hospital was determined based on the respective number of registrations, and 8,110 patients were eventually selected, whose medical records were thoroughly reviewed for details: age; sex; body mass index (BMI); smoking history; histopathologic tumor type; symptoms; pulmonary function test (PFT) including forced expiratory volume in 1 second (FEV1) and diffusing capacity of lung for carbon monoxide (DLCO); Eastern Corporative Oncology Group performance score; clinical and pathological stage according to the seventh edition of the TNM International Staging System [20]; treatment modality; results of molecular tests including epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase translocation; and survival status, respectively. The survival data were obtained from the Statistics Korea and the population resident registration data of the Ministry of the Interior and Safety [17]. Survival and recurrence status was followed up until December of 2019.
2. Patients selection and variables
From a total of 8,110 patients registered in the KALC-R database, 2,036 patients were diagnosed with clinical stage I NSCLC, of whom 382 patients who underwent curative SLR (n=339) or SBRT (n=43) were selected for the present study (S1 Fig.). Clinical and pathological data, including age, sex, BMI, smoking status, PFT, clinical and pathological stage, treatment modality, and survival and recurrence status were extracted from the KALC-R to compare the SLR and SBRT groups.
FEV1 and DLCO were categorized into two groups: 80% or higher and less than 80%. According to the European Respiratory Society/European Society of Thoracic Surgery clinical guidelines on fitness for radical therapy in lung cancer patients, additional exercise tests are recommended when FEV1 or DLCO is less than 80%. Therefore, radical therapy was considered appropriate for patients with FEV1 or DLCO greater than or equal to 80%, while patients with FEV1 or DLCO less than 80% were regarded as having impaired lung function in this study [21]. The information on tumor size was derived from clinical tumor size determined by pretreatment computed tomography scan, in order to reflect the clinical decision-making process.
3. Statistical analysis
The primary outcome of this study was overall survival (OS), and the secondary outcomes included recurrence-free survival (RFS), cumulative incidence of recurrence (CIR) including locoregional recurrence and distant metastasis, and the independent prognostic factors for OS, respectively. The survival durations were defined as the intervals from the base of follow-up (date of surgery or SBRT initiation) till the dates of event (death or recurrence) or censoring, respectively.
The baseline characteristics were analyzed using the Wilcoxon rank sum test for the continuous variables and the chi-square or Fisher’s exact test for the categorical variables. The Kaplan-Meier method and the log-rank test were used to estimate OS. Competing risk analysis and the Gray’s method were used to assess and compare the CIR. Death was regarded as the competing risk for recurrence. A multivariable Cox proportional hazard analysis was performed to determine the independent prognostic factors for the all-cause death. The covariates that had p < 0.05 in the univariate Cox proportional hazard were used for the multivariate Cox proportional hazard analysis. To compare two groups with balancing potential confounding variables, we performed propensity score matching analysis using R package Matchit. After excluding cases with unknown T category (n=2), nearest neighbor matching was conducted including the following variables of clinical significance: age, sex, FEV1, DLCO, and clinical T category. A p-value of < 0.05 was considered statistically significant.
Results
1. Study cohort
The comparative baseline characteristics based on the treatment groups are summarized in Table 1. When compared with the SLR group, the SBRT group patients were older (median age, 78 vs. 67 years; p < 0.001), and were associated with poor PFT more frequently (median FEV1 [%], 69.6% vs. 87%, p < 0.001; proportion of patients with FEV1 < 80%, 58.1% vs. 34.2%, p < 0.001; median DLCO (%), 73% vs. 86.5%, p=0.002; proportion of patients with DLCO < 80%, 51.2% vs. 31%, p < 0.001, respectively). The SBRT group patients had larger median clinical tumor size (2.3 vs. 1.5 cm, p < 0.001), included cT2 tumor more frequently (27.9% vs. 7.5%, p < 0.001), and had adenocarcinoma histology less frequently (46.5% vs. 82.4%, p < 0.001), respectively.
In the SBRT group, the median total radiation dose was 60 Gy (range, 48 to 64 Gy) in 4 fractions (range, 3 to 4 fractions) (Table 1). All 43 SBRT patients received BED10 (biologically effective dose with α/β=10) greater than 100 Gy10 [22]. Among 339 SLR patients, 187 underwent wedge resection, while 152 did segmentectomy, and lymph node sampling or dissection was performed on 241 patients (71.1%) (50% of patients who underwent wedge resection; and over 90% of patients who underwent segmentectomy, respectively) (S2 Table). Pathologic lymph node metastasis was confirmed in 15 patients: seven following wedge resection; and eight following segmentectomy, respectively. A total of 23 patients underwent adjuvant treatment following SLR.
2. Survival outcomes
The median follow-up period for the entire cohort was 41.6 months. The median follow-up period for SLR group and SBRT group were 41.6 (95% confidence interval [CI], 39.8 to 44.3) months and 40.8 (95% CI, 35.7 to 49.7) months, respectively (p=0.590). Upon study completion, 64 patients died (17 in SBRT group and 47 in SLR group, respectively) and 51 experienced recurrences (six in SBRT group and 45 in SLR group, respectively). The 3-year OS rate was significantly higher in the SLR group (86.6% vs. 57%, log-rank p < 0.001) (Fig. 1A). Similarly, the 3-year RFS rate was also significantly higher in the SLR group (79.4% vs. 51.7%, log-rank p < 0.001) (Fig. 1B). On the other hand, the CIR was not significantly different between the groups (p=0.899) (Fig. 1C).
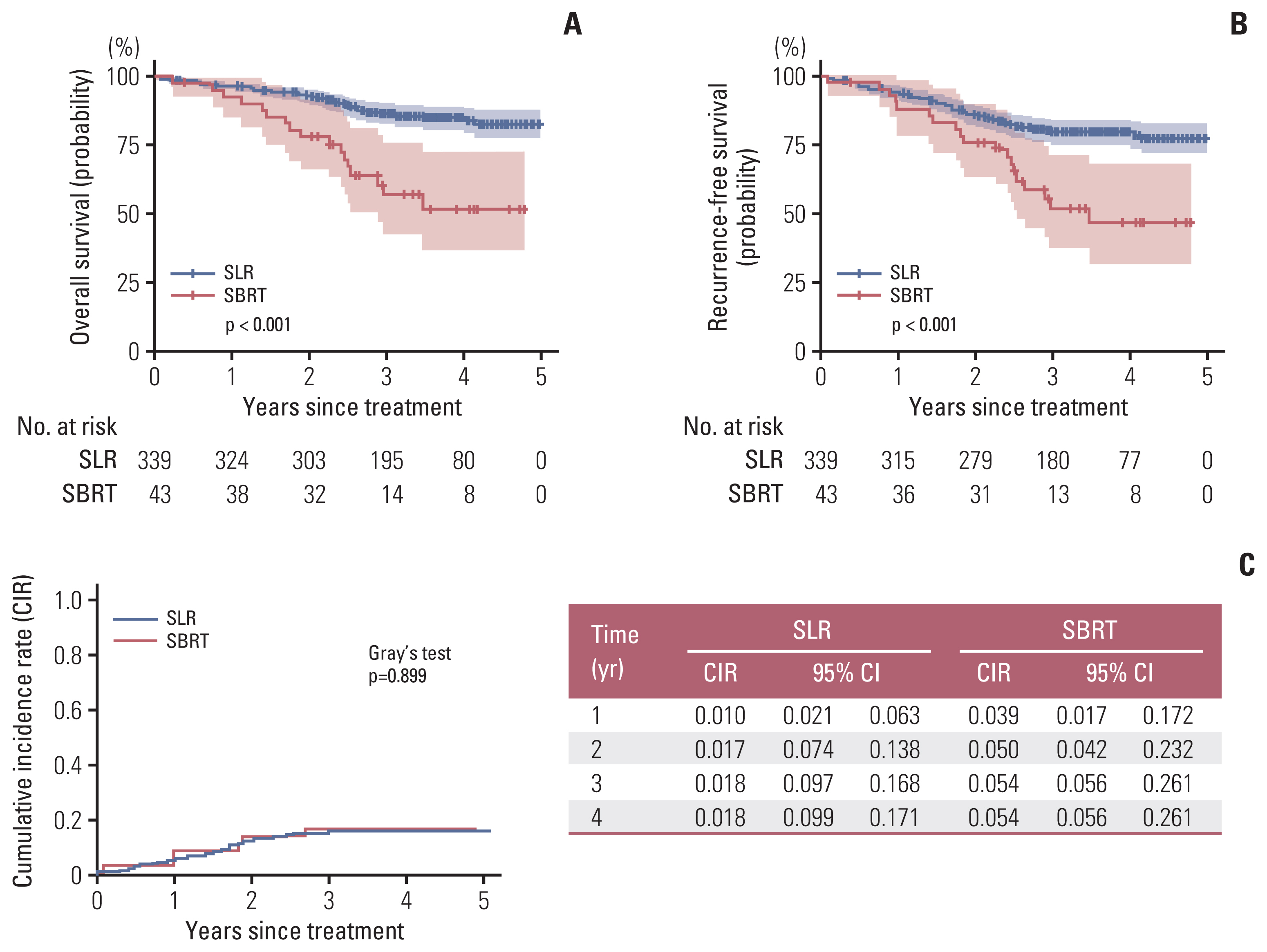
Survival outcomes. The sublobar resection (SLR) group has better overall survival (A) and recurrence-free survival (B) compared with those in the stereotactic body radiation therapy (SBRT) group. However, CIR (C) was similar between the two groups. CI, confidence interval.
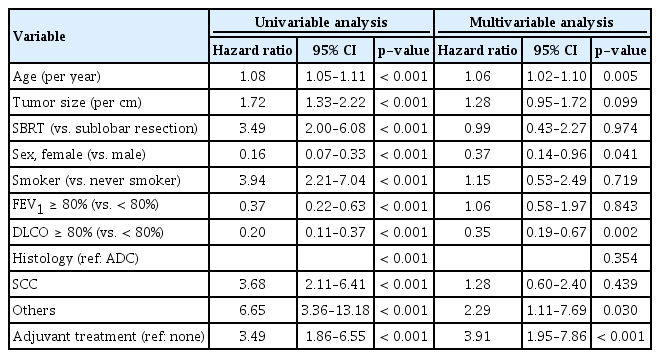
In the multivariate Cox proportional hazard model analysis for OS, older age, male sex, low DLCO, histology other than adenocarcinoma or squamous cell carcinoma, and adjuvant treatment were the independently poor prognostic factors. Treatment strategy (SBRT or SLR) was not an independent factor for survival after adjusting for age, sex, tumor size, smoking status, FEV1, DLCO, tumor histology, and adjuvant therapy (Table 2, Fig. 2). We have checked the correlation between variables using multicollinearity plot, and there was no significant collinearity (i.e., exceeding 0.4) between variables (S3 Fig.).
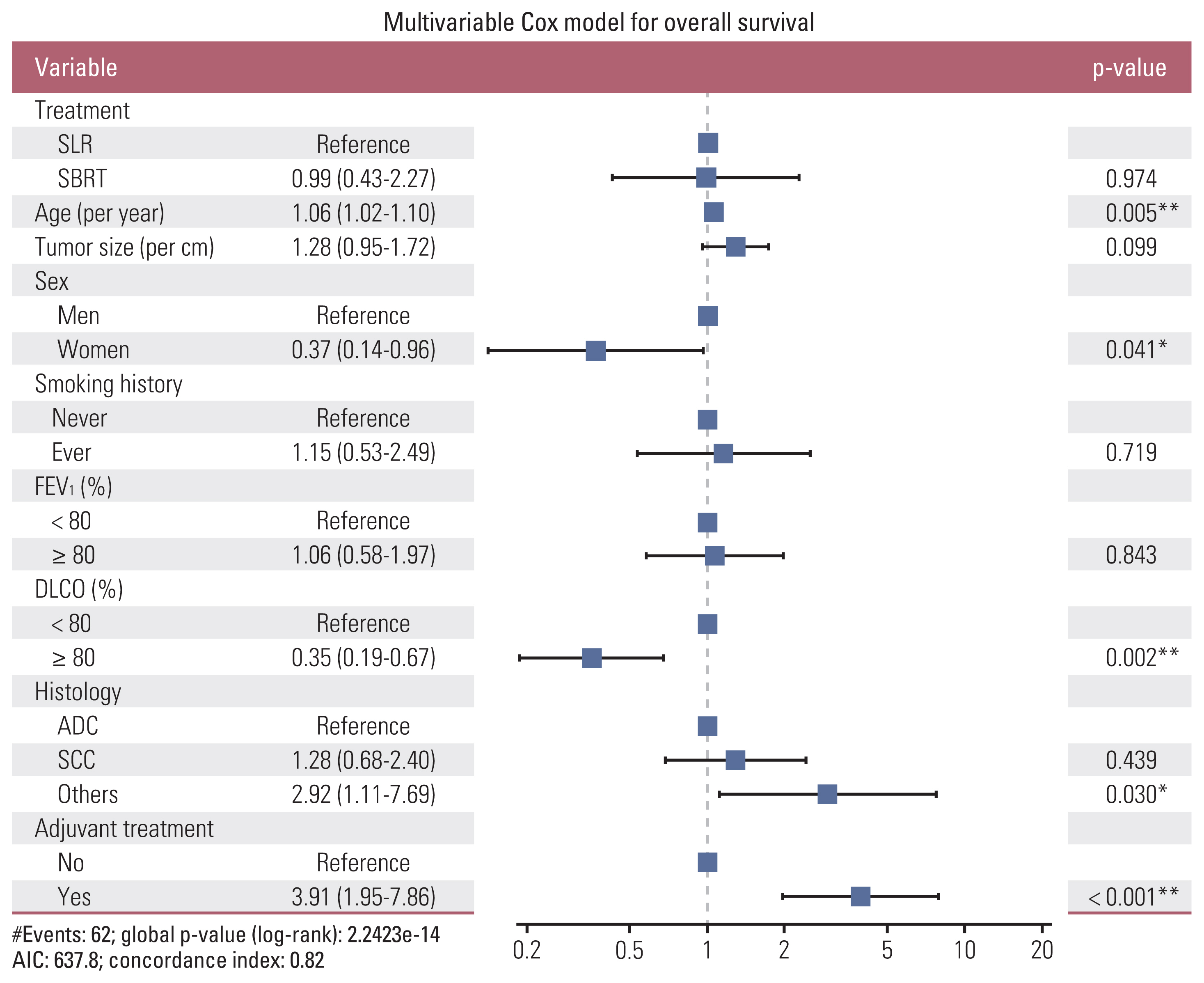
Forest plot. Multivariable Cox proportional hazard analysis was performed to determine the independent prognostic factors of overall survival. Older age, male sex, low diffusing capacity of lung for carbon monoxide (DLCO), histology other than adenocarcinoma (ADC) or squamous cell carcinoma (SCC), and adjuvant treatment were the independent poor prognostic factors. Treatment strategy (stereotactic body radiation therapy [SBRT] or surgery) was not an independent prognostic factor for survival. AIC, akaike information criterion; FEV1, forced expiratory volume in 1 second; SLR, sublobar resection. *p < 0.05, **p < 0.01.
We further analyzed the survival outcomes of two groups, SBRT versus SLR, using propensity score matching. After matching, there are no significant differences between the two groups in terms of age, sex, pulmonary function tests (FEV1 and DLCO), and clinical T category (S4 Table). After confirming that the standardized mean differences were not exceed 0.2, OS and RFS were compared between the matched pairs of SLR and SBRT groups (n=41 for each group). There were no significant differences in OS (log-rank p=0.530) as well as RFS (log-rank p=0.561) between the two groups (S5 Fig.).
3. Survival outcomes in the elderly patients
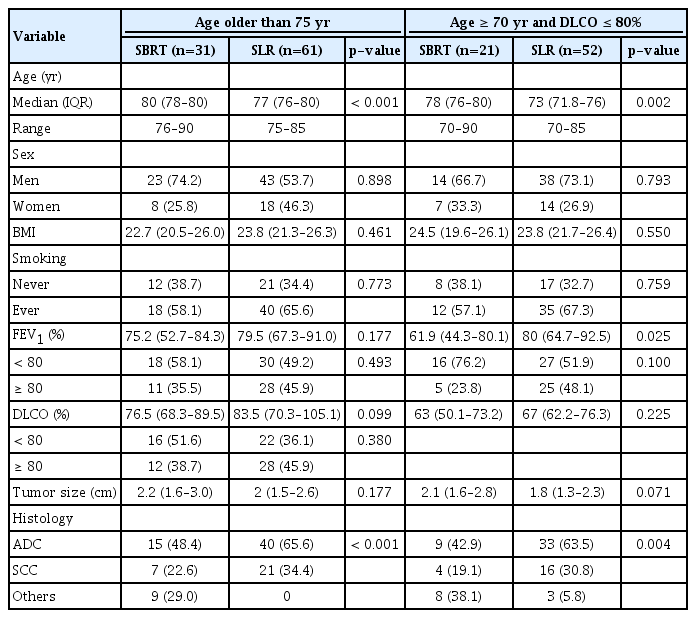
Since age and DLCO were identified as the factors that had the greatest influence on OS in the multivariable analysis, we performed subgroup analysis to determine whether the SLR group still as associated with better survival than the SBRT group in elderly patients.
The first subgroup analysis was performed in 92 patients who were older than 75 years: 31 underwent SBRT; and 61 did SLR, respectively (Table 3). There were no significant differences in the 3-year OS (SLR 74.2% vs. SBRT 65.7%, p=0.402) and in the 3-year RFS (SLR 67.8% vs. SBRT 61.5%, p=0.570), respectively (Fig. 3A).
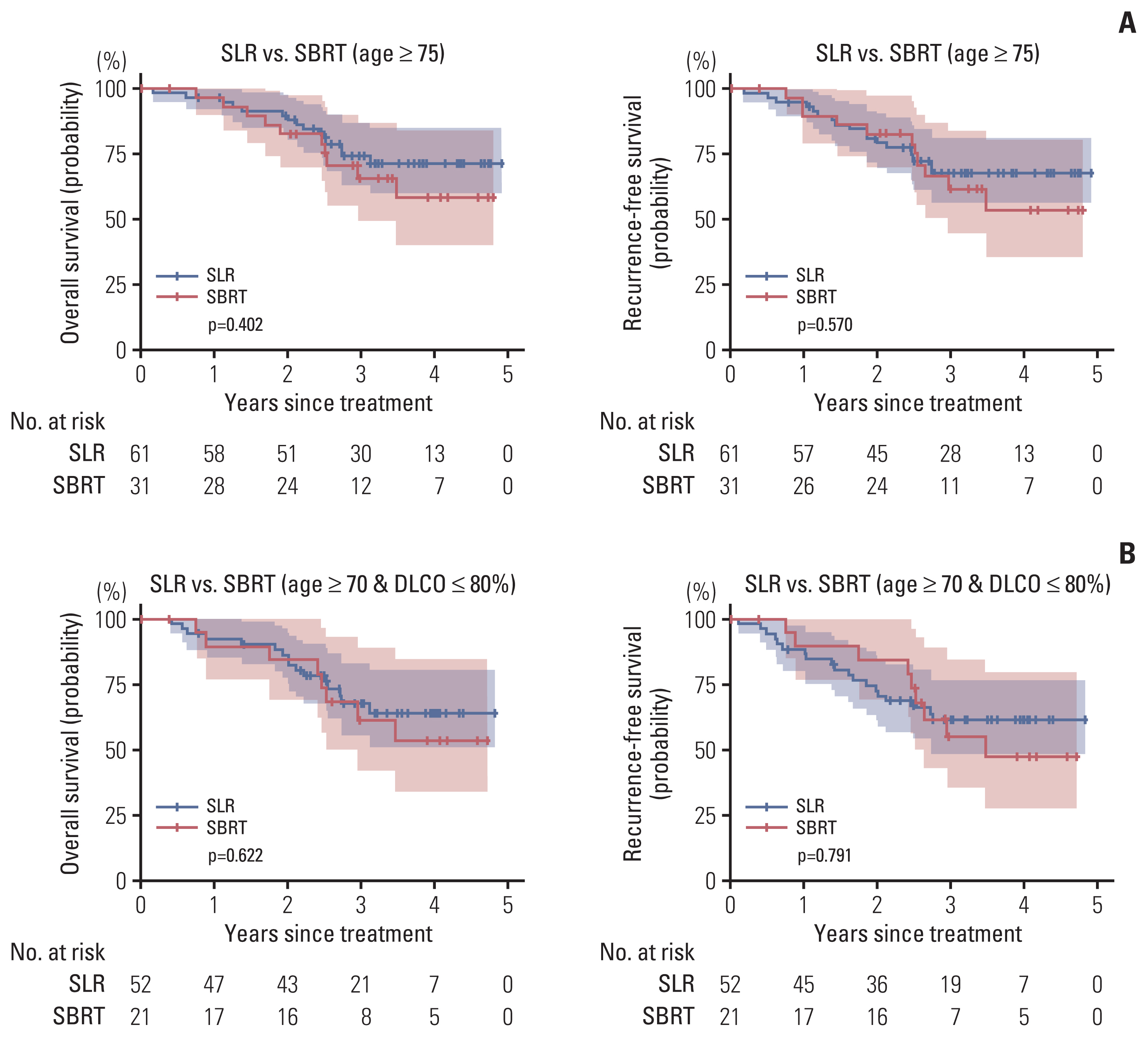
Subgroup analysis. There was no significant difference in overall survival and recurrence-free survival between the sublobar resection (SLR) and stereotactic body radiation therapy (SBRT) groups in both subgroup of patients ≥ 75 years (A) and the subgroup of patients ≥ 70 years of age and with a diffusing capacity of lung for carbon monoxide (DLCO) ≤ 80% (B).
The second subgroup analysis was performed in 73 patients who were older than 70 years and had DLCO ≤ 80%: 21 underwent SBRT; and 52 did SLR, respectively (Table 3). There were no significant differences in the 3-year OS (SLR 67.8% vs. SBRT 61.2%, p=0.622) and in the 3-year RFS (SLR 61% vs. SBRT 55%, p=0.791), respectively (Fig. 3B).
4. Comparison of survival outcomes between SBRT and wedge resection
Further analysis using the same statistical method was performed to compare the survival outcomes between SBRT and wedge resection (n=187) for clinical stage I NSCLC. Compared with the wedge resection group, the SBRT group were older (median age, 78 years vs. 67 years; p < 0.001) and had larger tumor (median tumor size, 2.3 vs. 1.5 cm; p < 0.001), respectively. More than half of the patients who underwent SBRT and approximately 30% of patients who did wedge resection had decreased pulmonary function (S6 Table).
OS was significantly better in the wedge resection group than in the SBRT group (log-rank p < 0.001) (S7A Fig.). RFS was also better in the wedge resection group (log-rank p=0.005) (S7B Fig.). On the other hand, the CIR was not significantly different between groups (p=0.936) (S7C Fig.). In the multivariable Cox proportional hazard model analysis for OS, SBRT did not independently increase the risk of death compared to wedge resection (hazard ratio, 0.95; 95% confidence interval, 0.35 to 2.61; p=0.926) (S8 Fig.).
5. Relative survival to general population
We performed a relative survival analysis using a national life expectancy table to assess any potential survival advantage of SLR or SBRT in elderly and medically compromised patients. The relative survival rates of the SLR and SBRT groups among male were 96.4% versus 98.3% at 1-year and 98.3% versus 53.0% at 3-year, respectively. In female, the relative survival rates of the SLR and SBRT groups were 100% and 94.2% at 1 year and 97.3% versus 100% at 3 years, respectively (S9 Table).
Discussion
Local control of the primary tumor is an essential component for the cure of lung cancer patients. Based on the Lung Cancer Study Group trial result [23], lobectomy has been the gold standard in the treatment of early-stage NSCLC. However, the optimal treatment for early-stage NSCLC in the medically compromised patients with insufficient pulmonary reserve, cardiac dysfunction, poor performance status, or medical comorbidities has remained still controversial. Both SBRT and SLR are valuable alternative options, however, there have been no clear guidelines for the treatment modality selection for these compromised patients. Thus, various institutions have chosen their favored treatment modality based rather on inconsistent criteria. In this study, we analyzed the characteristics of the stage I NSCLC patients who underwent SBRT or SLR based on the Korean nationwide registry database, in order to minimize the institutional selection bias, and then compared the survival outcomes following two different treatment modalities.
The results of this study showed that OS and RFS were better in the SLR group, which, however, could have been by virtue of selection bias: surgery was more frequently chosen for the younger and healthier patients than SBRT (S10 Fig.). In addition, some patients with unexpected lymph node metastasis found during surgery may have been selectively excluded from the SLR group due to the intraoperative plan change. Furthermore, some patients in the SLR group, whose pathological findings predicted poor prognosis, received adjuvant treatment, which might have altered the survival outcomes in this group. When adjusting for other potential covariates that could affect outcomes, SBRT or SLR was not an independent prognostic factor for OS. Instead, age and DLCO were the most influential factors for OS in multivariable Cox regression. Two subgroup analyses for elderly patients also showed no significant difference in OS and RFS between the SBRT and SLR groups consistently: ≥ 75 years; and ≥ 70 years with a DLCO ≤ 80%, respectively. These results indirectly proved that SBRT was a reasonable and valuable treatment option comparable to SLR in the elderly and/or compromised patients.
In the real-world practice, it seems that the clinicians have well selected the appropriate treatment modality according to the individual patients’ characteristics. According to this study’s results, about three-quarters of the patients who underwent SBRT were 75 years or older, while three-quarters of SLR group were younger than 75 years, and there was 12-year difference in the median age between groups. These suggest that the clinicians have considered age as an important factor when choosing the treatment option. These were reasonable decisions in accordance with the results of this study that showed no survival difference between groups in the elderly and/or lung function compromised patients.
There are a few limitations in this study. First, this study is a retrospective study based on rather a small sample size. For demonstrating the non-inferiority of SBRT, our findings need to be validated in a large independent cohort. Second, the database lacked information on the patients’ detail comorbidities, tumor location (central or peripheral), treatment-specific complication, actual causes of death, and recurrence pattern, respectively, which could serve as important factors in determining treatment strategy and analyzing the therapeutic effect of each treatment more accurately. Specifically, the KALC-R did not provide information on the actual causes of death, making it challenging to establish a definitive association between the deaths and the disease. Given that various factors, aside from the treatment modality, can influence the survival outcome, the conclusions of this study should be interpreted with caution. Third, our study was not free from selection bias, because the intention of performing SLR was not clear in this study. In the SLR group, patients who underwent SLR despite of functionally tolerable PFT for lobectomy, and those who had to undergo SLR due to impaired function were mixed.
There were several strengths, however, in our study. First is the high quality of the database. The KALC-R contained quite detailed information, such as smoking history, BMI, pulmonary function test, and clinical tumor size, which were difficult to handle in the national database. This helped the researchers better understand the patients’ characteristics. Another strength was that the patient enrollment period of our study was shortened compared to other previously published studies. For example, a previous study compared surgery and SBRT performed at different time periods [9], and another study recruited the patients for 12 years from 2001 to 2012 [24]. The current study could better reflect the recent practice pattern and reduce the potential bias that could have arisen from too long enrollment period. Lastly, various subgroup analyses were performed, and it might be helpful in determining the appropriate treatment strategy based on different individual characteristics.
In our study, there were no significant differences in terms of survival or recurrence between SBRT and SLR in medically compromised stage I NSCLC patients. Our findings suggest that SBRT could be considered as a potential treatment option for selected patients.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study protocol was reviewed and approved by the institutional review board of the National Cancer Center (NCC2018-0193), which waived the requirement for informed consent because of its retrospective design.
Author Contributions
Conceived and designed the analysis: Yun J, Cho JH, Ahn YC, Kim HK.
Collected the data: Yun J, Cho JH, Kim HK, Korean Association for Lung Cancer, Korea Central Cancer Registry.
Contributed data or analysis tools: Yun J, Hong TH, Yang K.
Performed the analysis: Yun J, Hong TH, Yang K.
Wrote the paper: Yun J, Cho JH, Hong TH, Ahn YC, Kim HK.
Conflicts of Interest
Yong Chan Ahn, the editor-in-chief of the Cancer Research and Treatment, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Acknowledgments
The data used for this study were provided by the Korean Association for Lung Cancer & Ministry of Health and Welfare, Korea Central Cancer Registry.