Prognostic Value of the Evolution of HER2-Low Expression after Neoadjuvant Chemotherapy
Article information
Abstract
Purpose
Patients with human epidermal growth factor receptor 2 (HER2)–low advanced breast cancer can benefit from trastuzumab deruxtecan. Given the unclear prognostic characteristics of HER2-low breast cancer, we investigated the prognostic characteristics of HER2-low expression from primary tumor to residual disease after neoadjuvant chemotherapy (NACT).
Materials and Methods
The data of HER2-negative patients receiving NACT at our center were collected. Pathological complete response (pCR) rate were compared between HER2-0 and HER2-low patients. The evolution of HER2 expression from primary tumor to residual disease and its impact on disease-free survival (DFS) were examined.
Results
Of the 690 patients, 494 patients had HER2-low status, of which 72.3% were hormone receptor (HR)–positive (p < 0.001). The pCR rates of HER2-low and HER2-0 patients (14.2% vs. 23.0%) showed no difference in multivariate analysis regardless of HR status. No association was observed between DFS and HER2 status. Of the 564 non-pCR patients, 57 (10.1%) changed to HER2-positive, and 64 of the 150 patients (42.7%) with HER2-0 tumors changed to HER2-low. HER2-low (p=0.004) and HR-positive (p=0.010) tumors before NACT were prone to HER2 gain. HER2 gain patients had a better DFS compared with HER2-negative maintained patients (87.9% vs. 79.5%, p=0.048), and the DFS of targeted therapy group was better than that of no targeted therapy group (92.4% vs. 66.7%, p=0.016).
Conclusion
Although HER2-low did not affect the pCR rate and DFS, significant evolution of HER2-low expression after NACT creates opportunities for targeted therapy including trastuzumab.
Introduction
Human epidermal growth factor receptor 2 (HER2) is a prognostic factor for breast cancer (BC) and a predictor of targeted therapy. At present, BC is categorized into HER2-positive (20%) and HER2-negative (80%) according to HER2 expression [1]. Anti-HER2 targeted therapy drugs, represented by trastuzumab, have been shown to significantly improve the prognosis of patients with HER2-positive BC [2,3]. On the contrary, studies have reported that HER2-negative BC does not respond to conventional anti-HER2 targeted therapy [1,4,5]. HER2-low expression is defined as immunohistochemistry (IHC) 1+ or IHC 2+ and HER2 gene not amplified measured by in situ hybridization; HER2-0 expression is defined as IHC 0. More than half of HER2-negative patients have HER2-low expression [6]. Previous studies have established the efficacy of novel antibody-drug conjugates (ADCs) in treating HER2-low tumors [7,8]. The DESTINY-Breast04 phase 3 trial recently reported positive results for HER2-low advanced BC [9]. These findings suggest that HER2-low BC may be a distinct biological subtype.
However, the biological behavior of HER2-low BC is yet to be elucidated. HER2-low BCs exhibit significant heterogeneity [5,10,11]. According to previous studies, the prognostic value of HER2-low remains controversial [12–14]. Discrepancies are also found in the influence of the HER2-low status on the pathological complete response (pCR) rate of patients receiving neoadjuvant chemotherapy (NACT) [12,13,15]. The diverse evolution of HER2 status before and after NACT have been observed in previous studies [16–18]. These controversial results suggest that the clinicopathological characteristics and prognostic value of HER2-low patients with early BC receiving NACT warrant further research.
This study aimed to analyze the impact of the HER2-low status on pathological response and prognosis, to explore the biological characteristics of HER2-low BC, and to analyze the evolution of the HER2-low status after NACT and its impact on prognosis.
Materials and Methods
1. Patient selection
We retrospectively analyzed the clinicopathological data of early BC patients treated at the Henan Cancer Hospital from January 1, 2016 to December 31, 2020. This study was approved by the Medical Ethics Committee of Henan Cancer Hospital (Research Approval Number: 2022-299).
The inclusion criteria were as follows: (1) female sex; (2) age > 18 years; (3) HER2-negative BC. HER2-negative criteria were as follows: IHC 0 or IHC 1+ or IHC 2+ and HER2 gene not amplified by fluorescence in situ hybridization; (4) stages II-III invasive BC, and all patients having received anthracycline and taxane-based NACT, followed by a curable surgery; (5) available data on the HER2 status, estrogen receptor (ER), progesterone receptor (PR), and the Ki-67 index before and after NACT; (6) accurate clinical tumor (cT) and nodal (cN) staging according to the American Joint Committee on Cancer (AJCC) systems (7th edition); (7) available follow-up.
Exclusion criteria were as follows: (1) HER2 positive BC; (2) male BC; (3) metastatic BC; (4) patients without NACT; (5) bilateral BC; (6) inflammatory BC; (7) received targeted or endocrine therapy in the neoadjuvant setting; (8) combined with other primary tumors.
2. Information collection and follow-up
The present study collected clinical data about patients, including their age, menopausal status, clinical T and N staging, the expression of ER, PR, HER2 and Ki-67 index before and after NACT, operation information, radiotherapy, postoperative therapy, relapse time, relapse site, and disease-free survival (DFS).
Considering that the efficacy of endocrine therapy is not significant when hormone receptor (HR) expression < 10%, we defined the cut-off value of HR positivity as 10%. In this study, HR-positive was defined as ER ≥ 10% and/or PR ≥ 10% and HR-negative as ER < 10% and PR < 10%. Ki-67 ≥ 20% was defined as the high expression and Ki-67 < 20% as the low expression. pCR was defined as the absence of residual invasive tumor cells in the postoperative specimen from breast and regional lymph nodes (ypT0/ypTis ypN0). DFS was defined as the period from surgery to disease relapse or death from any cause.
3. Statistical analyses
Statistical analysis was performed using SPSS ver. 23.0 (IBM Corp., Armonk, NY). The influencing factors of pCR and HER2 status were analyzed by chi-square test and logistic regression model. The independent risk factors affecting the prognosis of HER2-negative BC were analyzed through univariate and multivariate Cox regression analyses. Two-sided p < 0.05 was considered to indicate statistical significance. Kaplan-Meier survival curve was applied to reflect the impact of risk factors on prognosis. The Mulberry diagram was completed by using the R software (ver. 4.0.3, R Foundation for Statistical Computing, Vienna, Austria).
Results
1. Clinicopathologic characteristics based on HER2 status
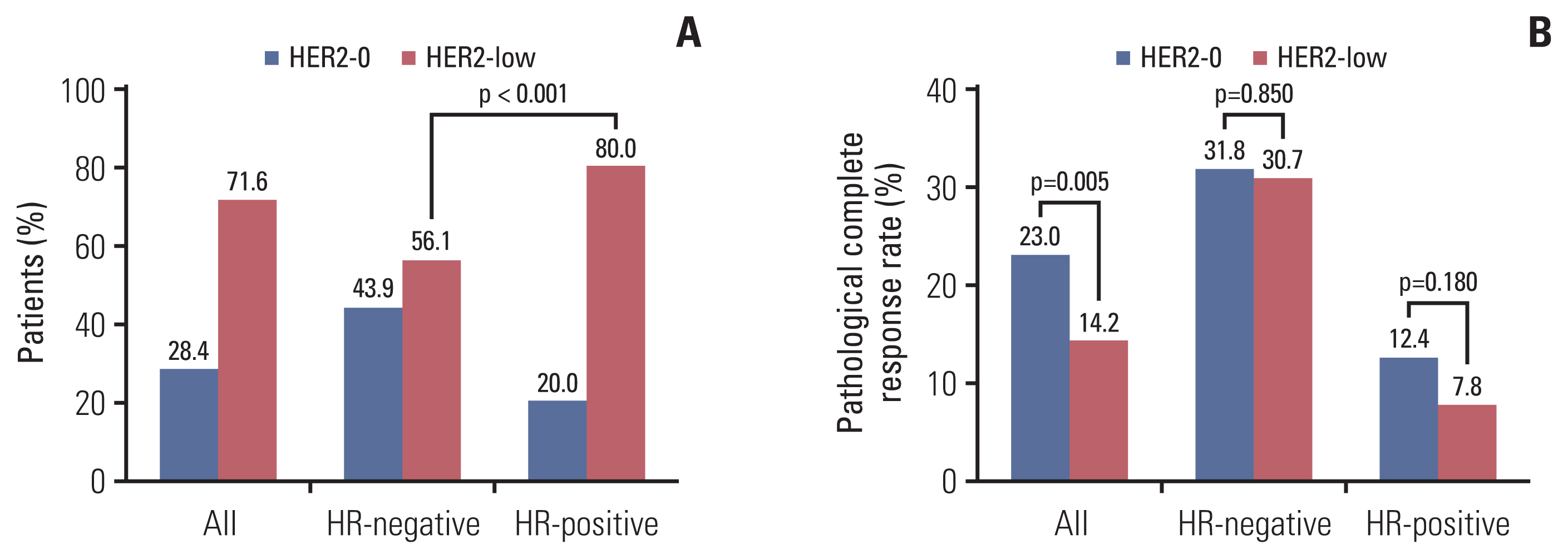
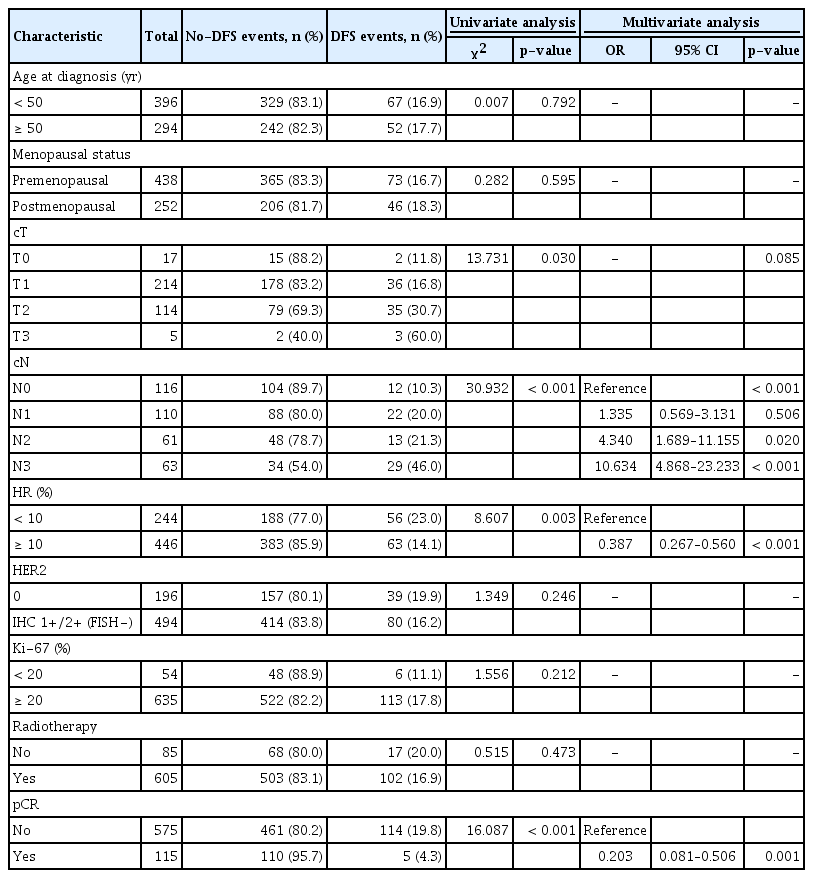
Of the 690 patients, 196 (28.4%) had HER2-0 tumors and 494 (71.6%) had HER2-low tumors (Table 1). The proportion of HER2-low was associated with HR status (80% in HR-positive patients vs. 56.1% in HR-negative, p < 0.001) (Fig. 1A). Furthermore, of the 690 patients, 641 (92.9%) had T2–T4 stage tumors and 562 (81.4%) had invasions of regional lymph nodes. Univariate analysis showed that the HER2-low status was also associated with age at diagnosis, menopausal status, and Ki-67 expression. No statistical difference was observed for the clinical T and N categories. In multivariate analysis, the HER2-low status was related to HR (odds ratio [OR], 3.389; 95% confidence interval [CI], 2.359 to 4.870; p < 0.001) and menopausal (OR, 2.111; 95% CI, 1.245 to 3.579; p=0.006) status (Table 1).
2. Effect of the HER2 status on pathological response
Of the 690 HER2-negative patients, 115 patients (16.7%) achieved pCR after NACT, 76 (31.1%) of the HR-negative patients achieved pCR, and 39 (8.7%) of the HR-positive patients achieved pCR (p < 0.001). In univariate analysis, achievement of pCR was associated with HR status, HER2 status, and clinical T and N categories (Table 2). The pCR rate of HER2-0 patients was significantly higher than that of HER2-low patients (23.0% vs. 14.2%, p=0.005) (Fig. 1B). In HR-negative patients, there was no significant difference in the pCR rate between HER2-0 and HER2-low tumors (31.8% vs. 30.7%, p=0.850) (Fig. 1B). In HR-positive patients, although the pCR rate of the HER2-0 and HER2-low groups did not achieve a statistical difference, the pCR of HER2-0 was higher than that of HER2-low numerically (12.4% vs. 7.8%, p=0.180) (Fig. 1B). The lower pCR rate of patients was associated with higher cT (p=0.018) and cN (p=0.003) categories. No significant difference was observed for Ki-67, age at diagnosis, and menopausal status (Table 2).
The variables with p-value < 0.05 in the chi-square test were included in the logistic regression model analysis. Multivariate analyses revealed that HR status and clinical T and N categories were independent predictors of pCR in patients with HER2-negative BC. No association was observed between HER2-low status and pCR rate (Table 2).
3. DFS based on the HER2 status before NACT
After a median follow-up of 40.2 months (95% CI, 38.9 to 41.5), the DFS of the overall population was 83.5%. Univariate analysis and Cox regression analyses revealed that clinical N category (p < 0.001), HR status (HR, 0.387; 95% CI, 0.267 to 0.560; p < 0.001), and pCR (HR, 0.203; 95% CI, 0.081 to 0.506; p=0.001) were independent prognostic factors (Table 3). HER2-0 and HER2-low patients had comparable DFS (81.1% vs. 84.1%, log-rank test p=0.387) (Fig. 2A).
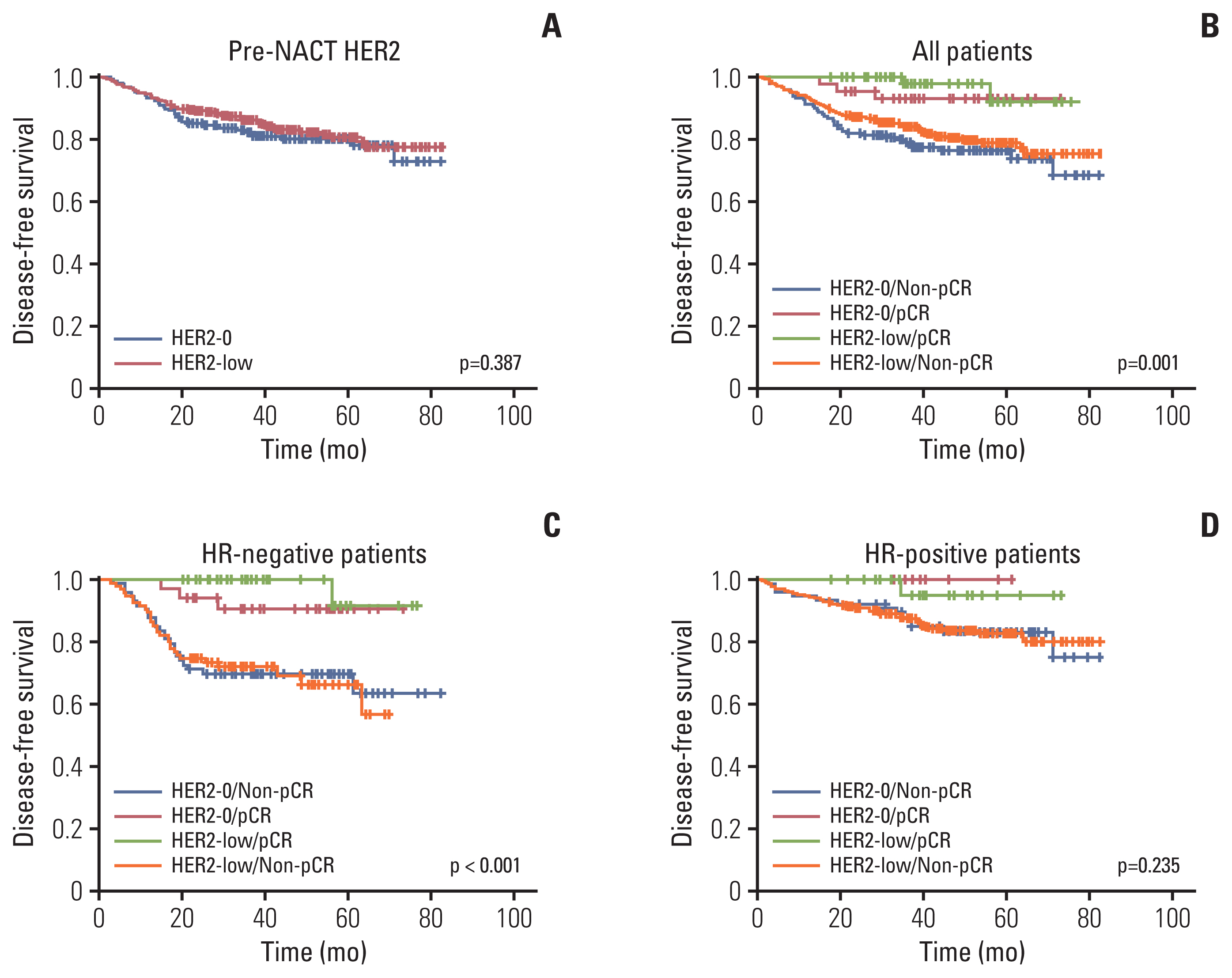
Kaplan-Meier survival analysis for disease-free survival according to pre-NACT human epidermal growth factor receptor 2 (HER2) status (A) and pathological complete response (pCR) status (B) in all, hormone receptor (HR)–negative (C), and HR-positive (D) patients.
The DFS events rate of HR-positive patients was lower than that of HR-negative patients (14.1% vs. 23.0%, p < 0.001). Patients with pCR had a lower DFS events rate than those without pCR (4.3% vs. 19.8%, p=0.001) (Table 3) regardless of the HER2-low or HER2-0 status (Fig. 2B). In HR-negative patients, there was no significant difference in DFS between HER2-0 and HER2-low tumors both in pCR and non-pCR groups (Fig. 2C). Similarly, no statistical difference was observed in DFS between HER2-low and HER2-0 tumors in HR-positive patients (Fig. 2D). Additionally, pCR patients had a better DFS rate compared with non-pCR patients in the HR-negative (p < 0.001) and HR-positive (p=0.041) subgroups (Fig. 2C and D).
4. HER2 evolution from primary tumors to residual diseases after NACT
Re-testing of the HER2 status was performed in 564 of the 575 non-pCR patients after NACT (Table 4), of which 57 (10.1%) became HER2-positive (HER2 gain) after NACT. Of these patients, 150 (26.6%) were HER2-0 and 414 (73.4%) were HER2-low before NACT (Table 4). Compared with primary tumors before NACT, the samples of residual diseases after NACT showed significant discordance in HER2 expression; both HER2-low and HER2-0 status changed to HER2-positive, HER2-low or HER2-0 status (Fig. 3). Of the 150 HER2-0 patients, 64 (42.7%) changed to HER2-low and three (2.0%) changed to HER2-postive. Of the 414 HER2-low patients, 58 (14%) changed to HER2-0 and 54 (13.0%) changed to HER2-positive (Fig. 3).

Clinicopathological characteristics associated with HER2-negative and HER2-positive status after NACT
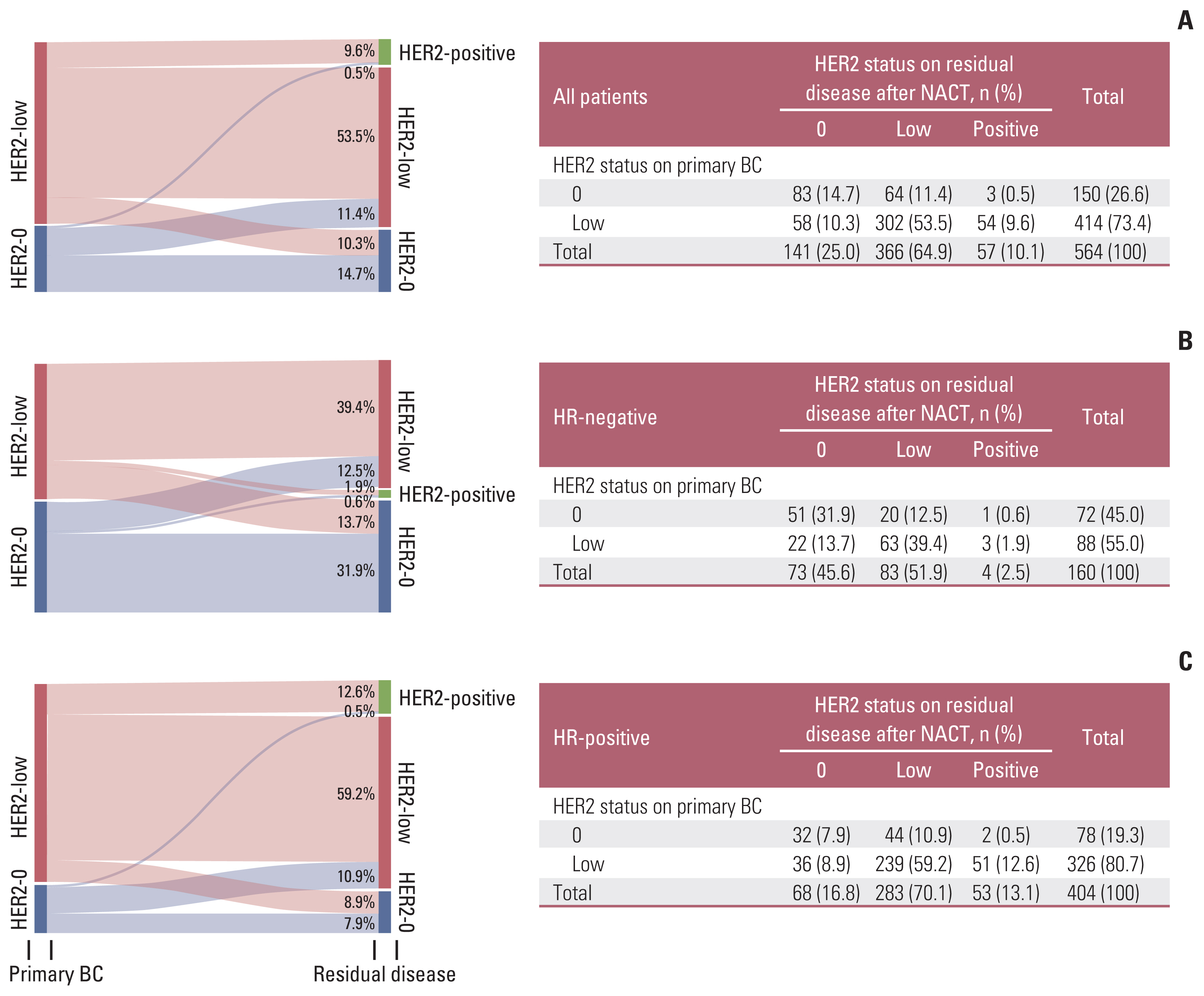
Evolution of human epidermal growth factor receptor 2 (HER2) status from primary breast cancer (BC) tumors to residual diseases after neoadjuvant chemotherapy (NACT) in all (A), hormone receptor (HR)–negative (B), and HR-positive (C) patients.
HR-positive and HER2-low tumors had a high rate of HER2 gain (Fig. 4A). The ratio of HER2 gain in HR-positive patients after NACT was higher than that in HR-negative patients (13.1% vs. 2.5%; OR, 3.995; 95% CI, 1.391 to 11.469; p=0.010) (Table 4). HER2-low tumors were more inclined to achieve HER2-positive status after NACT than HER2-0 tumors, with and without stratification based on the HR status (HER2-low 13.0% vs. HER2-0 2.0%; p=0.004; HR−/HER2-low 1.9% vs. HR−/HER2-0 0.6%; HR+/HER2-low 12.6% vs. HR+/HER2-0 0.5%) (Table 4, Fig. 3).
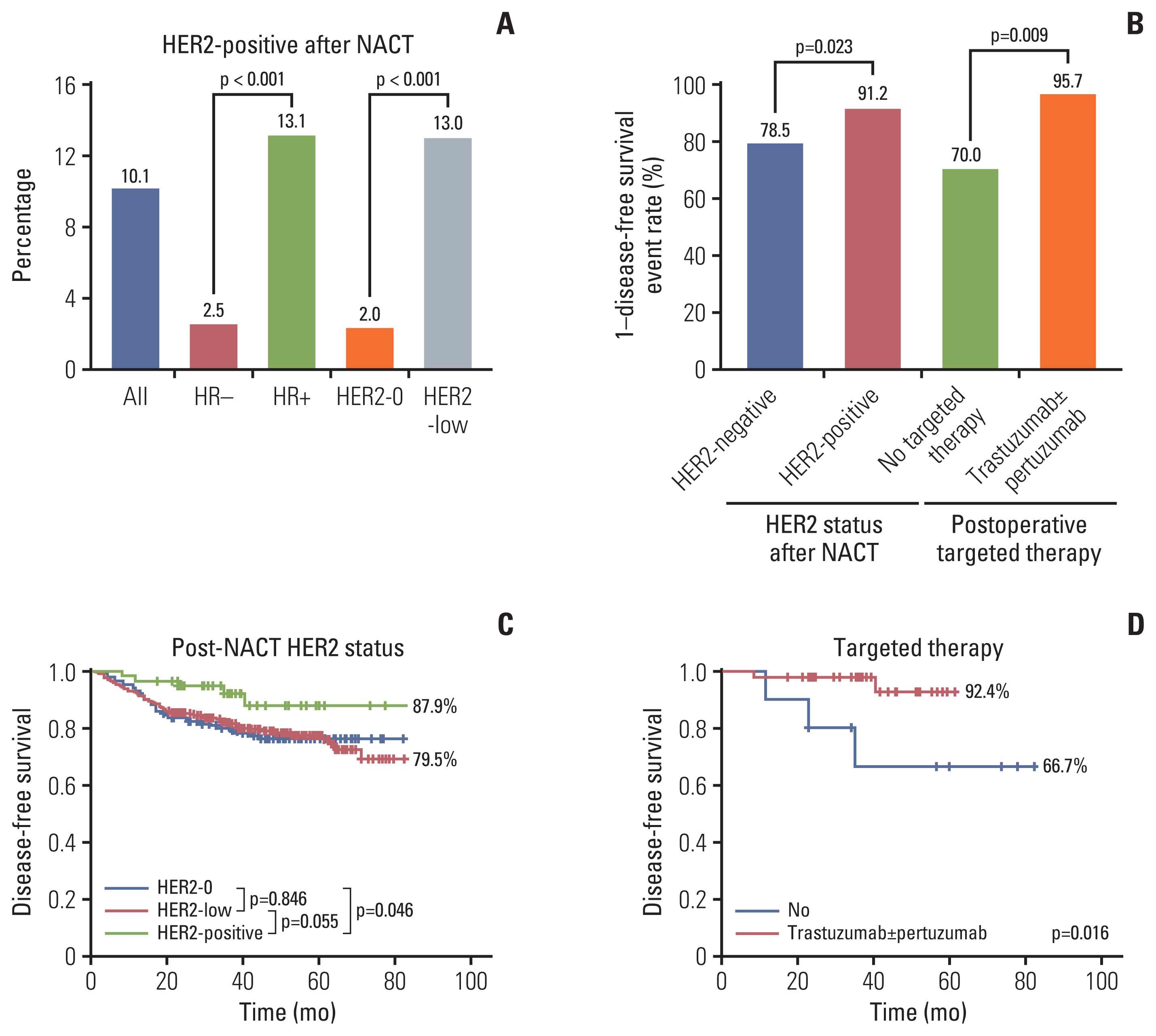
Human epidermal growth factor receptor 2 (HER2) status and disease-free survival analysis after neoadjuvant chemotherapy (NACT). (A) Distribution of HER2-positive according to hormone receptor (HR) and HER2 status before NACT. (B) The impact on disease-free survival events rate of HER2 gain and targeted therapy. (C) Kaplan-Meier survival analysis for disease-free survival according to HER2 status after NACT (HER2-positve 87.9% vs. HER2-negative maintained 79.5%; p=0.048). (D) Kaplan-Meier survival analysis for disease-free survival according to targeted therapy in HER2-positive patients after NACT.
5. DFS based on the HER2 status after NACT
Of the 564 patients with HER2 re-testing results, DFS events occurred in five of the 57 HER2 gain patients and 109 of 507 the HER2-negative maintained patients (χ2 test p=0.023) (Fig. 4B). The DFS of patients with HER2-positive status after NACT was significantly better than that of HER2-negative maintained patients (87.9% vs. 79.5%; log-rank test p=0.048) (Fig. 4C). Compared with HER2-0 patients, HER2-positive patients had a better DFS rate (log-rank test p=0.046). When comparing HER2-0 and HER2-low patients with residual diseases, there was no significant difference in DFS (log-rank test p=0.846). Although DFS was not statistically different between HER2-low and HER2-positive patients, the trend was that the DFS of the HER2-positive group was better (Fig. 4C).
Of the 57 patients with HER2-positive tumors after NACT, DFS events occurred in three of the 10 patients who did not receive targeted therapy, two of the 30 patients who received trastuzumab therapy, and 0 of the 17 patients who received trastuzumab combined with pertuzumab therapy after the surgery (χ2 test p=0.009) (Fig. 4B). The DFS of the targeted therapy group was significantly better than that of the no targeted therapy group (92.4% vs. 66.7%; log-rank test p=0.016) (Fig. 4D).
Discussion
Although studies have shown that HER2-low BC is heterogeneous [5,10,11], the DESTINY-Breat04 trial observed that patients with HER2-low metastatic BC gain survival benefits from trastuzumab deruxtecan, a novel ADC [9]. These findings suggest that the intrinsic characteristics of HER2-low BC have not yet been discovered. Our study found that the HER2-low status was remarkably correlated with HR-positive expression. In univariate analysis, the pCR rate of HER2-low patients was lower than that of HER2-0 patients in the entire population; however, the difference was not statistically significant in multivariate analysis. No difference was noted in DFS between HER2-low and HER2-0 tumors in overall population analysis or stratified analysis based on HR status and pCR status. It is noteworthy that our study observed that the HER2 status changed significantly after NACT. Nearly half of the HER2-0 tumors before NACT changed to HER2-low status. These patients would probably benefit from novel ADCs. The change of the HER2 status from negative to positive was common in HR-positive and HER2-low groups. HER2 gain and targeted therapy with trastuzumab alone or trastuzumab plus pertuzumab improved the DFS of patients with HER2-negative expression before NACT.
The proportion of HER2-low expression in HER2-negative patients reported in previous large-scale studies ranged from 31% [19] to 64% [20]. The proportion of HER2-low expression in our study was 71.6%, which is close to a previous report on the Asian population [20]. Such a large variation in the HER2-low ratio may be influenced by racial differences, discrepancies in the interpretations of IHC scores between pathologists, etc. One study pointed out that the consistency rate of IHC 0 and 1+ between pathologists was only 26% [21]. This inconsistent result may be linked to the linear continuous distribution of HER2 expression. In the past, this inconsistency did not affect the choice of targeted therapy. However, the inconsistency may affect the results of retrospective studies on HER2-low expression and the screening of patients with indications for novel ADCs.
As the biological characteristics of patients with low HR expression (1%–9%) were closer to those of patients with triple-negative breast cancer (TNBC) [22], our study defined low HR expression (1%–9%) as HR-negative. In this study, the correlation between HER2-low and HR-positive status was similar to that of other large-sample retrospective studies [12,13,15,19]. In previous studies, the results of the relationship between HER2-low status and pCR rate were inconsistent, which was common in subgroup analysis based on HR status [12,13,15]. In these studies, including ours, there is a trend that HER2-low expression is associated with low pCR rate in the general and HR-positive population. This observation suggests that patients with HER2-low expression may have insufficient treatment in the neoadjuvant therapy setting.
Two studies with large sample size on the influence of HER2-low expression on DFS and overall survival (OS) have been published recently by Denkert et al. [12] in Lancet Oncology and by Tarantino et al. [13] in JAMA Oncology. The two studies reported differing results. Denkert et al. [12] found that DFS and OS of HER2-low patients were significantly better than those of HER2-0 patients in the overall as well as TNBC population. On the contrary, Tarantino et al. [13] observed that there was no statistical difference in DFS and OS between HER2-low and HER2-0 tumors. In addition to the differences in follow-up time, the sample size and proportion of TNBC included in the two studies varied. In the former study, 50.3% of the 2,310 patients had TNBC, whereas, in the latter study, only 14.1% of the 5,235 patients had TNBC. These differences may explain the discrepancy between the results of the two studies. In a report with a median follow-up time of 148 months, the analysis of 6,934 patients with TNBC found that the BC-specific survival of HER2-low patients was better than that of HER2-0 patients [19]. Kang et al. [23] stated that the 5-year DFS of patients with HER2-low tumors was better than that of patients with HER2-0 tumors in the HR-negative subgroup. Studies reporting negative results were generally characterized by a short follow-up time or a relatively small sample size of TNBC [10,20].
Some studies have documented that HER2-low expression is associated with poor prognosis in HR-positive patients [14,24], which may be related to the fact that the HER2 pathway is involved in endocrine therapy resistance [25]. The de novo endocrine resistance is related to the co-existence of HER2 pathway, while the acquired endocrine resistance of endocrine therapy may be related to the acquired HER2 gene amplification [26]. In a previous study, PAM50 data showed that the level of HER2 gene in HER2-low group was higher than that in HER2-0 group in HR-positive population [10]. This suggests that HER2-low patients in the HR-positive population are prone to endocrine resistance. This study found that HER2-low and HR-positive tumors are more likely to change to HER2-positive tumors after neoadjuvant chemotherapy, which may also be one of the mechanisms of tumor resistance. For patients with endocrine-resistant HR-positive BC with HER2-low expression, novel ADCs may be useful. Another study opined that the risk of brain metastasis in HER2-low tumors is higher than that of HER2-0 tumors [24], which is similar to the characteristics of HER2-positive tumors. Another study discovered that the DFS and OS of the HER2-low group were better than those of the HER2-0 group in patients with HR-positive BC of Oncotype DX who have a high genetic risk [27]. From the results of the above studies, it appears that HER2-low tumors are associated with a better prognosis than HER2-0 tumors in TNBC and high-risk HR-positive BC, whereas HER2-low tumors have a worse prognosis than HER2-0 tumors in low-risk HR-positive BC. However, Almstedt et al. [28] found that DFS and OS of HER2-low patients were significantly better than those of HER2-0 patients (15-year DFS, 67.5% vs. 47.3%, p < 0.001; 15-year OS, 75.4% vs. 66.8%, p=0.009), and OS analysis was also significant in the HR-positive group (p=0.039) [28].
These diverse findings could be attributed to tumor heterogeneity, and another possible reason is the inconsistent interpretations of IHC 0 and 1+ among pathologists. The success of trastuzumab deruxtecan in the treatment of advanced BC makes it imperative to accurately define the HER2-low status. According to the latest guidelines, HER2-0 status includes tumors that faintly express HER2 in ≤ 10% of tumor cells [1]. The current definition of the HER2-low status may not permit accurate screening of the population suitable for novel ADCs treatment. The presently used IHC detection method is not perfect for distinguishing HER2-low and HER2-0 expressions. We need a better detection method to objectively and accurately determine the HER2 expression level, and quantitative real-time polymerase chain reaction may be a candidate.
Our study observed that the evolution of HER2 expression from primary tumors to residual diseases is related to HR and HER2 status before NACT. In another study, it was also noted that the HER2-low and HER2-0 expressions changed significantly after neoadjuvant treatment [18]. This significant alteration may be caused by the spatial heterogeneity of the tumor or the abovementioned limitations of the detection methods and the inconsistencies in the interpretations of pathologists. Other studies had established that the HER2-low expression differs significantly between primary and recurrent tumors [29,30]. We prefer to use tumor heterogeneity to explain the significant changes in HER2 expression because the evolution happened in so many patients.
In our study, the DFS of the 57 HER2-positive patients after NACT was significantly better than that of the HER2-negative maintained patients. The majority of the HER2-positive patients received trastuzumab (with or without pertuzumab) therapy after NACT. In the other study, no difference was observed in DFS between HER2 gain and HER2-negative maintained patients after NACT (p=0.26) [31]. The number of HER2 gain patients was too small (n=11), and only three patients (27.8%) received trastuzumab in this study. This finding suggests that the use of IHC can be continued until the new HER2 detection technology is confirmed, especially in patients with non-pCR. The result also emphasizes the need to retest the HER2 status after NACT. For tumors with spatial heterogeneity, NACT may not kill HER2-positive or HER2-low tumor cells, which can be treated with trastuzumab or novel ADCs.
The advantage of this research is that most of the patients enrolled in the neoadjuvant therapy study had a high risk of recurrence, which can yield convincing data on the biological characteristics of HER2-low BC. The novelty of the study is that it has analyzed the influence of the HER2 status of the residual disease on DFS after NACT. The findings provide clinical support for the selection of postoperative treatment options and the necessity to retest the HER2 status in residual diseases after NACT for HER2-negative BC. Nonetheless, our research has some limitations. Our study was retrospective research from a single central database. Furthermore, the median follow-up time was only 40.2 months.
HER2-low was associated with HR positivity. The HER2-low status did not have a significant effect on pCR and DFS, and the evidence is insufficient to consider HER2-low tumors as a distinct biological subtype of BC. The HER2 status changed significantly after NACT, which may offer targeted therapy opportunities, such as trastuzumab. HER2-low tumors were more likely to change to HER2 positivity after NACT when compared with HER2-0 tumors, and the evolution was more common in the HR-positive group. HER2 gain patients benefited from targeted therapy, with a better DFS than HER2-negative maintained patients.
Notes
Ethical Statement
This study was conducted in accordance with the standards set out in the Declaration of Helsinki. This study was approved by the Medical Ethics Committee of Henan Cancer Hospital (Research Approval Number: 2022-299). The Medical Ethics Committee of Henan Cancer Hospital granted exemption from informed consent for the study given its retrospective nature.
Author Contributions
Conceived and designed the analysis: Ma Y, Chen X, Liu Z.
Collected the data: Ma Y, Zhu M, Zhang J, Lv M.
Contributed data or analysis tools: Ma Y, Zhu M, Zhang J, Chen X.
Performed the analysis: Ma Y, Zhu M, Zhang J, Lv M, Liu Z.
Wrote the paper: Ma Y, Zhu M, Chen X, Liu Z.
Funding acquisition: Ma Y, Liu Z.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This research was sponsored by the Henan Provincial Medical Science and Technology Research Project (grant number: LHGJ20220204) and Training Program for Young and Middle-aged Health and Technology Innovation Leaders in Henan Province (grant number: YXKC2022005).
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.