Clinical Significance of bZIP In-Frame CEBPA-Mutated Normal Karyotype Acute Myeloid Leukemia
Article information
Abstract
Purpose
We evaluated the characteristics of CCAAT/enhancer-binding protein α (CEBPA) mutations and the significance of a basic leucine zipper in-frame mutation (bZIPin-f) of CEBPA in patients with acute myeloid leukemia with a normal karyotype.
Materials and Methods
Based on updated knowledge of CEBPA mutations, we conducted next-generation sequencing analyses in a previously established real-world cohort.
Results
Among 78 of a total of 395 patients (19.7%), 50 had bZIPin-f CEBPA, and 28 had non-bZIPin-f CEBPA. In the multivariate analysis, patients with NPM1mut, those with bZIPin-f CEBPA, and those who underwent allogeneic hematopoietic cell transplantation (allo-HCT) had favorable overall survival (OS), but FLT3-ITDmut was a poor prognostic indicator. For relapse-free survival (RFS) and cumulative incidence of relapse, bZIPin-f CEBPA, and allo-HCT were associated with favorable outcomes; FLT3-ITDpos was associated with worse outcomes. In the CEBPA double-mutated group (CEBPAdm), bZIPin-f CEBPA was associated with superior outcomes in terms of OS (p=0.007) and RFS (p=0.007) compared with non-bZIPin-f CEBPA. Of 50 patients with bZIPin-f CEBPA, 36 patients had at least one mutation. When grouped by the presence of mutations in chromatic/DNA modifiers (C), cohesion complex (C), and splicing genes (S) (CCS mutations), CCS-mutated bZIPin-f CEBPA was associated with poor OS (p=0.044; hazard ratio [HR], 2.419) and a trend in inferior RFS (p=0.186; HR, 1.838).
Conclusion
Only bZIPin-f CEBPA was associated with favorable outcomes in patients with CEBPAdm. However, some mutations accompanying bZIPin-f CEBPA showed inferior OS; thus, further studies with larger numbers of patients are required for clear conclusions of the significance of bZIPin-f CEBPA.
Introduction
CCAAT/enhancer-binding protein α (CEBPA) is a member of the family of basic leucine zipper (bZIP) transcription factors. It regulates tissue-specific gene expression and proliferation arrest and is critical for neutrophil development [1–3]. CEBPA mutations occur in 7.5%–11% of cases of de novo acute myeloid leukemia (AML) and predominantly in patients with a normal karyotype (NK-AML) with 15%–18% [4–6].
Two major types of CEBPA mutations have been identified in AML, and patients can have double or single mutations. CEBPA mutations affect either the N-terminus with nonsense mutations, leading to a dominant negative protein, or the C-terminus, resulting in decreased DNA binding of a leucine zipper region. Single mutations of CEBPA (CEBPAsm) occur in either the N- or C-terminus of the gene, and double mutations of CEBPA (CEBPAdm) dominantly involve both N-terminal frameshift mutations and C-terminal in-frame (bZIP) mutations, resulting in a lack of CEBPA wild-type allele expression [6,7].
The World Health Organization (WHO) Working Group classified AML with mutated CEBPA as a provisional entity [8,9]. NK-AML with a CEBPA mutation is associated with a favorable prognosis, similar to that of core binding factor AML [10]. Several studies have demonstrated that CEBPAdm is associated with a specific gene signature and a favorable outcome in patients with NK-AML, whereas CEBPAsm is not significantly different from wild-type (CEBPAwild) [11–13]. Due to advancements in understanding of CEBPA mutations and accumulation of clinical data, a favorable prognosis in patients with CEBPA mutations depends on the bZIP location of the CEBPA mutation but not on whether there is a single or double mutation [14–16]. The reason that CEBPAdm has been reported to be a good prognostic factor because most cases of CEBPAdm (94%) harbor bZIP mutations [15]. In particular, cases with an in-frame mutation of bZIP (bZIPin-f) show favorable outcomes; this is accompanied by an in-frame mutation in approximately 81% of bZIP mutations [16]. Approximately 36% of patients with CEBPAsm carry bZIPin-f and share a similar spectrum of co-mutations and clinical factors with patients with CEBPAdm. Based on these results, the 2022 European LeukemiaNet (ELN) classified only bZIPin-f CEBPA as a favorable risk category rather than including existing biallelic CEBPA mutations [17].
In a previous study, we reported on the clinical significance of single or double CEBPA mutations in NK-AML, and CEBPAdm was associated with a favorable prognosis with consolidation chemotherapy alone without allogeneic hematopoietic cell transplantation (allo-HCT) [13]. Based on the updated knowledge about CEBPA mutations, we conducted next-generation sequencing (NGS) analyses in a previously established real-world cohort to evaluate the characteristics of CEBPA mutations and the significance of bZIPin-f CEBPA. To clarify the prognostic significance of bZIPin-f CEBPA in AML, we directly compared the treatment results according to the consolidation type (consolidation chemotherapy alone or allo-HCT) in patients with bZIPin-f CEBPA. In addition, we analyzed the clinical significance of accompanying mutations in bZIPin-f CEBPA NK-AML.
Materials and Methods
1. Patients and treatment
The previous study included a total of 404 patients diagnosed with NK-AML from October 1998 to September 2012 at seven participating institutes [13]. A total of 395 samples of 404 were available for additional targeted NGS [18]. All patients met the following eligibility criteria: (1) age ≥ 15 years; (2) diagnosis of NK-AML confirmed by conventional cytogenetic analysis; and (3) treatment with induction chemotherapy using a standard protocol (a 3-day course of anthracyclines with a 7-day course of cytosine arabinoside). Patients who achieved complete remission (CR) received consolidation chemotherapy with or without allo-HCT depending on the availability of a matched related or unrelated donor. Genetic factors were not considered when choosing allo-HCT as a consolidation treatment.
2. CEBPA mutations and other genetic analyses
Cryopreserved bone marrow or peripheral blood samples taken at diagnosis were archived prior to genomic DNA extraction using QIAamp DNA blood mini-kits (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Mutation analysis was performed for CEBPA using Sanger sequencing employing polymerase chain reaction methodology [13]. For NGS analysis, Agilent custom probes were designed to cover the entire exon regions of targeted genes (92 genes) and sequenced according to the manufacturer’s protocol using an Illumina HiSeq 2000 sequencer [18]. The variant calling was performed under identical conditions to a previously reported study with a detection threshold of 3% variant allele frequency [18].
3. Endpoints of response and survival
The CR criteria were defined as follows [19]: The incidence of relapse was defined as the time from attainment of remission to the date of relapse in all patients who achieved CR, considering the competing risk of death without relapse. Non-relapse mortality (NRM) was defined as death occurring in the absence of relapse. Relapse-free survival (RFS) was defined as the time from attainment of remission to the date of death from any cause or relapse, whichever occurred first. Overall survival (OS) was defined as the time from commencement of induction chemotherapy to the date of last follow-up or death from any cause.
4. Statistical analysis
The chi-squared test was used to compare differences in the distributions of categorical data, and Student’s t test was used to evaluate the significance of differences in continuous variables. RFS and OS were estimated using Kaplan-Meier survival curves; differences among groups were compared using the log-rank test. The prognostic impacts of various risk factors on RFS and OS were evaluated using a Cox proportional hazard model. Time-dependent Cox proportional hazard models were used to examine time-dependent covariates by considering allo-HCT as a time-dependent covariate. To evaluate the risk of relapse and NRM, the cumulative incidence of relapse (CIR) and NRM were calculated using the Gray method and Fine–Gray proportional hazard regression model, considering competing events [20]. To clarify the immortal time bias, we performed a landmark analysis to compare the consolidation type. Covariates with parameters that were significant in the univariate analyses were included in the multivariate analyses. p-values of < 0.05 were considered to indicate statistical significance, and mutations detected with a frequency of 5% or more were analyzed. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using a predetermined reference risk value of unity. All statistical analyses were performed using EZR software using the ‘R’ language (available at http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html) [20].
Results
1. Reclassification according to the location of CEBPA mutations
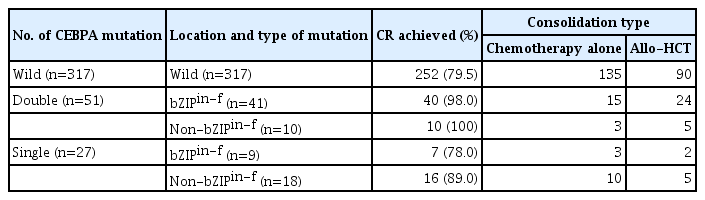
CEBPA mutations were observed in 78 of 395 patients (19.7%). Fifty patients had bZIPin-f CEBPA, and 28 patients had non-bZIPin-f CEBPA. CEBPAdm and CEBPAsm were obser-ved in 51 and 27 patients, respectively. Among patients with CEBPAdm, bZIPin-f CEBPA was detected in 41 patients (80%), and non-bZIPin-f CEBPA was observed in 10 patients (20%). Of the 51 patients with CEBPAdm, double C-terminal bZIPin-f was observed in only one patient. In 27 patients with CEBPAsm, nine (33%) instances of bZIPin-f CEBPA and 18 (67%) instances of non-bZIPin-f CEBPA were detected. In patients with CEBPA mutations (n=78), the risk stratification changed in 24% (from CEBPAdm [favorable] to non-bZIPin-f CEBPA [intermediate]:10 patients, from CEBPAsm [intermediate] to bZIPin-f CEBPA [favorable]: 9 patients) according to the location and type of mutation rather than the accompanying number of CEBPA mutations (Table 1).
2. Clinical characteristics and their associations with mutations
Younger patients were more likely to have bZIPin-f CEBPA mutations than CEBPAwild (p < 0.001) and non-bZIPin-f (p=0.001) (Table 2). Non-bZIPin-f CEBPA mutations were associated with high peripheral blast counts (p=0.031) in comparison with CEBPAwild.

Clinical characteristics and mutation status in patients with normal karyotype acute myeloid leukemia
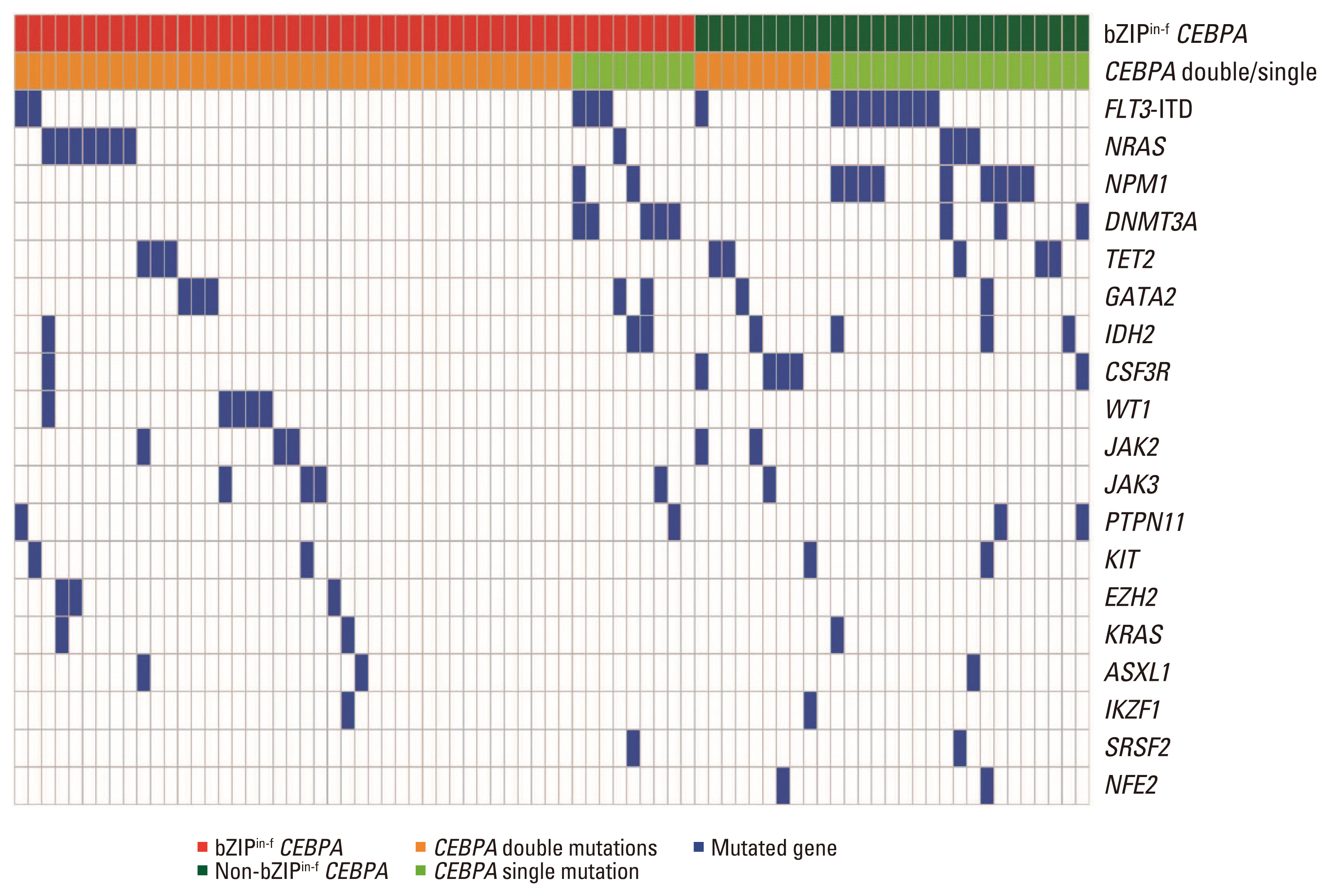
bZIPin-f CEBPA mutations and their associations with other molecular mutations are described in Table 1 and Fig. 1. bZIPin-f CEBPA mutations were associated with GATA2 mutations (p=0.029) and less frequently associated with FLT3-ITD (p=0.003), NPM1 mutations (p < 0.001), DNMT3A mutations (p=0.008), and IDH1 mutations (p=0.013) than CEBPAwild. bZIPin-f CEBPA was also less frequently associated with NPM1 mutations (p=0.001) and FLT3-ITD (p=0.028) than bZIPin-f CEBPA and non-bZIPin-f CEBPA (Table 2).
3. Favorable prognostic impact of bZIPin-f CEBPA mutations
CR was achieved in 325 of 395 patients (82.3%). The CR rate was 94% in patients with bZIPin-f CEBPA, 93% in patients with non-bZIPin-f CEBPA, and 79.5% in patients with CEBPAwild. The bZIPin-f CEBPA mutation was associated with a higher CR rate than CEBPAwild (p=0.011). Allo-HCT at first CR (CR1) was performed in 39.1% (127/325) of patients who achieved CR after induction chemotherapy. Allo-HCT in CR was performed in 26/50 (52%) patients with bZIPin-f CEBPA, in 10/28 (36%) patients with non-bZIPin-f CEBPA, and in 91/252 (36.1%) patients with CEBPAwild.
The median follow-up was 79 months (range, 1.5 to 219 months) among survivors. The 5-year RFS and OS rates were 37.0% (95% CI, 31.5 to 42.5) and 33.9% (95% CI, 29.1 to 38.9), respectively. Among 325 patients who achieved CR, the 5-year CIR was 43.6% (95% CI, 38.0 to 49.1), and the 5-year NRM was 18.1% (95% CI, 14.4 to 23.1). The long-term outcomes were subsequently analyzed by CEBPA mutational status. The 5-year OS rates were 60.8% (95% CI, 45.5 to 73.0) in patients with bZIPin-f CEBPA (n=50), 50.0% (95% CI, 30.6 to 66.6) in patients with non-bZIPin-f CEBPA (n=28), and 26.9% (95% CI, 24.4 to 35.0) in patients with CEBPAwild group (n=317) (Fig. 2A). bZIPin-f CEBPA was associated with a statistically favorable OS compared with CEBPAwild (p < 0.001). However, there was no statistical difference between patients with bZIPin-f and those with non-bZIPin-f CEBPA mutations (p=0.106) or between patients with non-bZIPin-f CEBPA and those with CEBPAwild (p=0.336) (Fig. 2A). The 5-year RFS rates were 60.7% (95% CI, 44.9 to 73.2) in patients with bZIPin-f CEBPA (n=47), 45.5% (95% CI, 25.8 to 63.2) those with non-bZIPin-f CEBPA (n=26), and 32.1% (95% CI, 26.0 to 38.2) in those with CEBPAwild (n=252). Patients with bZIPin-f CEBPA also showed a statistically favorable RFS compared with those with CEBPAwild (p=0.001), and there was a trend of a favorable outcome compared with those with non-bZIPin-f CEBPA (p=0.082). However, there was no statistical difference between patients with non-bZIPin-f CEBPA and those with CEBPAwild (p=0.587) (Fig. 2B). The 5-year CIRs among patients with bZIPin-f CEBPA, non-bZIPin-f CEBPA, and CEBPAwild were 21.6%, 39.2%, and 48.4%, respectively. Patients with bZIPin-f CEBPA showed a lower relapse rate than those with CEBPAwild (p < 0.001); they also showed a trend of a lower CIR than those with non-bZIPin-f CEBPA (p=0.057). However, there was no statistical difference between patients with non-bZIPin-f CEBPA and those with CEBPAwild (p=0.597) (Fig. 2C). There was no difference in the 5-year NRM in all three groups (Fig. 2D).
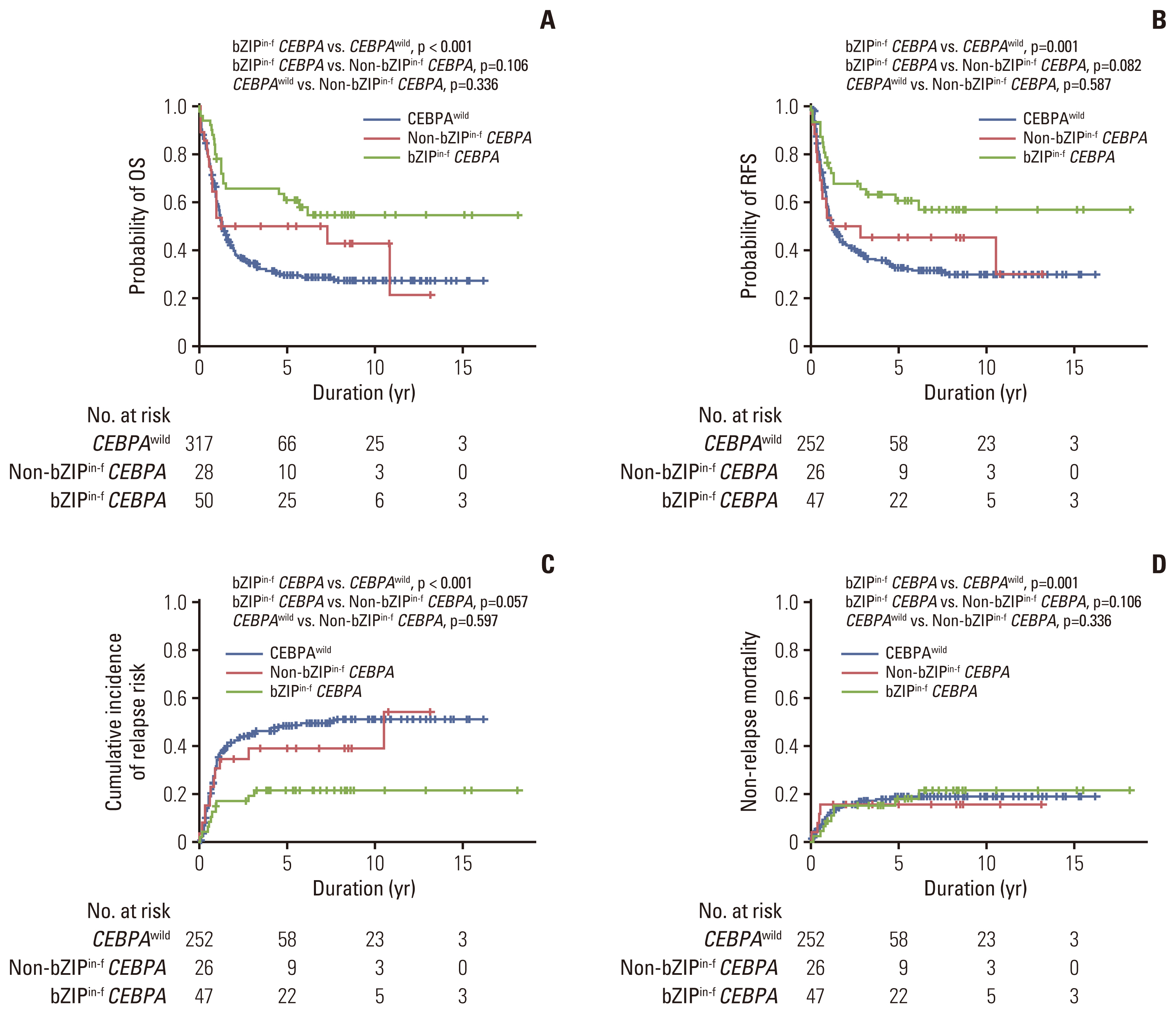
Prognostic significance according to the CEBPA mutation status for all 395 patients with normal karyotype acute myeloid leukemia. OS (A), RFS (B), cumulative incidence of relapse (C), and non-relapse mortality (D). bZIPin-f CEBPA, bZIP in-frame CEBPA mutation; CEBPA, CCAAT/enhancer-binding protein α; CEBPAwild, CEBPA wild-type; non-bZIPin-f CEBPA, non bZIP or non-in-frame CEBPA mutation; OS, overall survival; RFS, relapse-free survival.
4. Multivariate analyses on achievement of CR, OS, RFS, and CIR
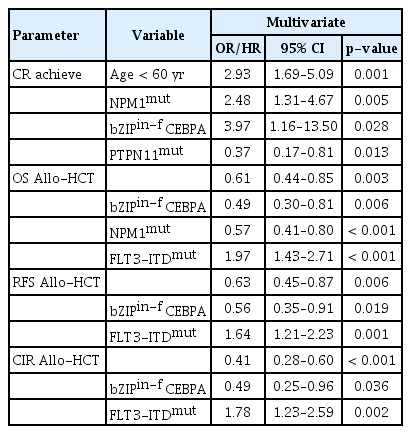
Multivariate analyses were conducted using the p-value–based stepwise selection method for achievement of CR, OS, RFS, and CIR. Allo-HCT was included as a time-dependent covariate for OS and RFS. In the multivariate analysis, a high rate of achieving CR was observed in younger patients (age < 60 years), those with NPM1mut, and those with bZIPin-f CEBPA, while patients with PTPN11mut showed low achievement of CR. In terms of OS, NPM1mut, bZIPin-f CEBPA, and allo-HCT were favorable factors, but FLT3-ITDpos was a poor prognostic factor. For RFS and CIR, bZIPin-f CEBPA, and allo-HCT were associated with favorable outcomes; FLT3-ITDpos was associated with worse outcomes (Table 3).
5. Comparison of outcomes according to bZIPin-f mutations in patients with CEBPAdm
Among patients with CEBPAdm (n=51), bZIPin-f CEBPA was detected in 41 patients (80%), and non-bZIPin-f CEBPA was observed in 10 patients (20%). All patients with CEBPAdm except one patient with a bZIPin-f CEBPA mutation achieved CR. Among them, patients with bZIPin-f CEBPA mutations showed superior outcomes in terms of OS (p=0.007) and RFS (p=0.007) compared with patients with non-bZIPin-f CEBPA mutations (Fig. 3). Patients with bZIPin-f CEBPA also showed a trend of a lower CIR (p=0.101) and a lower NRM (p=0.349); however, these findings were not statistically significant.
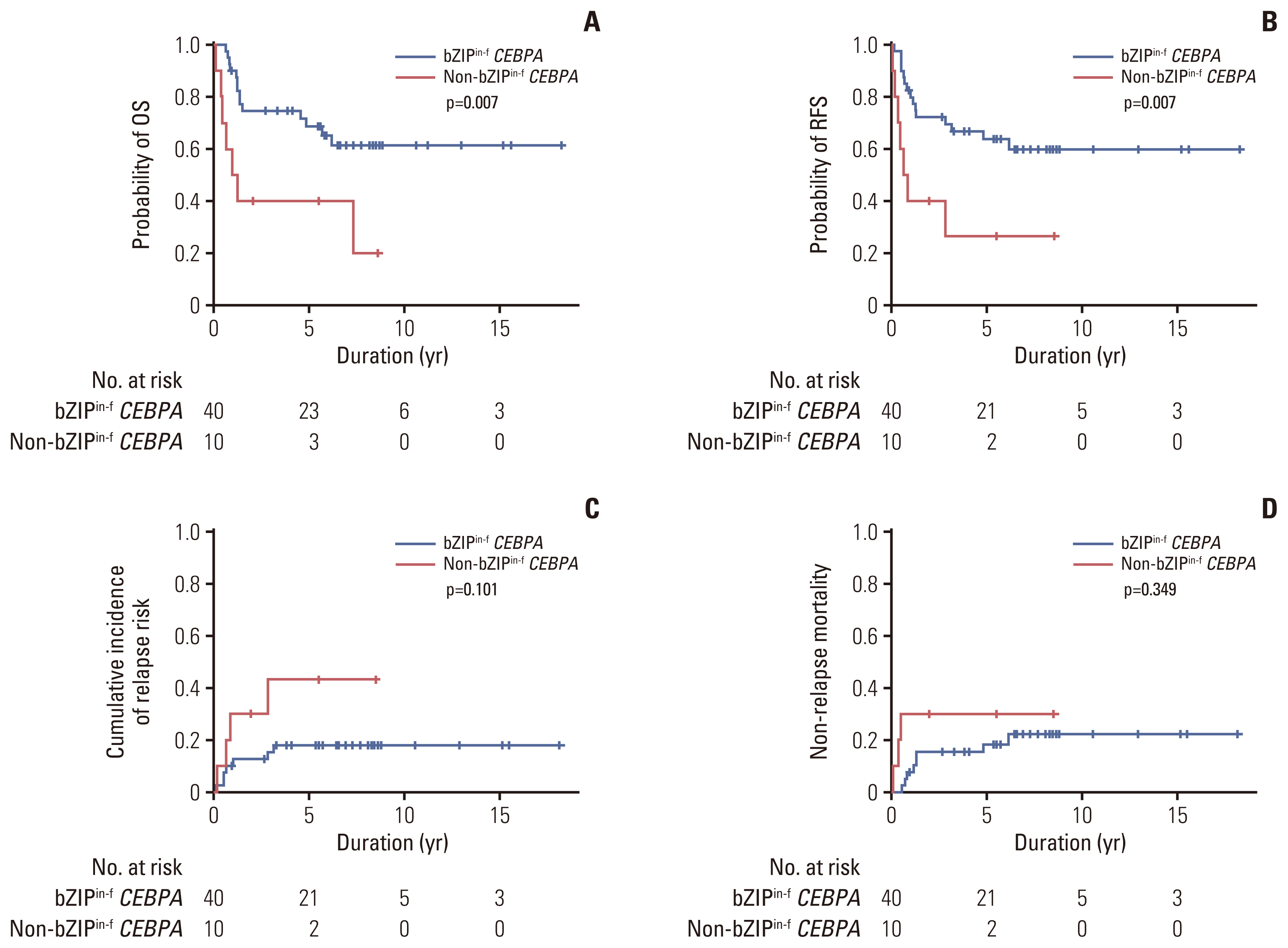
Prognostic significance according to bZIPin-f or non-bZIPin-f CEBPA in patients with CEBPA double mutations. OS (A), RFS (B), cumulative incidence of relapse (C), and non-relapse mortality (D). bZIPin-f CEBPA, bZIP in-frame CEBPA mutation; CEBPA, CCAAT/enhancer-binding protein α; non-bZIPin-f CEBPA, non bZIP or non-in-frame CEBPA mutation; OS, overall survival; RFS, relapse-free survival.
Direct comparison of the long-term outcomes by CEBPA mutational status had limitations in our cohort because the proportion of patients who received allo-HCT as a consolidation treatment was higher in patients with bZIPin-f CEBPA mutations. Therefore, we compared the outcomes to identify the role of either consolidation chemotherapy or allo-HCT in patients with bZIPin-f CEBPA mutations alone.
6. Favorable outcomes of consolidation chemotherapy alone compared with allo-HCT in patients with bZIPin-f CEBPA mutations
Of 50 patients with bZIPin-f CEBPA mutations, 47 patients achieved CR. We analyzed the treatment outcome by consolidation modalities in patients with bZIPin-f CEBPA mutations who achieved CR. The median duration from allo-HCT to the achievement of CR was 4.0 months. Therefore, we excluded three patients in the chemotherapy group who relapsed (n=2) or died (n=1) within four months. Twenty-six patients underwent allo-HCT, and 18 patients received consolidation chemotherapy alone. Of these 18 patients, six patients relapsed. Five of six patients received intensive reinduction chemotherapy, two patients achieved 2nd CR, and one of them underwent allo-HCT. Three patients died of complications during consolidation chemotherapy. Of 26 patients who underwent allo-HCT, only two patients relapsed and died of leukemia. The other six patients died of complications of allo-HCT including chronic graft versus host disease. The 5-year RFS was 54.5% (95% CI, 29.2 to 74.2) vs. 72.5% (95% CI, 50.6 to 85.8) in those who received chemotherapy alone vs. those who received allo-HCT (p=0.143) (Fig. 4B). The CIR was significantly different in favor of patients who underwent allo-HCT (p=0.026) (Fig. 4C). However, both groups of patients showed a similar OS, RFS, and NRM (all p > 0.05) (Fig. 4A and D).
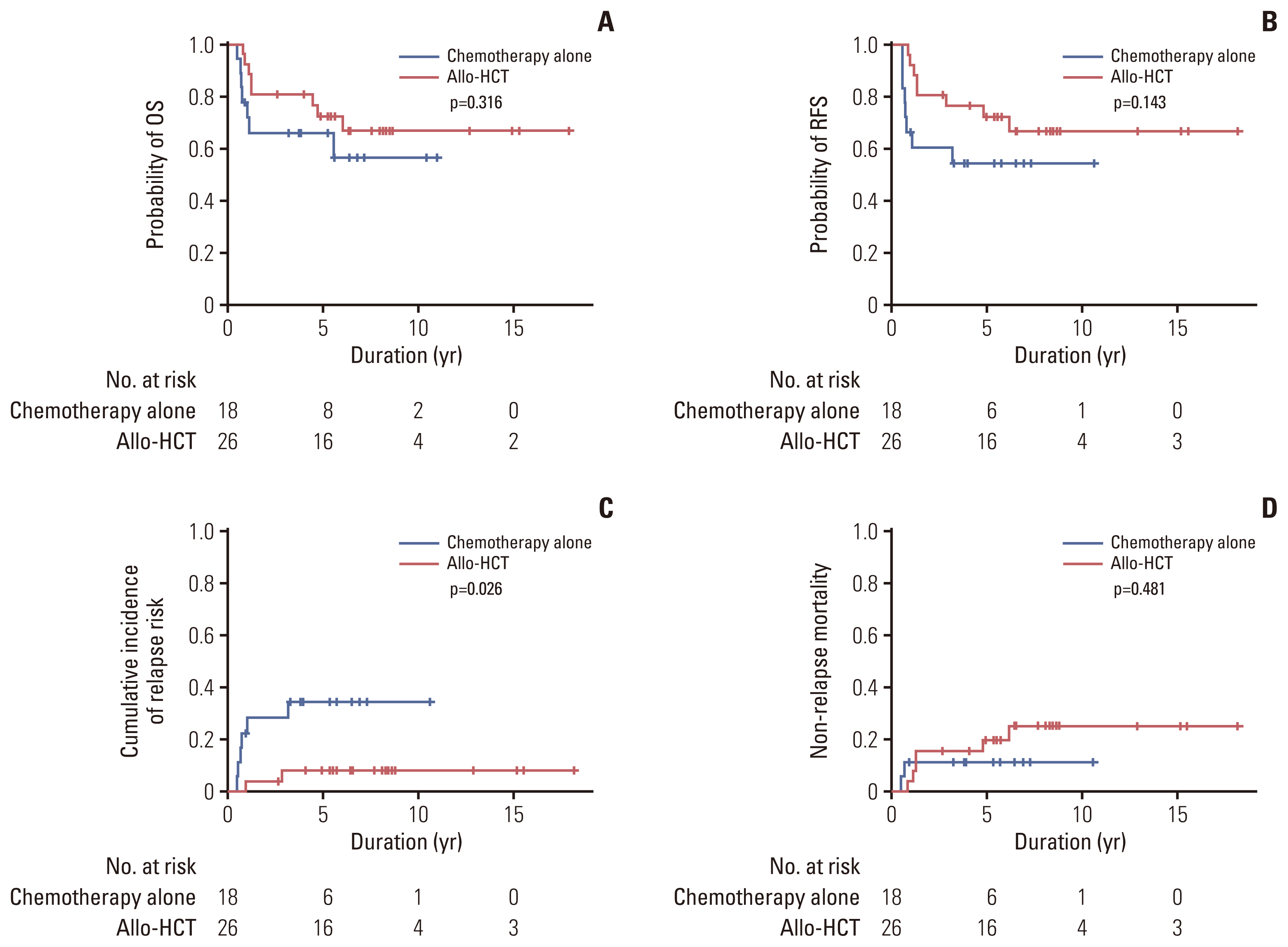
OS (A), RFS (B), cumulative incidence of relapse (C), and non-relapse mortality (D) by landmark analysis according to the type of consolidation therapy in patients with bZIPin-f CEBPA with normal karyotype acute myeloid leukemia. Allo-HCT, allogeneic hematopoietic cell transplantation; bZIPin-f CEBPA, bZIP in-frame CEBPA mutation; OS, overall survival; RFS, relapse-free survival.
7. Inferior outcomes of co-occurrence of some mutations in bZIPin-f CEBPA
Of 50 patients with bZIPin-f CEBPA mutations, 36 patients had at least one mutation. However, the number of patients in each co-mutated group was too small to compare them directly. By grouping the presence of mutations in chromatic/DNA modifiers (C), cohesion complex (C), and splicing genes (S) (CCS mutations), CCS-mutated bZIPin-f CEBPA showed poor outcomes in terms of OS (p=0.044; HR, 2.419; 95% CI, 1.025 to 5.709) and NRM (p=0.043; HR, 3.984; 95% CI, 1.042 to 15.23), and a trend of inferior outcomes without statistical significance in terms of RFS (p=0.186; HR, 1.838; 95% CI, 0.746 to 4.531) (Fig. 5).
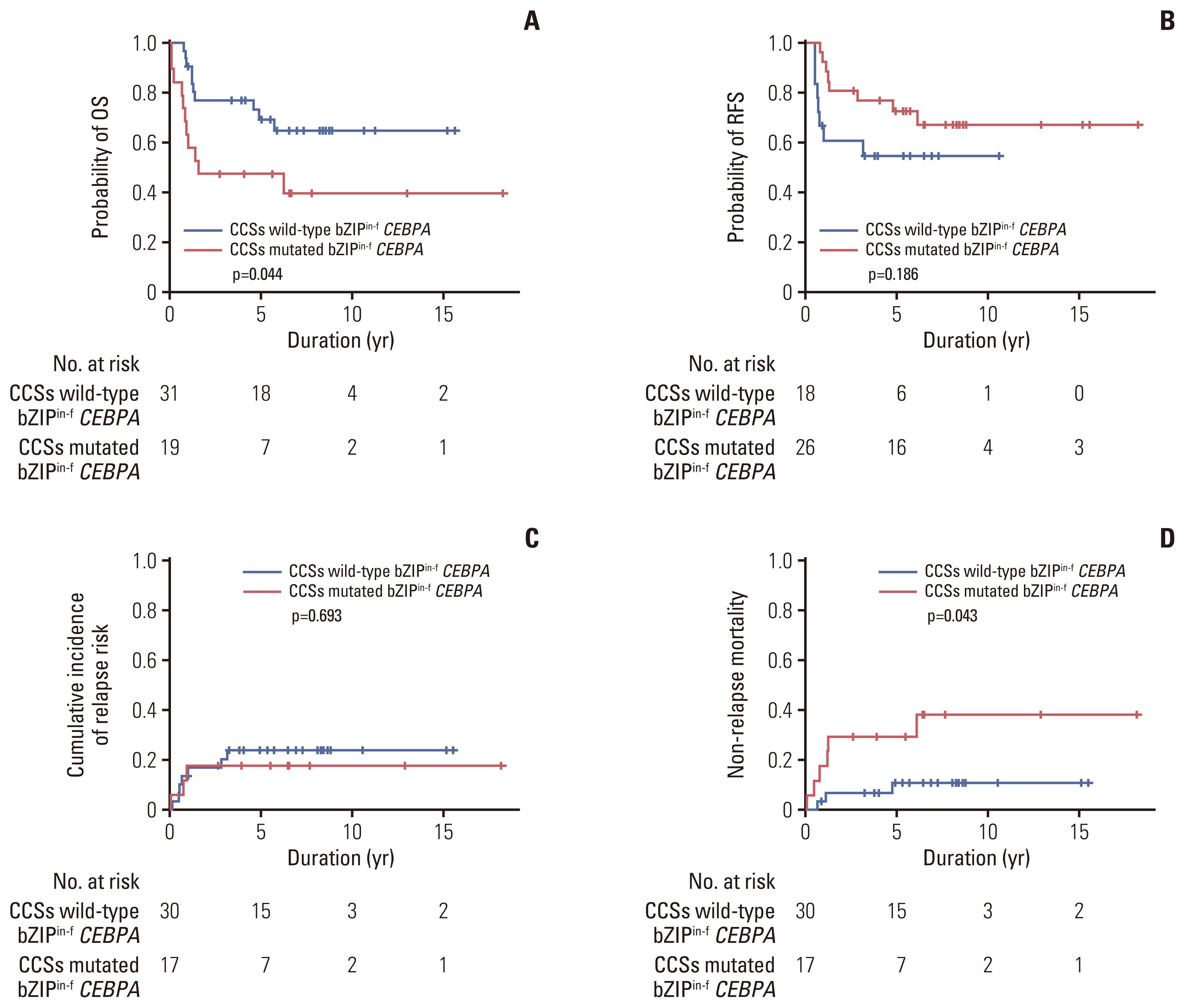
Prognostic significance by co-occurrence of mutations in patients with bZIPin-f CEBPA–mutated normal karyotype acute myeloid leukemia. OS (A), RFS (B), cumulative incidence of relapse (C), and non-relapse mortality (D). bZIPin-f CEBPA, bZIP in-frame CEBPA mutation; CEBPA, CCAAT/enhancer-binding protein α; CCSs mutations, mutations in chromatic/DNA modifiers (C), cohesion complex (C), and splicing genes (S).
Discussion
We evaluated the clinical impact of CEBPA mutations in 395 patients with NK-AML. In patients with CEBPA mutations (n=78), the risk stratification changed in 24% (n=19) according to the location and type of mutation rather than the number of CEBPA mutations. In patients with CEBPAdm, only those with bZIPin-f CEBPA mutations showed favorable outcomes. The results showed that consolidation chemotherapy alone showed similar results to allo-HCT in patients with bZIPin-f CEBPA mutations. However, some accompanying mutations in patients with bZIPin-f CEBPA were associated with inferior OS.
The frequency of CEBPA mutations in AML is 7%–20%, and these mutations are mostly associated with a normal karyotype, and more patients present with CEBPAdm in Asian populations [21,22]. Since the first report of CEBPA mutations in AML was published in 2001, understanding of CEBPA mutations has improved due to advancements in biological aspects and the accumulation of clinical data [3]. Recently, gene expression profile studies have suggested that bZIPin-f CEBPA is associated with a unique gene expression profile and can be distinguished from non-bZIPin-f CEBPA or CEBPAwild; in contrast, CEBPAsm with bZIPin-f does not express a discriminating signature from bZIPin-f CEBPAdm [16]. The favorable outcomes associated with CEBPA mutations in patients with AML are known to be restricted in those with bZIPin-f CEBPA. In previous studies, favorable outcomes associated with CEBPAdm were reported in patients with AML, which can be interpreted as showing a good prognosis because approximately 90% of cases of CEBPAdm are accompanied by bZIPin-f [16]. In our cohort, bZIPin-f CEBPA was detected in 80% and 33% of patients with CEBPAdm and CEBPAsm, respectively. The locations of bZIP and in-frame CEBPA mutations were prominently favorable in patients with CEBPAdm.
Allo-HCT is not recommended at first CR in patients with AML with bZIPin-f CEBPA according to the 2022 ELN recommendations [17]. In our study, the relapse incidence was significantly reduced in the allo-HCT group compared with chemotherapy alone group among patients with bZIPin-f CEBPA; however, there were no statistically significant differences in OS. The benefit of reducing the relapse rates in the allo-HCT group was likely neutralized by the higher transplant-related mortality (26%). On the other hand, the consolidation chemotherapy alone group may have overcome the higher relapse rates by reduced treatment mortality (11%).
Although patients with AML with bZIPin-f CEBPA showed favorable outcomes, relapse after treatment was inevitable in some patients. The co-occurrence of other genetic mutations may be a clue to predicting relapse in patients with bZIPin-f CEBPA. Approximately 70%–87% of patients with CEBPA mutations had other mutations detected by NGS [14–16]. Our results were similar to those of a previous report that bZIPin-f CEBPAdm was accompanied by rare concurrent mutations such as FLT3-ITDmut, NPM1mut, and DNMT3Amut compared with CEBPAwild or non-bZIPin-f CEBPA [14–16]. Some studies have reported that the associated mutations in patients with CEBPA-mutated AML can be used to predict prognosis [14,15,23]. In CEBPA-mutated AML, GATA2mut is relatively common, which is reported to be associated with a favorable outcome, whereas CSF3Rmut or TET2mut is associated with a poor prognosis [14,15,23]. In our study, 78 patients had CEBPA mutations, which is an insufficient sample size to demonstrate the significance of associated mutations. An effective way to solve the issue of a small cohort is to combine patients with mutations according to genetic pathways. As reported by Konstandin et al. [23], our study also showed poor OS in cases of CCS mutations in patients with bZIPin-f CEBPA. This result may provide evidence for considering alternative treatment in patients with poor survival through precise risk classification by NGS analysis performed at diagnosis.
The present study has several limitations in interpreting the clinical significance of bZIPin-f CEBPA mutations. This was a retrospective study that included patients treated at several centers where consolidation therapies were not uniform. Interestingly, the current cohort showed that a higher proportion of patients with bZIPin-f CEBPA who underwent allo-HCT than those in other populations. Patients with bZIPin-f CEBPA may have had more opportunities to undergo an allo-HCT because they were younger and had higher CR achievement after intensive induction chemotherapy. In addition, the number of patients with CEBPA mutations was small, limiting the power to demonstrate the role of consolidation therapy. In our cohort, bZIPin-f mutations in CEBPAsm did not significantly improve survival, which is a limitation to demonstrate the significance of bZIPin-f mutations in patients with CEBPAsm because only a small number of patients were included in this analysis. However, this work has significant clinical relevance in that the selection of consolidation therapy was relatively randomized since genetic factors were not considered when choosing allo-HCT as a consolidation treatment. Furthermore, the association with other mutations according to CEBPA mutational status and the prognostic significance of bZIPin-f CEBPA mutations were clarified.
Our results are considered good evidence in support of the 2022 ELN new risk stratification. The present study demonstrated that consolidation therapy alone was associated with similar OS to allo-HCT in patients with bZIPin-f CEBPA with NK-AML. However, precaution is needed in patients with accompanying mutations in bZIPin-f CEBPA because some mutations may be associated with poor outcomes. Further studies with larger numbers of patients are required for clear conclusions of the significance of bZIPin-f in the CEBPAsm subgroup.
Notes
Ethical Statement
The study was approved by the Institutional Review Board (IRB) of Chonnam National University Hwasun Hospital, Korea (IRB reference number: CNUHH-2014-153). All study participants provided written informed consent.
Author Contributions
Conceived and designed the analysis: Ahn SY, Kim HJ (Hyeoung-Joon Kim), Ahn JS, Kim DDH.
Collected the data: Ahn SY, Kim M, Song GY, Jung SH, Yang DH, Lee JJ, Kim MY, Jung CW, Jang JH, Kim HJ (Hee Je Kim), Moon JH, Sohn SK, Won JH, Kim SH, Ahn JS, Kim DDH.
Contributed data or analysis tools: Ahn SY, Kim T, Ahn JS, Kim DDH.
Performed the analysis: Ahn SY, Kim T, Ahn JS.
Wrote the paper: Ahn SY, Ahn JS.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (NRF-2015R1A2A1A10054579) and the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1720160). This study was supported by a grant from (BCRI22008) Chonnam National University Hospital and (HCRI21006) Chonnam National University Hwasun Hospital Institute for Biomedical Science. This work was also supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2018R1A2A1A05078480). This research was supported by a Basic Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1F1A1063836). The biospecimens used in this study were provided by the Biobank of Chonnam National University Hwasun Hospital, a member of the Korea Biobank.