Anti–PD-L1 Antibody and/or 17β-Estradiol Treatment Induces Changes in the Gut Microbiome in MC38 Colon Tumor Model
Article information
Abstract
Purpose
17β-Estradiol (E2) supplementation suppresses MC38 tumor growth by downregulating the expression of programmed death-ligand 1 (PD-L1). This study aims to figure out the gut microbiota that respond to anti–PD-L1 and/or estrogen treatment in MC38 colon cancer model.
Materials and Methods
A syngeneic colon tumor model was developed by injection of MC38 cells into C57BL/6 background male and female mice. Three days before MC38 cells injection, E2 was supplemented to male mice daily for 1 week. Male and female mice with MC38 tumors (50–100 mm3) were injected with anti–PD-L1 antibody. Fresh feces were collected 26 days after injection of MC38 cells and 16S rRNA metagenomics sequencing of DNA extracted from feces was used to assess gut microbial composition.
Results
At the taxonomic family level, Muribaculaceae was enriched only in the MC38 male control group. In male mice, linear discriminant analysis effect size analysis at the species level revealed that the four microorganisms were commonly regulated in single and combination treatment with anti–PD-L1 and/or E2; a decrease in PAC001068_g_uc and PAC001070_s (family Muribaculaceae) and increase in PAC001716_s and PAC001785_s (family Ruminococcaceae). Interestingly, in the anti–PD-L1 plus E2 group, a decrease in opportunistic pathogens (Enterobacteriaceae group) and an increase in commensal bacteria (Lactobacillus murinus group and Parabacteroides goldsteinii) were observed. Furthermore, the abundance of Parabacteroides goldsteinii was increased in both males and females in the anti–PD-L1 group.
Conclusion
Our results suggest that gut microbial changes induced by the pretreatment of estrogen before anti–PD-L1 might contribute to treatment of MC38 colon cancer.
Introduction
New evidences are emerging that the gut microbiota is closely related to disease development in human and animal models. Since the gut microbiome keep the homeostasis of host immune and metabolic functions, imbalances in the gut microbiota have been linked with healthy status and disease including inflammatory bowel disease, diabetes, and colon cancer [1]. Metagenome analysis in patients with colorectal cancer (CRC) revealed that the occurrence of CRC is associated with an imbalance of gut microbiota. Opportunistic pathogens including Fusobacterium nucleatum, which are presumed to be the cause of CRC, have increased in rectal adenoma or CRC patients [2]. Reportedly, infection of F. nucleatum in CRC mice and CRC cells induced cancer metastasis by activating the autophagy pathway via enhanced expression of caspase activation and recruitment domain 3 [3]. Furthermore, infection of F. nucleatum in CRC cells enhanced pathway involving the toll-like receptor 4/nuclear factor-κB/baculovirus IAP repeat 3, thereby reducing chemosensitivity to 5-fluorouracil [4]. Although many studies are currently underway on the relationship between diseases and the gut bacteria, the definitive role of the gut bacteria in the development and progression of CRC has not yet been elucidated.
Males have a higher incidence rate of CRC than females [5], and estrogen, the female sex hormone, has been shown to inhibit colon cancer progression [6]. There are reports of bidirectional regulation that estrogen regulates gut bacterial composition and gut bacteria influence estrogen levels [7]. Additionally, disease status such as obesity, diabetes, and cancer influence the crosstalk between the gut microbiome and estrogen [8]. Compared to control females, the bilateral ovariectomy (OVX) rodent model in which endogenous estrogen was removed showed altered composition of the gut microbiota [9,10]. In particular, plasma short-chain fatty acids produced by gut bacteria were significantly suppressed in the OVX rodent model [11].
Recently, immune checkpoint inhibitors (ICIs) have been used along with anticancer drugs to effectively prolong the survival period of cancer patients [12]. Generally, ICIs targeting programmed cell death receptor-1 (PD-1), PD-ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated protein 4 are widely used [13]. PD-L1 on the surface of immune and antigen-presenting cells binds to PD-1 expressed on activated T cells and transmits an inhibitory signal, thereby limiting T cell activation [14]. In cancer cells, PD-L1 acts as an immunosuppressive factor by inducing immune escape from the tumor microenvironment [12]. In previous studies, we confirmed that 17β-estradiol (E2) inhibited cancer progression in CRC male mice induced by azoxymethane/dextran sulfate sodium (AOM/DSS)-treatment [15] and the microbial community and the ratio of Firmicutes to Bacteroidetes (F/B) of the gut microbiota were regulated by E2 [10]. In addition, in MC38 colon tumor mice, estrogen suppressed colon tumor development by suppressing the expression of PD-L1 and modulating tumor-associated cell populations, thereby enhancing the therapeutic effect of anti–PD-L1 [16]. From these backgrounds, we hypothesized that bacterial composition in a syngeneic MC38 colon cancer mouse model induced by anti–PD-L1 antibody or E2 treatment, finally contributing to the anti-tumor therapy. To evaluate the gut microbiome changes by the treatment of anti–PD-L1 and estrogen, we investigated the gut microbiome regulated by anti–PD-L1 antibodies and/or E2 in the MC38 colon cancer mouse model.
Materials and Methods
The present study was carried out in the stool samples from previous experiments (Fig. 1A) [16].
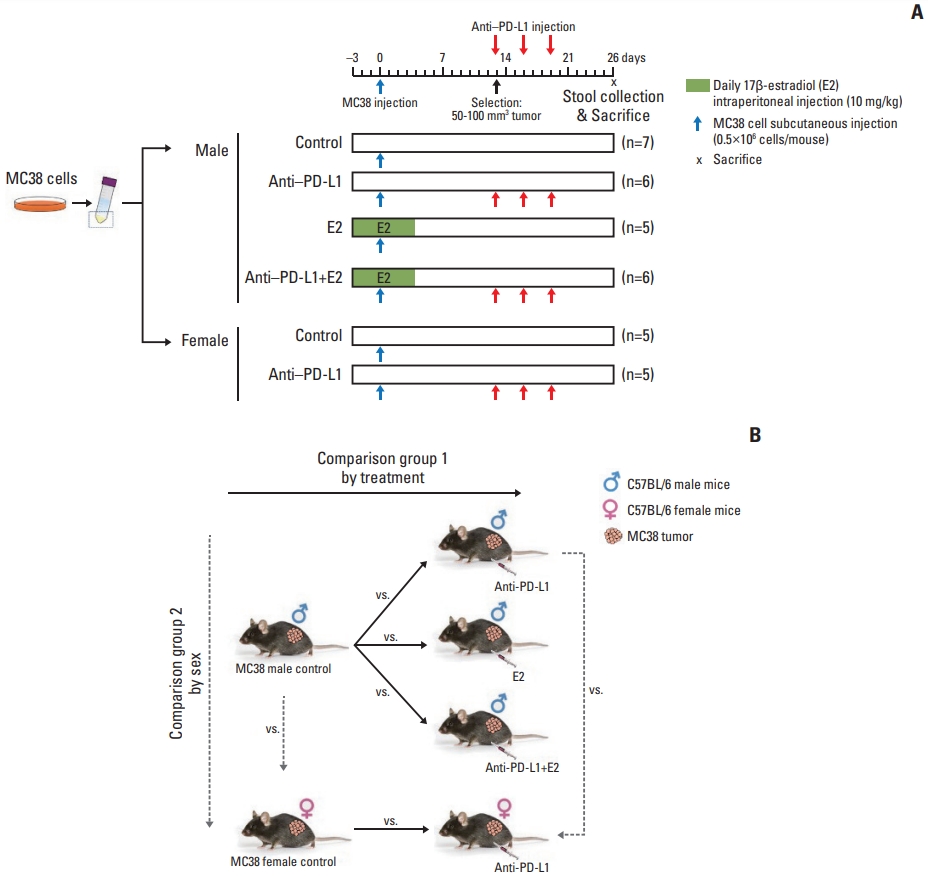
Experimental design to evaluate the effects of anti–PD-L1 antibody and/or E2 on gut microbiome in the MC38 colon tumor mouse model. (A) Experimental design. A total of 5×105 MC38 cells resuspended in 100 μL DPBS were injected subcutaneously into the right flank of 8-week-old C57BL/6 male mice. E2 (10 mg/kg) was intraperitoneally administered daily for 1 week from 3 days before injection of MC38 cells. Male and female mice bearing tumors (50–100 mm3) were selected and anti–PD-L1 or isotype control antibody was administered intraperitoneally at a dose of 10 mg/kg every 3 days for a total of three injections. Mice were sacrificed at day 26 after injection of MC38 cells. (B) Data analysis scheme. Investigation of changes in the gut microbiome composition according to treatment by comparing the gut microbiome in the MC38 males in the following groups: male control, anti–PD-L1–treated males, E2-treated males, males co-treated with anti–PD-L1 and E2. This analysis was used to examine the effect of anti–PD-L1 and/or E2 on the gut microbiome composition. Furthermore, sex-specific differences in the gut microbiome composition were analyzed by comparing the gut microbiome between male and female mice in the following groups: male control and female control, and anti–PD-L1 treated male and female groups. Based on this analysis, sex-specific changes in the gut microbiome composition were examined in MC38 colon tumor model. DPBS, Dulbecco’s phosphate-buffered saline; E2, 17β-estradiol; PD-L1, programmed death-ligand 1.
1. Reagents
E2 (# E8876) and olive oil (# O1514) were purchased from Sigma-Aldrich (St. Louis, MO) and E2 solution was prepared by dissolving in olive oil. Antibodies against anti-mouse PD-L1 (# BE0101) and its isotype control (# BE0090) for in vivo research were purchased from Bio X Cell (Lebanon, NH) and stored in a refrigerator at 4°C. MC38 cells (# ENH204-FP) were purchased from Kerafast Inc. (Boston, MA). Cell culture medium (Dulbecco’s modified Eagle’s medium [DMEM]) and reagents (Dulbecco’s phosphate-buffered saline [DPBS], fetal bovine serum [FBS], 200 mM L-glutamine [100× stock], 100 mM sodium pyruvate [100× stock], 1 M HEPES [100× stock], and antibiotic-antimycotic [A-A] [100× stock]) were purchased from Gibco BRL (Grand Island, NY).
2. MC38 cell culture
MC38 mouse colon adenocarcinoma cells were cultured in DMEM with 10% FBS, 1× L-glutamine, 1× sodium pyruvate, 1× HEPES, and 1× A-A, and maintained at 37°C, 5% CO2 incubator. For implantation, MC38 cells were prepared in the following manners: (1) washing with DPBS, (2) cell collection by trypsinization, (3) cell viability confirmation by trypan blue staining, and (4) final resuspension in DPBS.
3. Mouse housing condition
C57BL/6 male and female mice (7 weeks old) were purchased from Orient Bio (Seoul, Korea) and were maintained in filter-top cages in a specific pathogen-free facility at 23°C with a 12-hour light and dark cycles. After 1 week of acclimatization, male and female mice were randomly divided with three to five mice per cage according to sex. Each mouse was tracked with an ear tag. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Bundang Hospital (approval No. BA-2013-316-023-01), and were performed in accordance with the Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines.
4. Establishment of a syngeneic MC38 colon tumor mouse model and fecal DNA extraction
The right flanks of male and female mice (8 weeks old) were subcutaneously injected with 0.5×106 MC38 cells. Experiments were started with 10 mice per group for a total of 60 mice. As shown in Fig. 1A, 3 days before MC38 cells transplantation, E2 (10 mg/kg) was intraperitoneally administered to male mice daily for 1 week. The tumor size was assessed by “(minimum diameter)2×(maximum diameter)×1/2”. Following MC38 injection, male and female mice with tumors ranging in size from 50 to 100 mm3 were selected for anti–PD-L1 antibody injection [17–19], and mice with out-of-range tumors were omitted at this stage. Because, MC38 is the most variable model, which greatly increased the number of animals required, and mice with out-of-range tumors had to be removed to minimize variation to confirm the effects of various factors [20]. Finally, this study obtained results from 34 mice. Mice were administered by intraperitoneal injection of 10 mg/kg anti-mouse PD-L1 antibody or its isotype control antibody three times every 3 days. On day 26 post MC38 cells injection, feces were collected fresh and immediately frozen in liquid nitrogen and stored at −80°C. Genomic DNA was extracted using the QIAamp DNA stool mini kit (Qiagen, Hilden, Germany).
5. Metagenome sequencing
Polymerase chain reaction (PCR) and metagenome sequ-encing were performed on the DNA isolated from stool samples by CJ Bioscience, Inc. (Seoul, Korea). PCR reactions were performed under the following conditions (initial denaturation at 95°C for 3 minutes, followed by 25 cycles of denaturation at 95 °C for 30 seconds, primer annealing at 55 °C for 30 seconds, and extension at 72°C for 30 seconds, with a final elongation at 72°C for 5 minutes) using specific primers for the 16S rRNA V3–V4 regions (forward 341F primer, 5′-AATGATACGGCGACCACCGAGATCTACAC-XXXXXXXXTCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-CCTACGGGNGGCWGCAG-3′ and reverse 805R primer, 5′-CAAGCAGAAGACGGCATACGAGAT-XXXXXXXX-GTCTCGTGGGCTCGG-AGATGTGTATAAGAGACAG-GACTACHVGGGTATCTAATCC-3′). The PCR product was confirmed by using 1% agarose gel electrophoresis and visua-lized under a Gel Doc system (Bio-Rad, Hercules, CA). The amplified products were purified with the CleanPCR (CleanNA, Waddinxveen, Netherlands). Equal concentrations of purified products were pooled together and removed non-target short fragments with CleanPCR (CleanNA). The quality and product size were assessed on a Bioanalyzer 2100 (Agilent, Palo Alto, CA) using a DNA 7500 chip. Mixed amplicons were pooled and the sequencing was performed in CJ Bioscience, Inc., using the Illumina MiSeq sequencing system (Illumina, San Diego, CA) according to the manufacturer’s instructions.
6. Data processing and analysis
Data processing and analysis were carried out by CJ Bioscience, Inc. Briefly, raw data was processed in the following way: (1) filtering out of low quality (< Q25) reads using Trimmomatic ver. 0.32 [21], (2) merge of paired-end sequencing data using VSEARCH ver. 2.13.4 [22], (3) trimming out (similarity cutoff value: 0.8) of the primer sequence using Myers & Miller algorithm [23], (4) detection of non-specific amplicons using HMMER software [24], (5) extraction of unique reads using VSEARCH [22], (6) reference-based chimeric detection using the EzBioCloud 16S rRNA database [25] and the UCHIME algorithm [26], (7) after chimera filtering, reads not identified at the species level (< 97% similarity) in the EzBioCloud database were compiled and new clustering was performed using the cluster-fast command [22] to generate additional operational taxonomy units (OTUs), and (8) Finally, OTUs with single reads (singletons) were omitted from further analysis.
7. Gut microbial diversity analysis
OTUs were generated using EzBioCloud (CJ Bioscience, Inc.). For consistency of results, the same cutoff (12,919) for total reads was applied to the analysis. The alpha diversity indices (ACE, Chao1, jackknife, Shannon, NPShannon, Simpson, and phylogenetic diversity) were evaluated based on OTU counts. The differences between the groups were visualized using principal coordinate analysis (PCoA) and an unweighted pair group method with an arithmetic mean (UPGMA) tree. The distances of beta diversity such as PCoA and UPGMA tree were evaluated using the generalized UniFrac method at the species level. The statistical significance of beta diversity was evaluated using permutational multivariate analysis of variance (PERMANOVA). Relative taxonomic abundance (%) at the phylum and family level was plotted as a bar graph using GraphPad Prism (GraphPad Software, San Diego, CA). All analyses mentioned above were performed using EzBioCloud 16S-based MTP, which is a bioinformatics cloud platform of CJ Bioscience, Inc.
8. Determination of anti–PD-L1 and/or E2-specific and sex-specific gut microbiome
The linear discriminant analysis (LDA) effect size (LEfSe) method [27] was used to identify anti–PD-L1- and/or E2-specific gut microbiome based on relative taxonomic abundance (%) at the species level. LEfSe was performed using EzBioCloud (CJ Bioscience, Inc.) with the following general criteria: (1) alpha value for the factorial Kruskal-Wallis test among classes < 0.05; (2) the alpha value for the pairwise Wilcoxon test between subclasses < 0.05; (3) threshold on the logarithmic LDA score for discriminative features < 2.0; and (4) a multi-class analysis set as all-against-all. Bacterial characteristics were classified as “commensal bacteria”, “opportunistic pathogens” and “uncharacterized” according to previous reports. Venn diagrams were used to identify bacteria exhibiting common changes after anti–PD-L1 and/or E2 treatment or bacteria exhibiting sex differences based on LEfSe data.
9. Statistical analysis
The statistical significance of differences between the two groups or between more than two groups was calculated using the Mann-Whitney or Kruskal-Wallis H tests, respectively. The graphs were generated using GraphPad Prism 5.0 (GraphPad Software), and data were presented as the mean±standard error of the mean. All statistical analyses were performed using PASW statistics (2009, SPSS Inc., Chicago, IL). A p-value less than 0.05 is statistically significant.
10. Data availability
The raw 16S rRNA sequencing data generated from this study have been deposited in NCBI SRA (https://www.ncbi.nlm.nih.gov/sra) under the accession number PRJNA884174.
Results
The present study was carried out using the stool samples from previous experiments (Fig. 1A) [16], and the data were divided into group 1 (treatment in males) and group 2 (sex) according to the following analysis scheme (Fig. 1B).
1. Changes in microbial composition following anti–PD-L1 and/or E2 supplementation in MC38 colon cancer mice
First, we analyzed gut bacterial composition following anti–PD-L1 and/or E2 administration in MC38 colon cancer mice. At the phylum taxa level, the male group treated with anti–PD-L1 and/or E2 showed reduced Bacteroidetes and enhanced Verrucomicrobia compared to male colon cancer controls (Fig. 2A). Contrary to males, the anti–PD-L1 group of females tended to have an increase in Bacteroidetes and a decrease in Verrucomicrobia compared to female colon cancer controls (Fig. 2A). However, there was no significant difference between the groups. At the family level, in the male groups, the abundance ratio of Muribaculaceae (phylum: Bacteroidetes) was significantly lower in anti–PD-L1 group (p=0.003, 0%), E2 group (p=0.004, 0%), and anti–PD-L1 plus E2 group (p=0.002, 0%) compared to male colon cancer controls (3%) (Fig. 2B). Similar to phylum results, the abundance ratio of Bacteroidaceae (phylum: Bacteroidetes) and Akker-mansiaceae (phylum: Verrucomicrobia) decreased and increased, respectively, in both the anti–PD-L1 and/or E2 treatment groups compared to the male colon cancer control group (Fig. 2B). In the female groups, the abundance ratio of Muribaculaceae (phylum: Bacteroidetes) was decreased in the anti–PD-L1 group (0%) compared to female colon cancer controls (1%), but without statistical significance (Fig. 2B). Furthermore, Bacteroidaceae (phylum: Bacteroidetes) and Akkermansiaceae (phylum: Verrucomicrobia) increased and decreased in the anti–PD-L1 group compared to female colon cancer controls, similar to the phylum level (Fig. 2B). The ratios for abundance of the two major phyla, Firmicutes and Bacteroidetes, were further analyzed. Compared with each colon cancer controls, the F/B ratio increased in the male treatment group (anti–PD-L1, E2, and anti–PD-L1 plus E2) and decreased in the female anti–PD-L1 treatment group (Fig. 2C). However, no significant changes in the F/B ratio were observed.
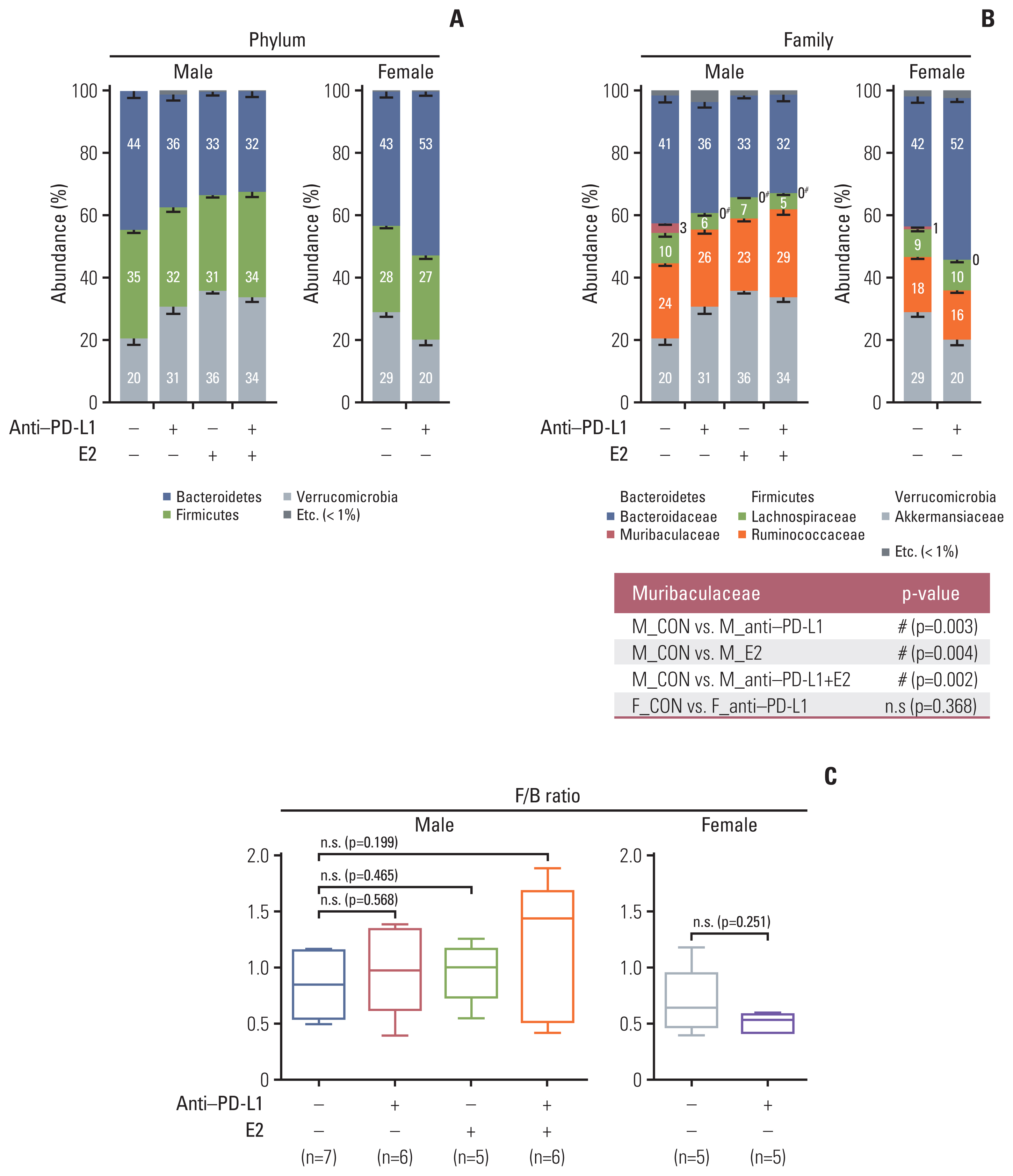
Taxonomic composition. (A) Gut microbiota compositions at the phylum level in MC38 male (left) and female (right) groups. (B) Gut microbiota compositions at the family level in MC38 males (left) and females (right). Mann-Whitney U test was used for comparison of differences between two independent groups. The p-value for Muribaculaceae, which showed significance in the comparison between groups, is presented as a table under the figure. #p < 0.05, male control vs. anti–PD-L1–treated male, male control vs. E2-treated male, and male control vs. male co-treated with anti–PD-L1 and E2. (C) F/B ratio in MC38 males and females. Data are expressed as the mean±SEM. Whiskers show the minimum and maximum values. The p-values were calculated using the Mann-Whitney U test for comparison difference between independent two groups. E2, 17β-estradiol; F/B, Firmicutes/Bacteroidetes; n.s., not significant; PD-L1, programmed death-ligand 1; SEM, standard error of the mean.
2. Effect of anti–PD-L1 and/or E2 on individual bacterial communities in MC38 colon cancer mice
Beta diversity for similarity between samples was analyzed using PCoA and UPGMA trees at the species level. In male mice, each distribution in the male colon cancer control sample was sporadically scattered (Fig. 3A). In contrast, after treatment with anti–PD-L1 and/or E2, each group was strongly clustered and separated upon treatment with anti–PD-L1, E2, and anti–PD-L1 plus E2 (Fig. 3A). PERMANOVA analysis of 2D PCoA revealed different but not significant beta diversity in MC38 male mice (p=0.060 for all groups) (Fig. 3A). Next, in female mice, the distribution and clustering were altered by anti–PD-L1 treatment compared to those in the female colon cancer control group (Fig. 3C). PERMANOVA analysis of 2D PCoA showed that the beta diversity differed between anti–PD-L1–treated mice compared to its female colon cancer controls, but the difference was not significant (p=0.282) (Fig. 3C). UPGMA results in male and female groups also showed a grouping pattern similar to PCoA (Fig. 3B and D). Collectively, beta diversity in male and female MC38 colon cancer mice was affected by anti–PD-L1, E2, or anti–PD-L1 plus E2 administration.
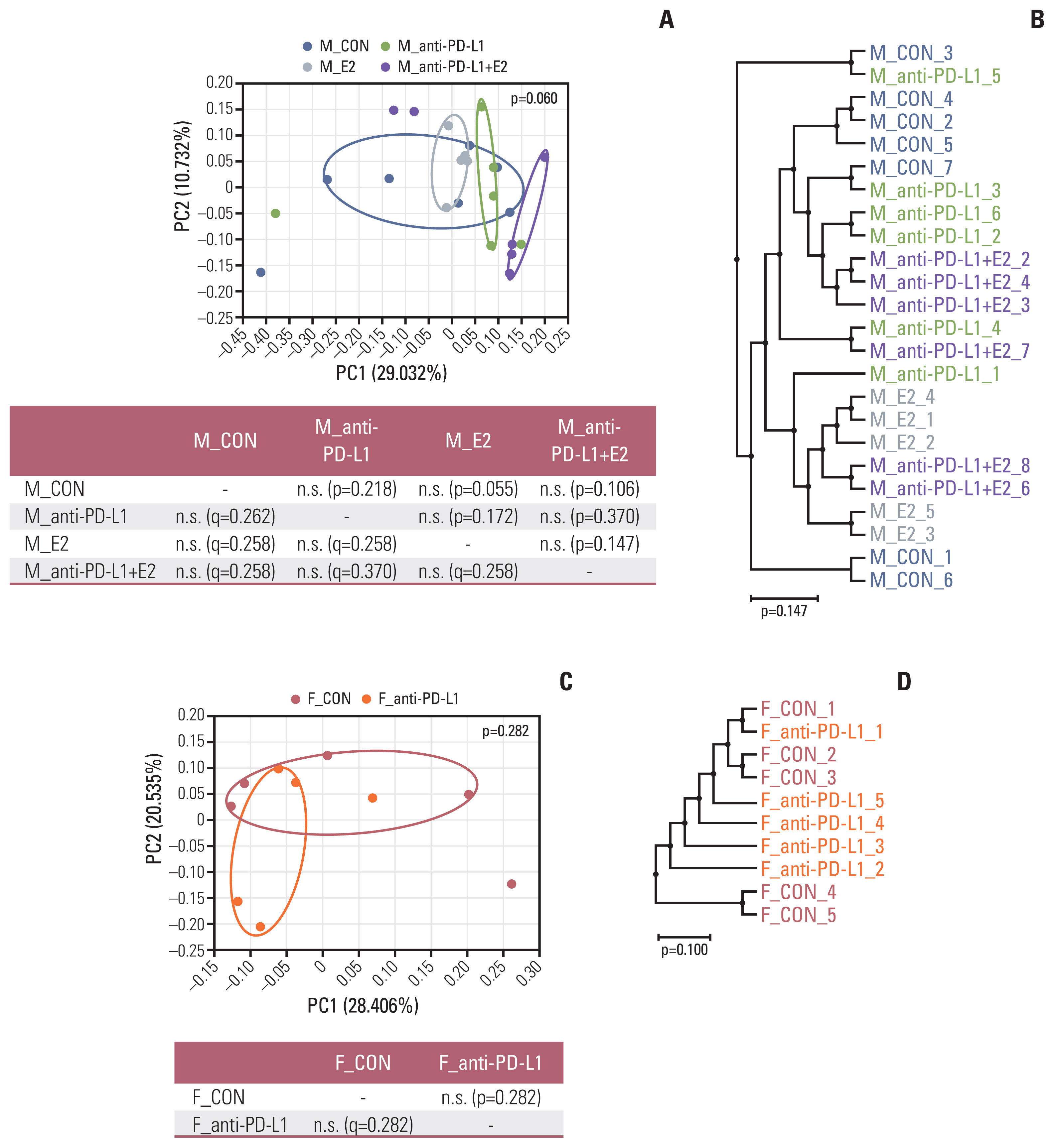
Beta diversity of gut microbiota. (A, B) Sample clustering using UniFrac-based PCoA and UPGMA tree at the species level in MC38 male groups. (C, D) Sample clustering using UniFrac-based PCoA and UPGMA tree at the species level in MC38 female groups. MC38 male and female samples were clustered using the Generalized UniFrac method at the species level. Significance of similarity of bacterial population structure was analyzed using PERMANOVA. The clustering and phylogenetic tree of each group are marked with a different color: M_CON, blue; M_anti-PD-L1, green; M_E2, grey; M_anti-PD-L1+E2, purple; F_CON, red; F_anti-PD-L1, orange color. CON, control; E2, 17β-estradiol; F, female; M, male; PCoA, principal coordinates analysis; PD-L1, programmed death-ligand 1; PERMANOMA, permutational multivariate analysis of variance; UPGMA, unweighted pair group method with arithmetic mean.
3. Identification of anti–PD-L1 or E2-specific microbiota in MC38 colon cancer mice
Next, we performed LEfSe analysis at the species level to identify gut microbiota that are regulated under specific conditions, such as anti–PD-L1 and/or E2. In LEfSe analysis compared with male colon cancer control mice, 15 bacteria (one commensal bacteria: Parabacteroides goldsteinii; 14 not characterized bacteria) in the anti–PD-L1 group, 17 bacteria (all not characterized bacteria) in the E2 group, and 17 bacteria (two commensal bacteria: Parabacteroides goldsteinii and Lactobacillus murinus group; one opportunistic pathogen: Enterobacteriaceae group; 14 not characterized bacteria) in the anti–PD-L1 plus E2 group were changed (Fig. 4A–C). Compared to the male colon cancer control group, the abundance ratio of P. goldsteinii was significantly increased, more than three times in the anti–PD-L1 treatment group (Fig. 4A). Unlike the anti–PD-L1 treatment group, only uncharacterized bacteria were identified in the E2 alone treatment group compared to the male colon cancer controls (Fig. 4B). In the anti–PD-L1 and E2 combination treatment group, the abundance ratio of commensal bacteria P. goldsteinii and L. murinus group increased, while the ratio of opportunistic bacteria, Enterobacteriaceae group, decreased compared to the male colon cancer controls (Fig. 4C). LEfSe analysis revealed that compared to the female colon cancer control group, the abundance ratio of the commensal bacteria P. goldsteinii was enhanced in the anti–PD-L1 group (Fig. 4D).
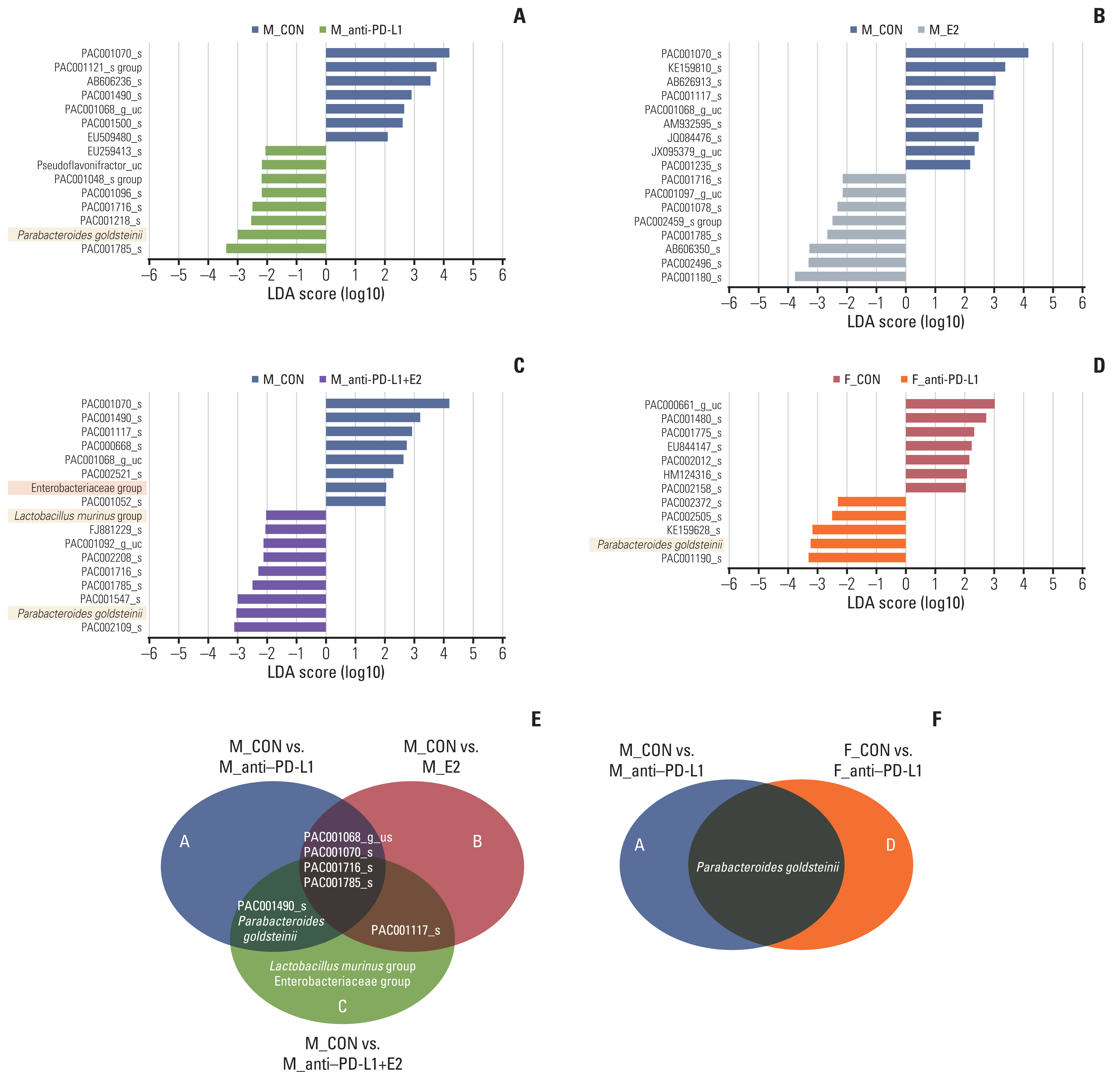
Identification of specific gut microbiome for anti–PD-L1 and/or E2 in MC38 colon tumor model. (A–D) LEfSe analysis. Bar plots of the LEfSe results, which were obtained based on the following criteria: (1) alpha value for the factorial Kruskal-Wallis test among classes < 0.05; (2) the alpha value for the pairwise Wilcoxon test between subclasses < 0.05; (3) threshold on the logarithmic LDA score for discriminative features < 2.0; and (4) a multi-class analysis set as all-against-all. Bacterial characteristics were classified as “commensal bacteria,” “opportunistic pathogens” and “uncharacterized” according to previous reports. The color bars show the LDA score (log10) of species that enriched in indicated conditions; (A–C) blue bar (male control), (A) block bar (anti–PD-L1–treated male), (B) green bar (E2-treated male), (C) purple bar (male co-treated with anti–PD-L1 and E2), (D) red bar (female control), gray bar (anti–PD-L1–treated female). The color on the species name indicates the characteristics of each species: yellow for commensal bacteria, orange for opportunistic pathogens, and no color for not characterized bacteria. (E, F) Venn diagrams based on LEfSe data. (E) Identification of bacteria exhibiting changes after anti–PD-L1 and/or E2 treatment in MC38 male mice. (F) Identification of bacteria exhibiting common changes after anti–PD-L1 treatment in MC38 male and female mice. CON, control; E2, 17β-estradiol; F, female; LDA, linear discriminant analysis; LEfSe, LDA effect size; M, male; PD-L1, programmed death-ligand 1.
Additionally, we attempted to identify the gut microbiome specific for anti–PD-L1 or E2 in the MC38 colon cancer mice by means of Venn diagram analysis. Compared with the male colon cancer controls, an increase in the abundance ratio of P. goldsteinii and a decrease in the abundance ratio of PAC001490 were confirmed in both the anti–PD-L1 alone and the anti–PD-L1 plus E2 combination treatment group (Fig. 4E). Compared with the male colon cancer controls, a decrease in the abundance ratio of PAC001117 was confirmed in both the E2 alone and the anti–PD-L1 plus E2 combination treatment group (Fig. 4E). Interestingly, four bacteria showing common changes in all the anti–PD-L1, E2, and anti–PD-L1 plus E2 treatment groups were identified; an increase in the abundance ratio of PAC001785 and PAC001716 and a decrease in the abundance ratio of PAC001070 and PAC00-1068 (Fig. 4E). Furthermore, the abundance of P. goldsteinii was commonly increased by anti–PD-L1 treatment compared to each colon cancer control group regardless of sex (Fig. 4). Taken together, P. goldsteinii was identified as a specific gut microbiota for anti–PD-L1 in both males and females. In males treated with anti–PD-L1 and E2, L. murinus was identified as an additional gut microbiota.
4. Sex effect on microbial composition in MC38 colon cancer mice
At the phylum level, in sex comparisons between colon cancer controls, Firmicutes were significantly lower in female controls (28%) than in male controls (35%) (p=0.042) (Fig. 5A). The level of Verrucomicrobia was higher in female controls (29%) than in male controls (20%), but the difference was not significant (Fig. 5A). Next, in sex comparisons between anti–PD-L1–treated groups, Bacteroidetes was significantly higher in anti–PD-L1–treated females (53%) than in anti–PD-L1–treated males (36%) (p=0.045) (Fig. 5A). The abundance of Firmicutes and Verrucomicrobia only tended to decrease in anti–PD-L1–treated females compared to males (Fig. 5A). At the family level, in sex comparisons between colon cancer controls, Muribaculaceae (phylum: Bacteroidetes) was significantly lower in female controls (1%) than in male controls (3%) (p=0.041) (Fig. 5B). The levels of Ruminococcaceae (phylum: Firmicutes) and Akkermansiaceae (phylum: Verrucomicrobia) showed only a tendency to decrease and increase, respectively, in female controls than in male controls (Fig. 5B). In sex comparisons between anti–PD-L1–treated groups, the abundance ratio of Bacteroidaceae (phylum: Bacteroidetes) (p=0.045, 36% in males and 52% in females) and Ruminococcaceae (phylum: Firmicutes) (p=0.045, 25% in males and 16% in females) significantly increased and decreased, respectively, in anti–PD-L1–treated female mice than in anti–PD-L1-treated males (Fig. 5B). As a result of F/B ratio analysis, the male mice showed higher F/B ratios than female mice in both colon cancer control group and anti–PD-L1 treatment group (Fig. 5C).

Taxonomic composition. (A) Gut microbiota compositions at the phylum level in control group (left) and anti–PD-L1 group (right) in MC38 male and female mice. (B) Gut microbiota compositions at the family level in control group (left) and anti–PD-L1 group (right) in MC38 male and female mice. Mann-Whitney U test was used for comparison of differences between two independent groups. The p-values for Phylum and Family level are presented as a table under the figure. *p < 0.05, male control vs female control, anti–PD-L1–treated male vs. anti–PD-L1–treated female. (C) F/B ratio in control groups and anti–PD-L1 treated groups in MC38 male and female mice. Data are expressed as the mean±SEM. Whiskers show the minimum and maximum values. The p-values were calculated using the Mann-Whitney U test for comparison of differences between two independent groups. F, female; F/B, Firmicutes/Bacteroidetes; M, male; n.s., not significant; PD-L1, programmed death-ligand 1; SEM, standard error of the mean.
5. Sex effect on individual bacterial communities in MC38 colon cancer mice
As a results of PCoA analysis in colon cancer control groups, the female group was well clustered, while the male group had sporadically scattered individuals, implying that segregation based on sex was not clear in the control group (Fig. 6A). Unlike the colon cancer control group, in the anti–PD-L1 treatment group, male and female clustering was clearly separated (p=0.024) (Fig. 6B). The UPGMA tree also showed a grouping pattern similar to that of PCoA (Fig. 6B).

Beta diversity of gut microbiota. (A) Sample clustering using UniFrac-based PCoA and UPGMA tree at the species level in MC38 male and female control groups. (B) Sample clustering using UniFrac-based PCoA and UPGMA tree at the species level in anti–PD-L1–treated MC38 male and female groups. MC38 male and female samples were clustered using the Generalized UniFrac method at the species level. Significance for similarity of bacterial population structure was analyzed using PERMANOVA. The clustering and phylogenetic tree of each group are marked with a different color: M_CON, blue; F_CON, red; M_anti-PD-L1, green; F_anti-PD-L1, orange color. CON, control; F, female; M, male; PCoA, principal coordinates analysis; PD-L1, programmed death-ligand 1; PERMANOMA, permutational multivariate analysis of variance; UPGMA, unweighted pair group method with arithmetic mean.
6. Identification of sex-specific microbiota in MC38 colon cancer mice
In the LEfSe analysis according to the sex of the colon cancer control group and the anti–PD-L1-treated group, there were changes in the abundance ratio of 11 bacteria (one opportunistic pathogen: Enterobacteriaceae group; ten not characterized bacteria) in the MC38 colon cancer control group and 45 bacteria in the anti–PD-L1 treatment group (all uncharacterized bacteria) (Fig. 7A and B). In the comparison based on sex in the MC38 colon cancer control group, the abundance ratio of the Enterobacteriaceae group was significantly increased showing a more than two-fold increase in the male controls compared to the female controls (Fig. 7A). Unlike the colon cancer control group, only bacteria that were not characterized were identified in the anti–PD-L1 treatment group (Fig. 7B).

Identification of sex-specific gut microbiome in control and anti–PD-L1 group in MC38 male and female mice. (A, B) LEfSe analysis. Bar plots of the LEfSe results, which were obtained based on the same criteria mentioned in Fig. 5. The color bars show the LDA score (log10) of species that were enriched in indicated conditions; (A) blue bar (male control) and red bar (female control), (B) block bar (anti–PD-L1–treated male) and gray bar (anti–PD-L1-treated female). (C) Venn diagrams based on LEfSe data. Identification of bacteria showing sex-dependent changes in control and anti–PD-L1 groups in male and female MC38 mice. CON, control; E2, 17β-estradiol; F, female; LDA, linear discriminant analysis; LEfSe, LDA effect size; M, male; PD-L1, programmed death-ligand 1.
Additionally, we attempted to identify the sex-specific gut microbiome in the MC38 colon cancer mice by means of Venn diagram analysis. A comparison of the colon cancer control group and anti–PD-L1 treated group confirmed that the two bacteria (PAC001294 and PAC002514 group) showed a common difference dependent on sex (Fig. 7C). Interestingly, in the control group, a decrease in PAC002514 and an increase in PAC001294 were observed in males compared to females, and a decrease in PAC001294 and an increase in PAC001294 were observed in males than in females in the anti–PD-L1 group (Fig. 7C).
Discussion
The gut microbiota is crucial for maintaining the homeostasis of the host’s immune and metabolic functions. An imbalance in gut microbiota could lead to the progression of diseases such as inflammatory bowel disease, diabetes, and colon cancer [1]. In a previous study, we evaluated the effects of co-treatment with anti–PD-L1 antibody and E2 and the effects of sex and estrogen in MC38 mice [16]. E2 treatment before injection of MC38 cells inhibited colon tumor burden through inhibition of PD-L1 expression and modulation of cell populations in male mice [16]. In addition, when the anti–PD-L1 antibody and E2 were co-administered, the colon tumor burden and the PD-L1–expressing cell populations were significantly suppressed in MC38 males compared to the anti–PD-L1 antibody or E2 alone group [16]. Co-treatment showed a decrease in tumor-associated macrophages (TAM) (CD11b+F4/80+) and an increase in M1 TAMs (CD11b+F4/80+CD86+) in the tumor burden [16]. The stool samples from this experiment frame (Fig. 1A) [16] were used in the present study to identify specific gut microbiome for anti–PD-L1 and/or E2.
P. goldsteinii belonging to the genus Parabacteroides is a known gut commensal bacteria. Wu et al. [28] reported that gut the bacterium P. goldsteinii is a novel probiotic that can be used to treat obesity and type 2 diabetes. Additionally, P. goldsteinii was reduced in high-fat diet (HFD)–fed mice [28]. They reported that oral gavage with live P. goldsteinii in HFD-fed mice suppressed obesity and was related with enhanced adipose tissue thermogenesis, improved intestinal integrity and decreased levels of inflammation and insulin resistance [28]. Another study reported that oral gavage of P. goldsteinii MTS01 to Helicobacter pylori–infected mice alleviated H. pylori–mediated gastritis by altering the gut microbial composition and strongly reducing serum cholesterol levels and improved gastric epithelial cells [29]. In addition, P. goldsteinii MTS01 also attenuated the pathogenic effects of H. pylori CagA and VacA in the gastric epithelium, suggesting the potential of P. goldsteinii as a probiotic [29]. In this study, we suggested that P. goldsteinii is anti–PD-L1–specific and PAC001117: family Lachnospiraceae is E2-specific gut microbiome. Interestingly, P. goldsteinii was enriched in the anti–PD-L1 therapy group regardless of sex. These results suggest that P. goldsteinii could induce tumor regression in CRC models and increase the effectiveness of ICIs. To establish its relevance for potential therapeutic use, investigation on the effect of P. goldsteinii alone or in combination with an anti–PD-L1 antibody on tumor growth regression in the MC38 colon tumor model is necessary in the future. In terms of PAC001117, there was no other report except the present experiment result. However, as estrogen has helped suppress the formation of the tumor microenvironment with synergistic effect in the use of ICIs in CRC, we made a hypothesis that PAC001117 could be related with estrogen somehow. As gut microbiome with β-glucuronidase activity contribute to estrogen reactivation through estrogen deconjugation and reabsorption [30], the microbiota such as PAC001117 which has possible synergistic effect of ICIs could have β-glucu-ronidase activity.
Lactobacillus is one of the genera that is used as probiotics, and members of this genus have anti-inflammatory and protective effects on gut barrier function. Lactobacillus murinus is the most abundant species of Lactobacillus. Tang et al. [31] reported that L. murinus could promote regulatory T cell development and inhibit the development of DSS-induced colitis in mice. Wilck et al. [32] observed that L. murinus was depleted by high salt intake, and reported that treatment of mice with L. murinus could prevent salt-induced exacerbation of actively induced experimental autoimmune encephalomyelitis and salt-sensitive hypertension by regulating T helper 17 cells. L. murinus V10 prevented neonatal dysbiosis and late-onset sepsis. In addition, we previously reported that the level of L. murinus was increased by E2 supplementation in AOM/DSS-mediated CRC males in ICR background mice [10]. Furthermore, the levels of L. murinus were decreased in the C57BL/6 WT CRC female and Nrf2 KO CRC male groups than in the control group, and L. murinus revealed a negative correlation with the number of colon tumors [33]. Contrary to previous reports, in the present study, the L. murinus group was not enriched in the E2 alone-treated group compared to the MC38 colon cancer controls of male mice. Interestingly, the L. murinus group was increased and Enterobacteriaceae group was decreased in the combination treatment group (anti–PD-L1 plus E2) compared to colon cancer control male mice. Collectively, these results suggest that regulating estrogen levels in males during CRC development may induce changes in the gut microbiome, thereby enhancing anti–PD-L1 CRC suppression. In addition, anti–PD-L1 could induce changes in the gut microbiome, suggesting that estrogen and anti–PD-L1 may act reciprocally on changes in the gut microbiome. To establish the relevance of L. murinus for potential therapeutic use, the investigation on the effect of L. murinus alone or in combination with an anti–PD-L1 antibody on tumor growth regression in the MC38 colon tumor model is necessary in the future.
Recently there was a report that that Bifidobacterium bifidum were significantly enriched in responders to immunotherapy in 96 patients with non–small cell lung cancer, whereas Akkermansia muciniphila and Blautia obeum were enriched in non-responders [34]. To establish relevance for potential therapeutic uses, the group found that when MC38 syngeneic mouse colon tumors were treated with commercial strains of B. bifidum, only certain B. bifidum strains (B. bifidum_K57) suppressed tumor growth synergistically with PD-1 inhibitor or chemotherapeutic agent oxaliplatin by inducing an anti-tumor host immune response such as lymphocyte activation and interferon-gamma production [34]. They also confirmed the therapeutic effect of B. bifidum_K57 in subcutaneous and orthotopic lung cancer mouse models using Lewis lung carcinoma (LLC1) cells [34]. Our results reveal the association between CRC therapy methods and the gut microbiome, which are secondary outcomes of previous experimental models [16]. As it is difficult to develop an animal model co-treated with anti–PD-L1 and estrogen, our results might be helpful for the understanding of the changes of the gut microbiome under the treatment of anti–PD-L1 and estrogen.
Based on the present and previously published data [16], we proposed a regulatory mechanism in which estrogen in MC38 colon tumor models modulates the tumor microenvironment and simultaneously changes the gut microbiome to increase the effect of anti–PD-L1, resulting in a reduction in tumor size (Fig. 8). Estrogen treatment before transplantation of MC38 cells inhibits the gut microbial community of the family Muribaculaceae, which is abundant in male MC38 colon tumors. At the same time, estrogens modulate the tumor microenvironment by reducing the PD-L1–expressing tumor cell populations, cancer-associated fibroblasts, and TAMs, and simultaneously increasing classically activated macrophages, M1 TAMs [16]. Anti–PD-L1 antibody treatment inhibits tumor immune evasion by interfering with the binding of PD-L1 in tumor cells to PD-1 in T cells along with an increase in the commensal gut bacteria P. goldsteinii, which is deficient in male MC38 colon tumors. Furthermore, estrogen co-treated with anti–PD-L1 antibody contributes to inhibition of tumor growth by further regulating the composition of gut microbiome, such as an increase in the L. murinus group and a decrease in the Enterobacteriaceae group, and synergistically regulating the tumor microenvironment.
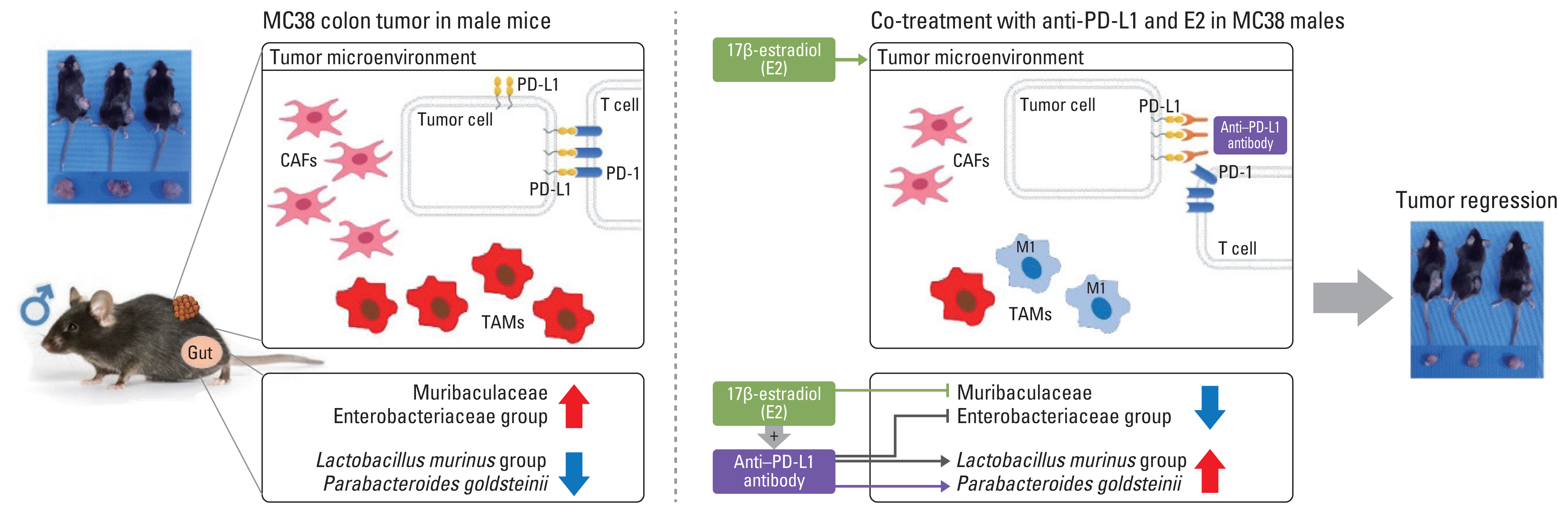
Regulatory mechanism by which estrogen modulates the tumor microenvironment and simultaneously alters the gut microbiome to increase the effect of anti–PD-L1 and contribute to tumor size reduction in the MC38 colon tumor model. CAFs, cancer-associated fibroblasts; E2, 17β-estradiol; PD-1, programmed cell death-1; PD-L1, programmed death-ligand 1; TAM, tumor-associated macrophages.
There are some limitations in this study. First, this study was performed in the M38 colon cancer mouse model instead of human thus it is not certain in the human CRC model. However, if more evidence is obtained in the future, these settings could be attempted in the clinical trials. Second, we did not suggest a direct role for the gut microbiome on the efficacy of CRC treatment in this study. However, it could be important to provide basic knowledge to explain the abundance of specific bacteria under specific conditions. In addition, we identified PAC002514 (family Ruminococcaceae) and PAC001294 (family Lachnospiraceae) as gut microbiota regulated by sex: increase of PAC002514 in female control and male anti–PD-L1 group and increase of PAC001294 in male control and female anti–PD-L1 group. However, not only are they not characterized bacteria, but nothing has been reported about them yet. It is hoped that future reports will reveal potential impacts and their functions. Third, since this study conducted group-by-group comparisons at the end-point, it was not possible to confirm the change of gut microbiota in the subject according to the treatment. Therefore, in future research, to make up for the limitations of this study, we are planning to check the changes of the gut microbiota before and after treatment. In addition, the specific bacteria found in these basic studies would be orally administered to CRC mice to confirm the direct effect on CRC treatment.
In summary, we present data of gut microbiomes of MC38 mouse models with colon tumors receiving anti–PD-L1, E2, or anti–PD-L1 plus E2 treatment. In the male mice, a decrease in the Enterobacteriaceae group and an increase in the L. murinus group and P. goldsteinii were observed in the anti–PD-L1 plus E2 group compared to the MC38 colon cancer control group. P. goldsteinii was increased in both male and female anti–PD-L1 treatment groups. In conclusion our results regarding the changes induced by anti–PD-L1 plus E2 suggest that there are gut microbiome which can be beneficial in the co-treatment of the anti–PD-L1 and estrogen for CRC therapy.
Notes
Author Contributions
Conceived and designed the analysis: Song CH, Kim N.
Collected the data: Song CH.
Contributed data or analysis tools: Song CH.
Wrote the paper: Song CH, Kin N.
Reviewed the manuscript critically: Choi J, Lee HN.
Performed the animal experiments: Nam RH, Choi SI, Jang JY.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the government of the Republic of Korea (2019R1A2C2085149).
