Image-Guided versus Conventional Brachytherapy for Locally Advanced Cervical Cancer: Experience of Single Institution with the Same Practitioner and Time Period
Article information
Abstract
Purpose
This study aimed to compare treatment outcomes and toxicity profile between imaged-guided brachytherapy (IGBT) versus conventional brachytherapy (CBT) performed by the same practitioner during the same time period.
Materials and Methods
Medical records of 104 eligible patients who underwent brachytherapy for locally advanced cervical cancer were retrospectively reviewed. Fifty patients (48.1%) underwent IGBT, and 54 (51.9%) patients underwent CBT. All patients underwent concurrent chemoradiation with cisplatin. High-dose-rate intracavitary brachytherapy with dose prescription of 25–30 Gy in 4–6 fractions was performed for all patients. Late lower gastrointestinal (GI) and urinary toxicities occurred more than 3 months after the end of brachytherapy were included for comparative and dosimetric analyses.
Results
The median follow-up period was 18.33 months (range, 3.25 to 38.43 months). There were no differences in oncologic outcomes between the two groups. The IGBT group had lower rate of actuarial grade ≥ 3 toxicity than the CBT group (2-year, 4.5% vs. 25.7%; p=0.030). Cumulative equieffective D2cc of sigmoid colon was significantly correlated with grade ≥ 2 lower GI toxicity (p=0.033), while equieffective D2cc of rectum (p=0.055) and bladder (p=0.069) showed marginal significance with corresponding grade ≥ 2 toxicities in the IGBT group. Half of grade ≥ 3 lower GI toxicities impacted GI tract above the rectum. Optimal thresholds of cumulative D2cc of sigmoid colon and rectum were 69.7 Gy and 70.8 Gy, respectively, for grade ≥ 2 lower GI toxicity.
Conclusion
IGBT showed superior toxicity profile to CBT. Evaluating the dose to the GI tract above rectum by IGBT might prevent some toxicities.
Introduction
Brachytherapy is essential for better treatment outcomes of locally advanced cervical cancer [1]. Even the recently developed external beam radiation therapy (EBRT) technique cannot substitute brachytherapy [2]. A significant advancement of gynecologic brachytherapy is utilization of three-dimensional (3D) images such as computerized tomography (CT) and magnetic resonance imaging (MRI). With image-guided technique, radiation dose delivery can be more precise than conventional brachytherapy (CBT) by defining tumor and organs-at-risk (OARs), resulting in improved local control (LC) and toxicity profile [3]. Results of prospective EMBRACE-I trial have shown that MRI-guided adaptive brachytherapy has effective and stable long-term LC with limited severe morbidity [4]. Although accumulation of evidence has shown superiority of image-guided brachytherapy (IGBT) over conventional technique, implementation of IGBT is still limited in some areas [5]. The proportion of radiation oncology centers offering brachytherapy is low in Korea partly caused by a deficit due to an imbalance of fee-to-source expenses [6]. It can be assumed that many Korean institutions still perform CBT for reasons related to cost [7].
IGBT has been implemented in our institution since 2018. Integration of MRI in brachytherapy planning was started in 2019. Due to limitation of staffing and equipment, only some patients with locally advanced cervical cancer were able to undergo IGBT, leading to a unique situation of our group: IGBT and CBT were practiced simultaneously by the same treating radiation oncologist. Usual comparison studies between IGBT versus CBT have two temporally distinguished cohorts, showing improvement of treatment outcomes and toxicity profile of the IGBT group [8–10]. We hypothesized that analyzing patient cohort in our institution might extract the impact of IGBT on clinical outcomes from that of advancement of other treatment modalities such as EBRT and chemotherapy. Thus, the purpose of this study was to compare treatment outcomes and toxicity profile between IGBT and CBT by evaluating patients who underwent IGBT or CBT by the same practitioner during the same time period.
Materials and Methods
1. Study population
Medical records of 148 patients who underwent intracavitary brachytherapy for locally advanced cervical cancer (International Federation of Gynecology and Obstetrics [FIGO] stage IB3, IIA2, IIB and above) from October 2018 to December 2021 were retrospectively reviewed. Interstitial brachytherapy was not performed during this time period. Twenty-four patients without follow-up record were excluded. Seven patients with induction chemotherapy and 10 patients without concurrent chemotherapy were also excluded. Two patients with incomplete brachytherapy and one patient treated with both image-guided and conventional planning were ineligible for this study. The remaining 104 patients were included in the analysis. Among them, 50 patients (48.1%) underwent IGBT, and 54 patients (51.9%) underwent CBT.
2. Treatment
All patients were treated with pelvic EBRT and concurrent cisplatin-based chemotherapy. Dose-fractionation schedule of pelvic EBRT was 45 to 50.4 Gy with 1.8 Gy of dose per fractions. Most EBRTs were planned by intensity-modulated radiation therapy (IMRT) technique. Twenty patients (19.2%) were treated by 3D conformal radiotherapy. Target volume delineation was based on previously published consensus guidelines [11]. Boost dose was delivered for gross metastatic lymph node. Parametrial or cervical tumor boost was performed in discretion of treating radiation oncologist or by protocol of other participating trials. Median total EBRT doses to gross lymph node, parametrium, and cervical tumor of the patients with boost to each site were 56.0 Gy (range, 48.6 to 64.0 Gy), 54.0 Gy (range, 50.0 to 55.0 Gy), and 50.5 Gy (range, 50.0 to 54.6 Gy), respectively. Paraaortic lymph node area was irradiated when suspicious metastatic common iliac or paraaortic lymph node was presented. Concurrent chemotherapy regimen was weekly cisplatin or tri-weekly cisplatin. Nine patients (18.0%) from the IGBT group enrolled in the randomized phase III CALLA trial, and durvalumab or placebo was administered during and after chemoradiation [12]. Fifty-eight patients (55.8%) underwent EBRT with concurrent chemotherapy at other institutions using similar principles of target delineation and dose prescription for radiotherapy. Thirteen patients (12.5%) underwent lymph node sampling or dissection on pelvic or paraaortic lymph node area before starting chemoradiation. The decision of lymph node surgery was made by the referring gynecologist when there was clinical suspicion of lymph node metastasis and exact staging was needed for decision of treatment modalities. Patients who were considered as unresponsive at 3 months after completion of chemoradiation by the referring gynecologist underwent additional treatment. Adjuvant chemotherapy was administered to two patients (1.9%) and total abdominal hysterectomy was performed for one patient (1.0%).
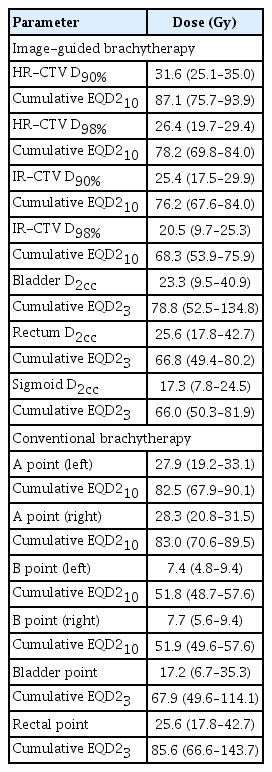
High-dose-rate (192Ir) intracavitary brachytherapy with dose prescription of 25 to 30 Gy in 4 to 6 fractions was performed for all patients. Response to EBRT and disease extent were re-evaluated by diagnostic MRI scan before brachytherapy planning. Applicator (tandem and ovoids) insertion and planning were performed by an experienced radiation oncologist (H.-C.K.). Allocation of patients to IGBT or CBT was in discretion of the treating radiation oncologist, and mainly based on the availability of staff and 3D image-compatible devices. For CBT, simulation and the first treatment were performed on the same day with fluoroscopy. Point A, Point B, bladder point, and rectal point were set based on the definition from International Commission on Radiation Units and Measurements (ICRU) Report 38 [13], and the dose was prescribed to point A. For IGBT, the patient underwent CT simulation scan with applicator in place. MRI-compatible Fletcher-type applicator and hybrid applicator (Venezia, Elekta AB, Stockholm, Sweden) were used. MRI-based treatment planning with the applicator in place was performed for 30 patients (60.0%). MRI simulation was performed after CT simulation was completed. The patient transferred to MRI room with applicator in place and underwent MRI scan. A 0.35 T MRI installed in MRI-guided radiation therapy system (MRIdian, ViewRay Inc., Oakwood Village, OH) was used for the most patients, as this treatment system is capable of MRI simulation scan separated with actual treatment sessions. One patient underwent 1.5 T diagnostic MRI simulation scan. Delineation of high risk (HR) clinical target volume (CTV) and intermediate risk CTV was according to recommendations of the Gynaecological GEC-ESTRO working group [14]. The dose was prescribed to D90% of HR-CTV. Rectum, sigmoid colon, and bladder were delineated as OARs.
To calculate the cumulative dose, dose from EBRT was assumed to be a prescribed dose to the pelvis. The equivalent dose in 2 Gy per fraction was calculated using α/β of 10 for tumor (EQD210) and 3 for OARs (EQD23). For CBT, Cumulative EQD23 of bladder and rectal point was intended to be limited to 75 Gy. For IGBT, cumulative EQD23 D2cc was limited to 75 Gy for rectum and sigmoid colon and 90 Gy for bladder. A treating radiation oncologist reviewed the plan and decided whether to adjust dose-fractionation scheme or affirm the existing plan when these doses exceeded the limitation. Dose calculation and treatment planning were performed by Oncentra Brachy (Elekta AB, Stockholm, Sweden). For every treatment session, fluoroscopy was used to check the location of the applicator.
3. Endpoints and statistics
Clinical endpoints of this study were LC, locoregional control (LRC), progression-free survival (PFS), and overall survival (OS). An LC event was defined as a recurrence in the treated cervix or parametrium, while LRC event was defined as a recurrence in the pelvic or paraaortic regional lymph node area. For PFS, the event was defined as any recurrence or death of the patient. For OS, the event was defined as death of the patient. These clinical outcomes were measured from the date of the completion of brachytherapy for each defined event. Rates were calculated using the Kaplan-Meier method. Clinical endpoints of two groups were compared by log-rank test. Univariate and multivariate analyses were performed for LC, LRC, PFS, and OS to identify covariates potentially affecting clinical outcomes. Statistically significant (p < 0.05) or marginally significant (p < 0.1) variables in univariate analysis and brachytherapy technology (IGBT vs. CBT) were incorporated into multivariate analysis using the Cox proportional hazards model.
New occurrence or worsening of urinary or lower gastrointestinal (GI) toxicities were recorded and graded using the Common Terminology Criteria for Adverse Events (CTCAE) ver. 5 [15]. Toxicities occurred more than 3 months after completion of brachytherapy were considered as late toxicities and included for the analysis. Symptoms suspected of being caused by other reasons such as recurred tumor (e.g., rectovaginal fistula with cervical recurrence) were excluded. Crude rates of toxicity were compared between two groups by chi-square test. Grade ≥ 3 toxicities were considered as severe toxicities. Actuarial rate of occurrence of severe toxicities of two groups were calculated by the Kaplan-Meier method and compared by log-rank test. Dose-response analysis was done separately by treatment groups as the methodology of reporting dose to OARs was different between IGBT and CBT. For IGBT, relationship between cumulative EQD23 D2cc of bladder and occurrence of any, grade ≥ 2, or ≥ 3 urinary toxicity was analyzed with a logistic regression model. The relationship between cumulative EQD23 D2cc of rectum/sigmoid and occurrence of any, grade ≥ 2, or ≥ 3 lower GI toxicity was analyzed similarly. For CBT, dose-response relationship was analyzed with the same method. However, cumulative EQD23 D2cc of bladder was substituted by cumulative EQD23 of bladder point and cumulative EQD23 D2cc of rectum was substituted by cumulative EQD23 of rectal point. Receiver operative characteristic (ROC) analysis was performed for relationships that showed statistical significance (p < 0.05) or marginal significance (p < 0.1) in the logistic regression model. Optimal cumulative dose threshold was found using the Youden index method. Categorical variables were compared between two groups by chi-square test with or without Yates’s continuity correction and numerical variables were compared by t-test. All statistical analyses were performed using R ver. 4.2.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
1. Patient characteristics and treatment specifics
Patient characteristics and treatment specifics are summarized in Table 1. The median follow-up period of all patients was 18.33 months (range, 3.25 to 38.43 months) from the completion of brachytherapy. There was no difference in follow-up period between the two groups. There were more patients referred from other institutions after chemoradiation in the CBT group (81.5% vs. 28.0%, p < 0.001). Baseline clinical factors were generally similar between the two groups. However, patients who underwent CBT were older (median, 59 years vs. 49.5 years, p < 0.001). Most patients in both groups had FIGO stage IIIC disease. A total of six patients (5.8%) had stage IVA disease. Five of them had disease spread to the bladder. One patient from the CBT group had a large tumor with adhesion to urethra, small bowel, and rectum. Three patients (2.9%) had distant metastasis at presentation. Two of them had supraclavicular lymph node metastasis and one patient from CBT group had suspected peritoneal seeding nodules in pelvis. These metastatic lesions were treated by EBRT.
Several differences between treatment specifics were observed. More patients in the CBT group underwent EBRT with pelvic dose fractionation of 50.4 Gy in 28 fractions (18.5% vs 4.0%, p=0.013). Other dose fractionation schemes were 44 Gy in 22 fractions and 46 Gy in 23 fractions. Parametrial boost was more frequent in IGBT group (52.0% vs. 13.0%, p < 0.001) partly due to the EBRT protocol of the CALLA trial which requires parametrial boost of 54 Gy in 27 fractions and boost to gross lymph node of 58 Gy in 29 fractions. In the IGBT group, tri-weekly cisplatin was more often administered as a concurrent chemoradiation (20.0% vs. 1.9%, p=0.007) with more patients undergoing lymph node surgery (22.0% vs. 3.7%, p=0.013) due to institutional preference. Dosimetric parameters of brachytherapy are summarized in Table 2. Median average dose of bilateral point A of the IGBT group was 23.25 Gy (range, 14.38 to 44.49 Gy). The average dose of bilateral point A was lower than prescribed dose in the IGBT group (p < 0.001), indicating that prescribing the same dose to point A instead of HR-CTV may have been resulted overtreatment in general.
We also compared patient characteristics between MRI- and CT-based IGBT. There were trends for more FIGO stage III disease (86.7% vs. 60.0%, p=0.088) and larger tumors (median 5.8 cm vs. 5.1 cm, p=0.054) in patients underwent MRI-based IGBT. Most (n=28, 93.3%) of the patients underwent MRI-based IGBT had dose-fractionation regimen of 30 Gy in five fractions, while 12 patients (60.0%) underwent CT-based IGBT had such regimen. Patient characteristics of MRI- and CT-based IGBT were summarized in S1 Table.
2. Treatment outcomes
Patterns of recurrence are summarized in Table 3. Crude rates of local recurrence, regional recurrence, and distant metastasis did not show significant difference. However, the CBT group showed higher crude rate of concurrent pelvic and paraaortic lymph node metastasis (0.0% vs. 11.1%, p=0.045). Kaplan-Meier curves of treatment outcomes are illustrated in Fig. 1. The 1-year and 2-year LC rates were 93.6% and 93.6% in the IGBT group and 94.2% and 86.5% in the CBT group, respectively, showing no statistically significant difference between the two groups (p=0.584). The 1-year and 2-year LRC rates were 83.4% and 78.8% in the IGBT group and 78.3% and 69.1% in the CBT group, respectively, showing no significant difference between the two groups (p=0.408). PFS rates at 1-year and 2-year were 77.3% and 72.7% in the IGBT group and 72.3% and 69.8% in the CBT group, respectively. OS rates at 1-year and 2-year were 93.8% and 88.4% in the IGBT group and 96.0% and 83.6% in the CBT group, respectively. No significant difference in PFS (p=0.777) or OS (p=0.475) was observed between the two groups. There was no difference of treatment outcomes between MRI- and CT-based IGBT (LC, p= 0.770; LRC, p=0.929; PFS, p=0.457; OS, p=0.347).
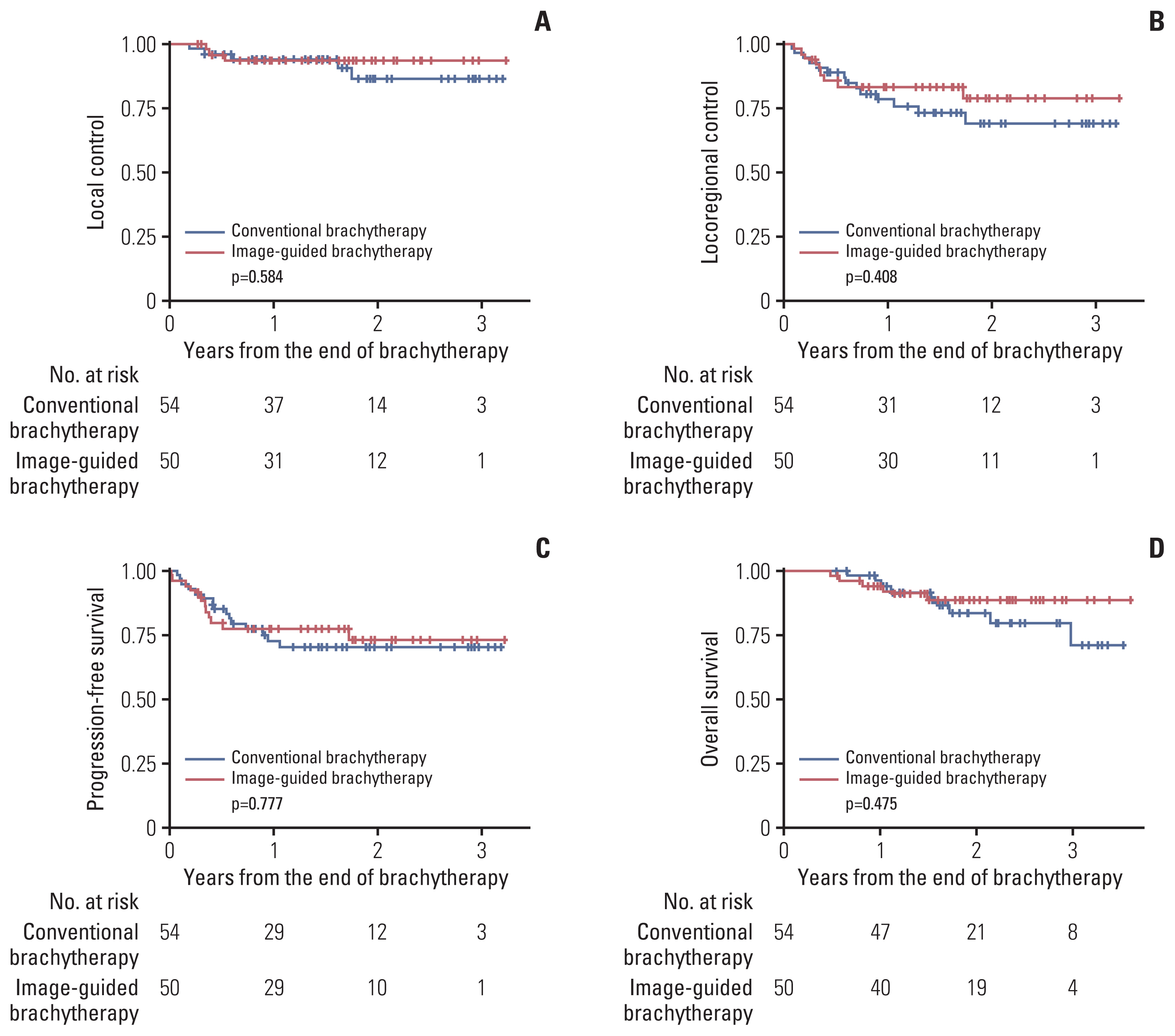
Kaplan-Meier curves of local control (A), locoregional control (B), progression-free survival (C), and overall survival (D) rates.
Results of univariate and multivariate analyses are summarized in S2 Table. In the multivariate analysis, lymph node dissection was associated with worse LC (hazard ratio, 13.24; 95% confidence interval [CI], 2.989 to 58.62; p=0.001). Distant metastasis at diagnosis was associated with worse PFS (hazard ratio, 4.738; 95% CI, 1.057 to 21.24; p=0.042). Initial tumor size was associated with worse LRC (hazard ratio, 1.288; 95% CI, 1.017 to 1.632; p=0.036) and OS (hazard ratio, 1.459; 95% CI, 1.090 to 1.952; p=0.011). Brachytherapy technology (IGBT vs. CBT) did not show statistically significant correlation with treatment outcomes in the multivariate analysis.
3. Toxicity profile
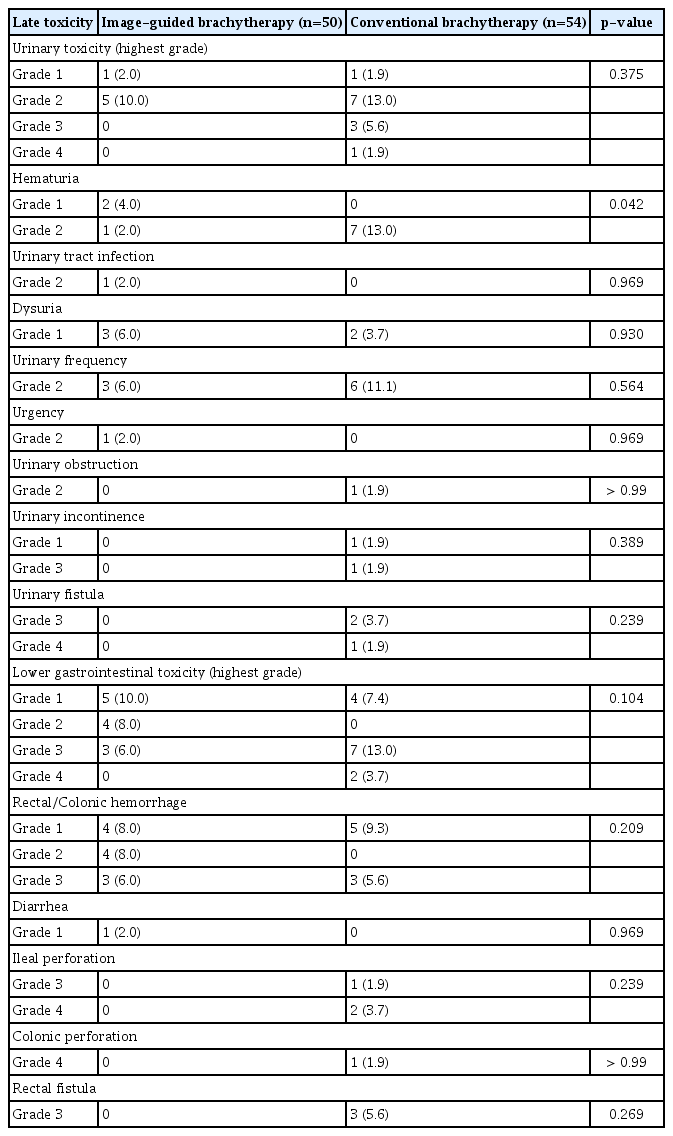
Overall, six urinary toxicities (12.0%) and 12 lower GI toxicities (24.0%) were reported in the IGBT group, and 12 urinary toxicities (22.2%) and 13 lower GI toxicities (24.1%) were reported in the CBT group. For severe toxicities with grade ≥ 3, there were three lower GI toxicities (6.0%) and 0 urinary toxicity (0.0%) in the IGBT group vs. nine lower GI toxicities (16.7%) and four urinary toxicities (7.4%) in the CBT group. All three patients with severe toxicities in the IGBT group underwent CT-based brachytherapy planning. There was no statistically significant difference in crude rate of overall or severe urinary/lower GI toxicities. Detailed toxicity profile is summarized in Table 4. The median time to occurrence of any urinary or lower GI toxicity was 11.93 months (range, 3.42 to 25.73 months). Treatment, details of toxicity, and disease status of severe toxicity cases are summarized in S3 Table. It should be noted that seven among 12 patients (1/3 in IGBT group, 6/11 in CBT group) with severe late lower GI toxicities had evidence of bleeding, perforation, or fistula involving GI tract above the rectum.
Actuarial rates of severe toxicities are illustrated in Fig. 2. The 1-year and 2-year rates for the occurrence of lower GI toxicities with grade ≥ 3 were 4.5% and 4.5% in the IGBT group and 17.8% and 20.4% in the CBT group, respectively, showing statistically significant differences between the two groups (p=0.046). For severe urinary toxicities, the 1-year and 2-year rates were 0.0% and 0.0% in the IGBT group and 7.1% and 13.7% in the CBT group, respectively. Marginally significant difference was observed between the two groups (p=0.061). The 1-year and 2-year actuarial rates of any grade ≥ 3 toxicities were 4.5% and 4.5% in the IGBT group and 17.8% and 25.7% in the CBT group, respectively, showing significant difference between the two groups (p=0.030). There was a trend for less any grade ≥ 3 toxicities in MRI-based IGBT when compared with CT-based IGBT (2-year rate, 0.0% vs. 10.6%; p=0.093).
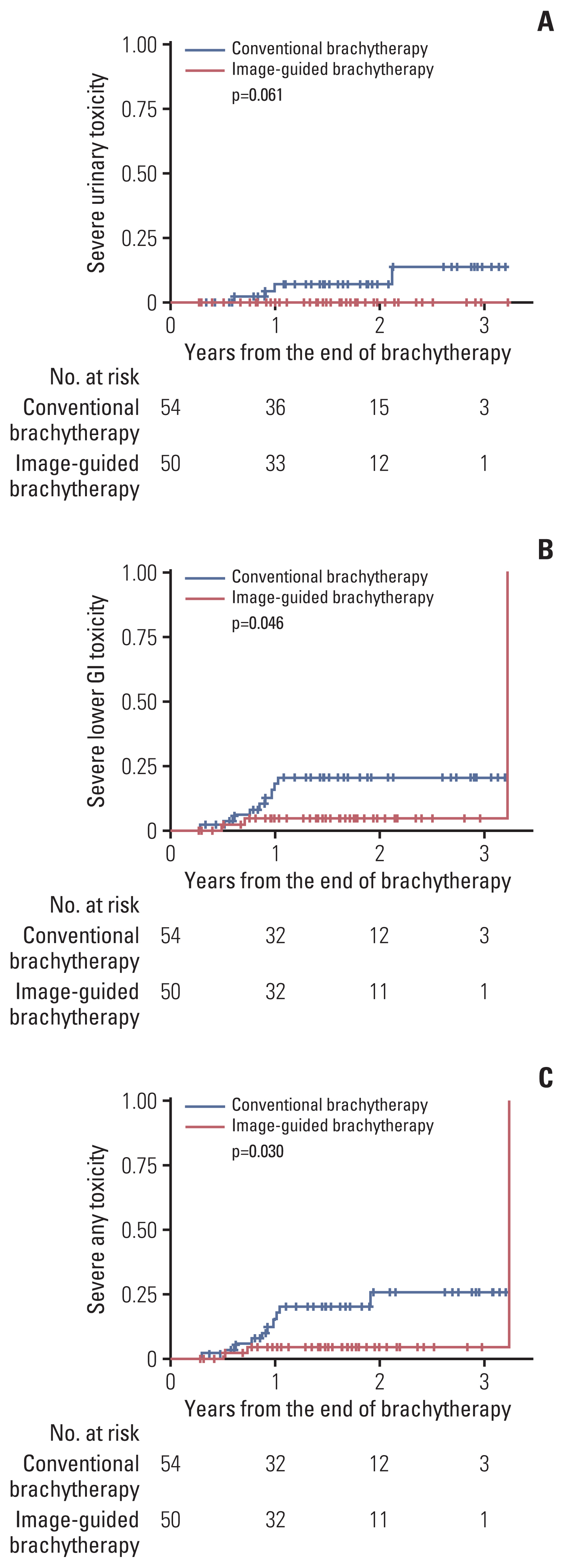
Actuarial rates of severe (grade ≥ 3) urinary (A), lower gastrointestinal (GI) (B), and any toxicities (C).
Logistic regression was performed between occurrence of toxicity and cumulative EQD23 D2cc of corresponding OAR in the IGBT group. Occurrence of grade ≥ 2 lower GI toxicities and cumulative EQD23 D2cc of sigmoid colon was significantly correlated (odds ratio [OR], 1.158; 95% CI, 1.012 to 1.326; p=0.033), while the relationship between grade ≥ 2 lower GI toxicities and cumulative EQD23 D2cc of rectum (OR, 1.160; 95% CI, 0.997 to 1.350; p=0.055) and that between grade ≥ 2 urinary toxicities and cumulative EQD23 D2cc of the bladder (OR, 1.049; 95% CI, 0.996 to 1.104; p=0.069) showed marginal significance. ROC analysis showed that cumulative EQD23 D2cc of rectum (area under the curve [AUC], 0.7542; 95% CI, 0.5975 to 0.9108) and sigmoid colon (AUC, 0.8173; 95% CI, 0.6777 to 0.9569) were predictive for grade ≥ 2 lower GI toxicities. Cumulative EQD23 D2cc of bladder did not show significance in ROC analysis (AUC, 0.6711; 95% CI, 0.3558 to 0.9864). The optimal threshold of cumulative EQD23 D2cc of rectum by Youden method was 69.7 Gy with a sensitivity of 85.7% and a specificity of 74.4%. The optimal threshold of cumulative EQD23 D2cc of sigmoid colon was 70.8 Gy with a sensitivity of 85.7% and a specificity of 76.7%.
For the CBT group, logistic regression between cumulative EQD23 of bladder point and occurrence of urinary toxicities did not show a significant or marginally significant correlation. The relationship between cumulative EQD23 of rectal point and lower GI toxicities with grade ≥ 2/3 showed a marginal significance (OR, 1.037; 95% CI, 0.999 to 1.077; p=0.054). Setting the endpoint as grade ≥ 2 or ≥ 3 had the same effect because late lower GI toxicity with grade 2 was not reported in the CBT group. In ROC analysis, cumulative EQD23 of rectal point was predictive for lower GI toxicities with grade ≥ 2/3 (AUC, 0.7111; 95% CI, 0.5206 to 0.9016). The optimal threshold of cumulative EQD23 of rectal point was 101.0 Gy with a sensitivity of 55.6% and a specificity of 84.4%. ROC curves are illustrated in S4 Fig.
Discussion
This study compared IGBT versus CBT performed by the same practitioner in the same time period. Oncologic outcomes were not significantly different between the two groups. However, the actuarial incidence of severe toxicities was higher in the CBT group. Cumulative EQD23 D2cc of sigmoid colon was significantly correlated with lower GI toxicity in the IGBT group, while cumulative EQD23 D2cc of rectum and bladder in the IGBT group and cumulative EQD23 of rectal point in the CBT group showed marginal significance with corresponding toxicities.
Although results from a randomized controlled trial have not been reported, several prospective and retrospective studies have compared efficacy of IGBT versus CBT, with many studies showing better treatment outcomes and toxicity profile of IGBT [16,17]. In this study, superior toxicity profile of IGBT to CBT was confirmed. However, contrary to the previous studies, there was no significant difference in LC, LRC, PFS, or OS between the two groups. This discrepancy might be due to changes of other treatment modalities over time in previous comparative studies and different implementation rate of MRI in IGBT planning. As previously stated, many comparative studies have two temporally distinguished cohorts. Some studies have clinically significant difference in treatment other than brachytherapy between two cohorts such as concurrent chemotherapy [10,18] and utilization of IMRT for EBRT [19]. Also, the IGBT group in this study included both CT-based and MRI-based planning. MRI-based planning might improve treatment outcomes of advanced tumors. EMBRACE-I trial, which implemented MRI-based brachytherapy planning for all patients, showed absolute improvement in LC or pelvic control in stage IIIB disease when compared with RetroEMBRACE cohort, which included both CT- and MRI-based planning [4,20].
Severe toxicity rate of IGBT (overall crude rate 6.0%) found in this study was comparable to rates from EMBRACE-I (overall grade 3 10.2%, grade 4 4.4%) and RetroEMBRACE (actuarial 3-year 4% for bladder, 6% for GI tract), considering the retrospective nature and a shorter follow-up period of this study [4,20]. Statistical significance or marginal significance of correlation between OAR dose and corresponding toxicity was observed in this study. It implies that estimating morbidity by calculating the OAR dose through 3D image-guidance technique is effective. The optimal threshold of cumulative EQD23 D2cc for lower GI toxicities in this study was 69.7 Gy for rectum and 70.8 Gy for sigmoid colon, consistent with the EQD23 D2cc limit of 70–75 Gy proposed by the American Brachytherapy Society Guideline [21]. Also, in the analysis of prospective EMBRACE trial, the equieffective D2cc for a 10% probability for grade ≥ 2 rectal toxicity was 69.5 Gy [22]. In contrast, this study could not establish an optimal threshold dose for bladder by ROC analysis. Retrospective and prospective studies have proposed bladder dose threshold based on clinical and dosimetry data [23,24]. In this study, hematochezia, fistula, and perforation were relatively well-documented than other toxicities, which might have made it feasible to evaluate lower GI toxicities more precisely than evaluating other types of toxicities. Additional recruitment and follow-up might reveal significant results.
The optimal threshold of cumulative EQD23 of rectal point in the CBT group was 101.0 Gy in this study, which is relatively high. Although rectal point dose of this study showed statistical significance for association with lower GI toxicities, this high optimal threshold may indicate that rectal point dose often overestimates actual rectal dose depending on clinical situations and practitioners. Although ICRU rectal point dose has some correlation with volumetric dose of the rectum, it also has limitations to evaluate rectal dose compared with volumetric dose calculation [25]. We considered that our data is concordant with this statement.
Half of severe GI toxicity cases in this study showed evidence of bleeding, perforation, or fistula involving colon and ileum. In contrast to IGBT, CBT cannot evaluate dose of GI tract above the rectum. This drawback might have caused severe consequence for some patients. Tandem can be unexpectedly placed proximity to the sigmoid colon, and dose to sigmoid colon is associated with the distance between the tandem and the sigmoid colon [26]. Furthermore, this study showed a dose-response relationship of sigmoid colon and lower GI toxicity. Without an image-guidance technique, it would be difficult to access the risk of colonic or ileal morbidity. Some severe colonic or ileal toxicities in the CBT group of this study might have been prevented if IGBT was applied.
One distinct feature of IGBT in this study was the utilization of 0.35 T MRI incorporated in MRI-guided radiation therapy system. A previous study has compared image quality of 0.35 T MRI, 1.5 T diagnostic MRI, and CT for brachytherapy planning and concluded that 0.35 T MRI-based planning is anticipated to give similar clinical benefit to diagnostic MRI-based planning [27]. In the current analysis, no severe toxicity was reported from patients who underwent 0.35 T MRI-based brachytherapy planning. This might indicate potential clinical benefit of applying 0.35 T MRI to brachytherapy planning compared with CT-based planning. Several practical obstacles such as longer time to acquire image, transporting of the patient, and needs of cooperation with other department exist for utilizing diagnostic MRI in most clinical settings. A 0.35 T MRI installed in MRI-guided radiation therapy system would provide fast and convenient way of MRI-based brachytherapy planning without acquiring an additional dedicated MRI simulator.
In the multivariate analysis of this study, lymph node dissection was correlated with worse LC. Considering definition of the event and covariates incorporated in the multivariate analysis for LC, this result was presumably due to patient selection for lymph node dissection. Other than lymph node dissection to LC, treatment specifics including brachytherapy technology did not significantly impact oncologic outcomes in this study.
Several limitations exist in this study mainly due to its retrospective nature. Patient characteristics and treatment specifics were not balanced between the two groups, such as age, concurrent chemotherapy, and lymph node surgery, as more patients were referred from other institutions after concurrent chemoradiation in the CBT group, although effects of different chemotherapeutic regimens [28] and lymph node surgeries [29] are not yet conclusive. Another undiscovered patient selection bias might have impacted the analysis. EBRT boost doses may also have been affected toxicities of brachytherapy, but these doses were not applied to calculation of cumulative dose in this study, as EBRT plans for the patients from other institutions were not available. Furthermore, there were patients participated in other prospective trials in the IGBT group, which might have impacted clinical efficacy, although AstraZeneca recently announced that CALLA trial did not achieve significant benefit of PFS [30]. A relatively short follow-up period might have hindered the comparability of late toxicity between the two groups. Although the follow-up period is relatively short, this study still has clinical implications as the follow-up period exceeds median times to local recurrence (less than 12 months) [31,32] and median times to occurrence of toxicity (around 12 months) [8,22] in the previous literatures. Nevertheless, this study showed superior toxicity profile of IGBT over CBT, concordant with previous literature.
In conclusion, lower actuarial severe toxicity rate in the IGBT group than in the CBT group was observed for locally advanced cervical cancer, although there were no significant differences in oncologic outcomes between the two groups. Dose to sigmoid colon, rectum, and bladder were significantly or marginally significantly correlated with occurrence of grade ≥ 2 corresponding toxicities in the IGBT group. Half of severe lower GI toxicities reported in this study impacted GI tract above the rectum. Evaluation of dose to GI tract above rectum including sigmoid colon by IGBT might prevent some lower GI toxicities. There is accumulated evidence including this study supporting the benefit of applying IGBT over CBT for locally advanced cervical cancer. Thus, policies to encourage implementation of IGBT in institutions need to be applied.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. H-2204-055-1314) before collecting patient information. Informed consent was waived due to its retrospective nature.
Author Contributions
Conceived and designed the analysis: Lee TH, Kang HC.
Collected the data: Lee TH, Kim KS, Kim HJ, Choi CH, Kang S, Kang HC.
Contributed data or analysis tools: Kim HJ, Eom KY, Wee CW, Song YS, Park NH, Kim JW, Chung HH, Kim HS, Lee M, Kang HC.
Performed the analysis: Lee TH, Kim KS, Choi CH, Kang S, Kang HC.
Wrote the paper: Lee TH, Kim KS, Kang HC.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.