Prognostic Factor and Clinical Outcome in Stage III Non–Small Cell Lung Cancer: A Study Based on Real-World Clinical Data in the Korean Population
Article information
Abstract
Purpose
The optimal treatment for patients with stage III non–small cell lung cancer (NSCLC) remains controversial. This study aimed to investigate prognostic factors and clinical outcome in stage III NSCLC using real-world clinical data in the Korean population.
Materials and Methods
Among 8,110 patients with lung cancer selected from 52 hospitals in Korea during 2014–2016, only patients with stage III NSCLC were recruited and analyzed. A standardized protocol was used to collect clinical information and Cox proportional hazards models were used to identify risk factors for mortality.
Results
A total of 1,383 patients (46.5% had squamous cell carcinoma and 40.9% had adenocarcinoma) with stage III NSCLC were enrolled, and their median age was 70 years. Regarding clinical stage, 548 patients (39.6%) had stage IIIA, 517 (37.4%) had stage IIIB, and 318 (23.0%) had stage IIIC. Pertaining to the initial treatment method, the surgery group (median survival period, 36 months) showed better survival outcomes than the non-surgical treatment group (median survival period, 18 months; p=0.001) in patients with stage IIIA. Moreover, among patients with stage IIIB and stage IIIC, those who received concurrent chemotherapy and radiation therapy (CCRT; median survival period, 24 months) showed better survival outcomes than those who received chemotherapy (median survival period, 11 months), or radiation therapy (median survival period, 10 months; p < 0.001).
Conclusion
While surgery might be feasible as the initial treatment option in patients with stage IIIA NSCLC, CCRT showed a beneficial role in patients with stage IIIB and IIIC NSCLC.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide and causes a substantial socio-economic burden [1]. Non–small cell lung cancer (NSCLC) accounts for more than 85% of all lung cancer cases, and stage III disease accounts for approximately one-third of patients with NSCLC at initial diagnosis [2]. However, stage III NSCLC is highly heterogeneous [3] and is divided into stage IIIA, IIIB, and IIIC according to the recent eighth edition of American Joint Committee on Cancer (AJCC) staging system [4].
Despite recent advances in this field, including immunotherapy, in the treatment and management of lung cancer [5], optimal management for stage III NSCLC remains controversial [6]. Traditionally, concurrent chemotherapy and radiation therapy (CCRT) has been recommended in these patients, especially in unresectable stage III NSCLC cases [7]. However, the beneficial role of surgical treatment has been reported in select patients with stage III NSCLC [8]. Moreover, upfront surgery for stage III NSCLC has been more commonly performed in Asian population than in populations of Western countries [9]. Indeed, a recent expert consensus statement in Asia suggested a proposed clinical algorithm for stage III NSCLC, which divided patients as resectable, potentially resectable, and unresectable cases [10].
Despite these previous lines of evidence, little is known about the clinical characteristics and treatment patterns of patients with stage III NSCLC in the real-world setting among Asian population. In this study, we aimed to investigate prognostic factors and clinical outcome in stage III NSCLC using real-world clinical data in the Korean population.
Materials and Methods
1. Study populations and methods
During 2014–2016, the Korean Central Cancer Registry (KCCR) registered the data of patients who were newly diagnosed with lung cancer (24,354 patients in 2014, 24,502 patients in 2015, and 25,780 patients in 2016). Among the eligible patients, the final survey population comprised 13 regional cancer centers and 39 hospitals in Korea, from which a significant number of registrations were made. In total, 8,110 patients with lung cancer were selected from each of the 52 hospitals using a systematic sampling method [11], and only patients with clinical information which could be converted to the 8th edition of the TNM International Staging System were analyzed in this study.
A standardized protocol was used to collect information about clinical characteristics including age, sex, body mass index (BMI), smoking history, histopathologic tumor type, symptoms, performance status (PS), Eastern Corporative Oncology Group (ECOG) score, clinical stage (according to the 8th edition of the TNM International Staging System) [4], treatment modality, results of molecular tests including epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) translocation, and survival status. Information on BMI and PS was obtained on the initial date of visit at the time of diagnosis. Patients were followed up until December 2018.
2. Statistical analysis
All data of continuous variables are expressed as mean± standard deviation or median (interquartile range [IQR]), and the data of categorical variables are expressed as percentages. One-way analysis of variance was used to compare continuous variables, while Pearson’s chi-square test was used to compare categorical variables. Cox proportional hazards models were used to identify risk factors for mortality, and variables with a p-value of < 0.20 on univariate analysis were used for the multivariate analysis. Survival was analyzed using the Kaplan-Meier method and compared using log-rank tests. All p-values were two-tailed, with statistical significance set at p < 0.05. All statistical analyses were performed using SPSS ver. 20.0 (IBM Corp., Armonk, NY).
Results
1. Patient characteristics
A total of 1,383 patients with stage III NSCLC were enrolled at 52 sites in South Korea. The baseline characteristics of the study patients are presented in Table 1. Median patient age was 70 years (IQR, 61 to 76 years), and 79.4% of them were male. According to histopathology, 46.5% had squamous cell carcinoma and 40.9% had adenocarcinoma. Among the study patients, 548 (39.6%) had stage IIIA, 517 (37.4%) had stage IIIB, and 318 (23.0%) had stage IIIC. There were no significant differences in age, sex, smoking history, PS, and histopathology between stage groups. The proportion of patients who experienced weight loss was higher in stage IIIC (9.7%) than in stage IIIB (7.4%) or stage IIIC (3.6%, p=0.001).
2. Risk factor for mortality according to baseline characteristics
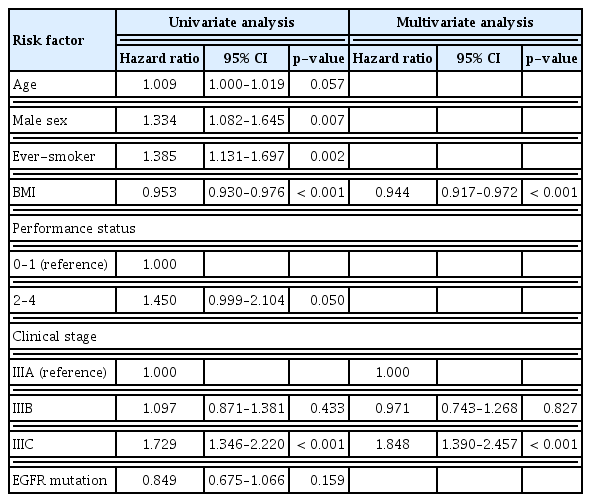
The median follow-up period for the study patients was 16 months (IQR, 7 to 39 years), and 1,052 patients (76.1%) died during follow-up period. In patients with squamous cell cancer, univariate Cox analysis revealed that old age, poor PS, and clinical stage IIIC (compared to stage IIIA) were significant predictors of mortality (Table 2). In multivariate Cox analysis, old age (hazard ratio [HR], 1.031; 95% confidence interval [CI], 1.019 to 1.043; p < 0.001), poor PS (HR, 1.353; 95% CI, 1.010 to 1.813; p=0.043), and stage IIIC (compared with stage IIIA; HR, 1.720; 95% CI, 1.328 to 2.229; p < 0.001) were independently associated with mortality.

Risk factors for mortality in patients with squamous cell carcinoma assessed using the Cox proportional hazards model
Among patients with adenocarcinoma, univariate Cox analysis revealed that old age, male sex, ever-smoker, lower BMI, poor PS, and clinical stage IIIC (compared with stage IIIA) were significant predictors of mortality (Table 3). On multivariate Cox analysis, lower BMI (HR, 0.944; 95% CI, 0.917 to 0.972; p < 0.001), and clinical stage IIIC (compared with stage IIIA; HR, 1.848; 95% CI, 1.390 to 2.457; p < 0.001) were independently associated with mortality.
3. Initial treatment modality
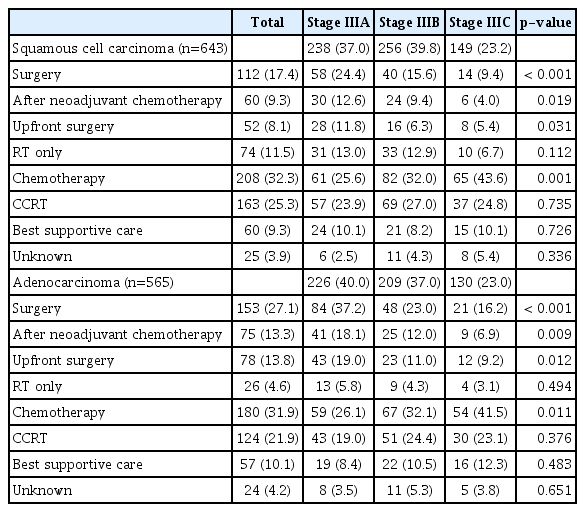
The initial treatment modality among the study patients is listed in Table 4. Among patients with squamous cell carcinoma, 17.4% received surgery as the initial treatment (9.3% of the patients underwent surgery after neoadjuvant chemotherapy, and 8.1% of the patients received upfront surgery). In addition, 11.5% of the patients received radiation therapy, 32.3% of the patients received chemotherapy, 25.3% the patients received CCRT, and 9.3% received no specific anti-cancer treatment as the initial treatment.
Among patients with adenocarcinoma, 27.1% underwent surgery as the initial treatment (13.3% of patients underwent surgery after neoadjuvant chemotherapy, and 13.8% of patients underwent upfront surgery). Moreover, 4.6% of patients received radiation therapy, 31.9% were treated with chemotherapy, 21.9% were received CCRT, and 10.1% received no specific anti-cancer treatment as the initial treatment.
4. Survival analysis
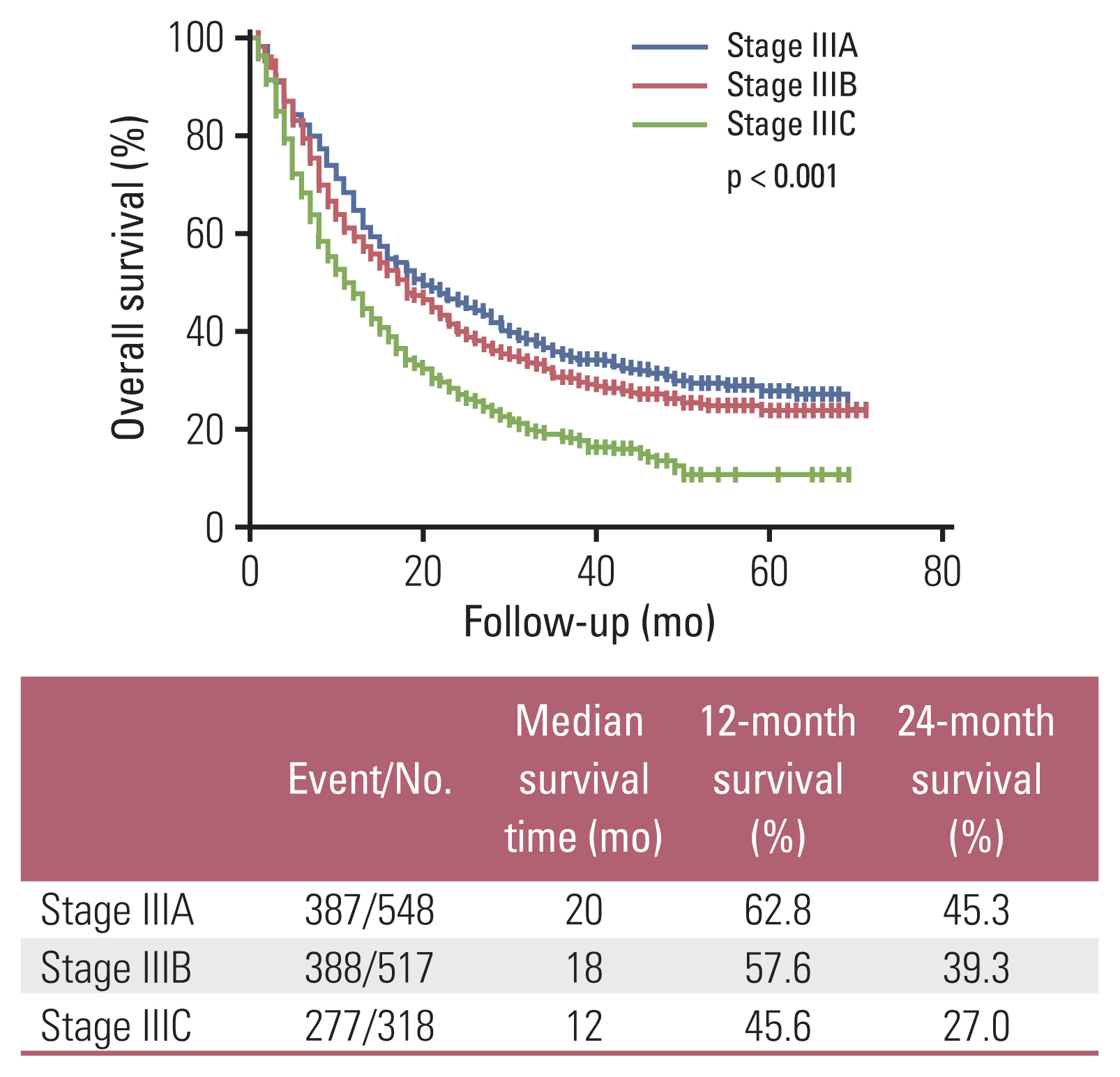
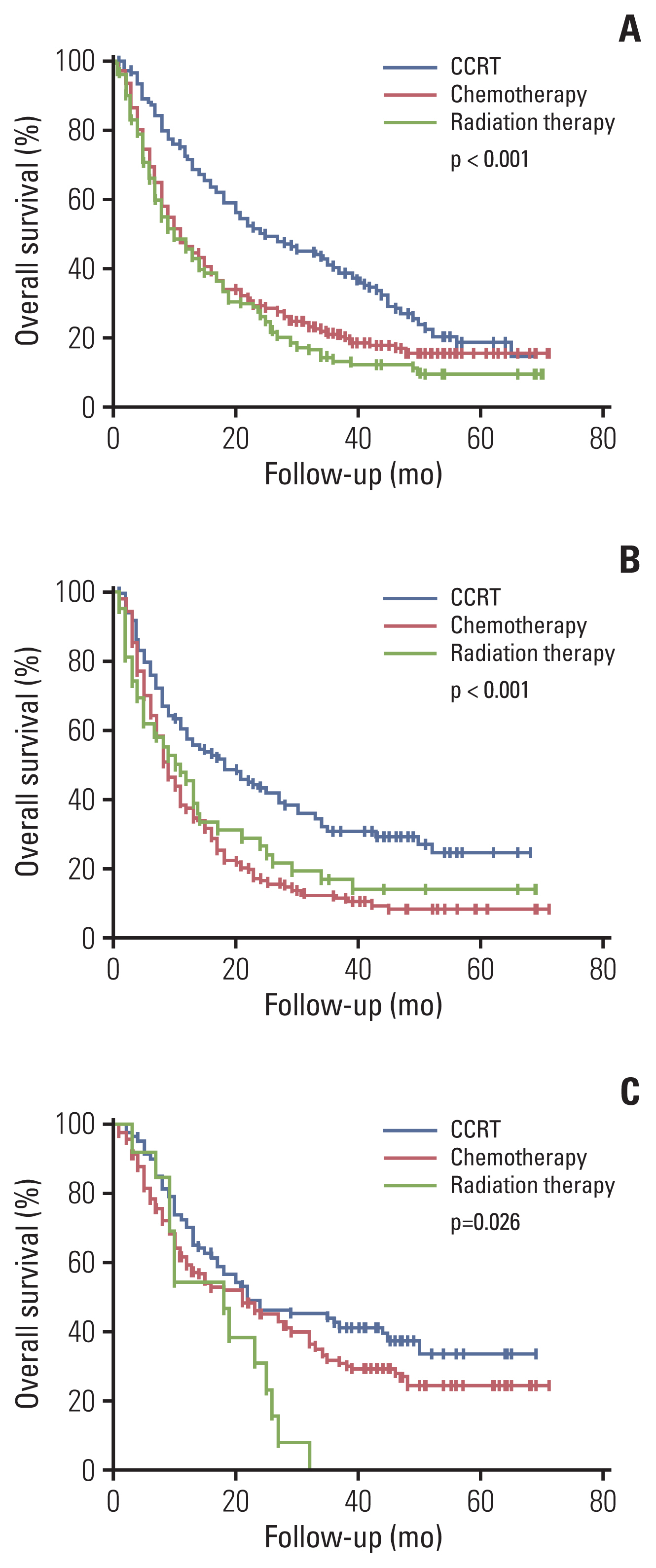
Among the total subjects, patients with stage IIIC (median survival period, 12 months) had worse survival compared to patients with stage IIIA (median survival period, 20 months) or patients with stage IIIB (median survival period, 18 months; p < 0.001) (Fig. 1). According to the initial treatment method in patients with stage IIIA, the surgery group (median survival period, 36 months) showed better survival than the non-surgical treatment group (median survival period, 18 months; p=0.001) (Fig. 2A). These results were consistent for squamous cell carcinoma (Fig. 2B), and the surgery group tended to have better survival than the non-surgical treatment group in adenocarcinoma (Fig. 2C). A comparison of baseline characteristics between the surgery group and the non-surgical treatment group is shown in S1 Table. Patients in the surgery group had younger age, higher BMI, and better PS than those in the non-surgical treatment group. Although, stage N1 group tended to have better survival than stage N2 group among stage IIIA who have undergone surgery on stage IIIA, there were no significant statistical difference in survival time between nodal stages N1 and N2 (median survival, 41 months vs. 30 months; p=0.703). In addition, there was no significant statistical difference in survival time between pneumonectomy group (n=7) and lobectomy group (n=84) in who have undergone surgery on stage IIIA (median survival, 55 months vs. not reached; p=0.773). Among patients with stage IIIB or stage IIIC, those who received CCRT (median survival period, 24 months) showed better survival than those who received chemotherapy (median survival period, 11 months), radiation therapy (median survival period, 10 months; p < 0.001) (Fig. 3A). These results were also consistent for squamous cell carcinoma and adenocarcinoma (Fig. 3B and C). A comparison of patients’ baseline characteristics according to the treatment modality is shown in S2 Table. Patients in the CCRT group had younger age and better PS than those in the chemotherapy group or the radiation therapy group.
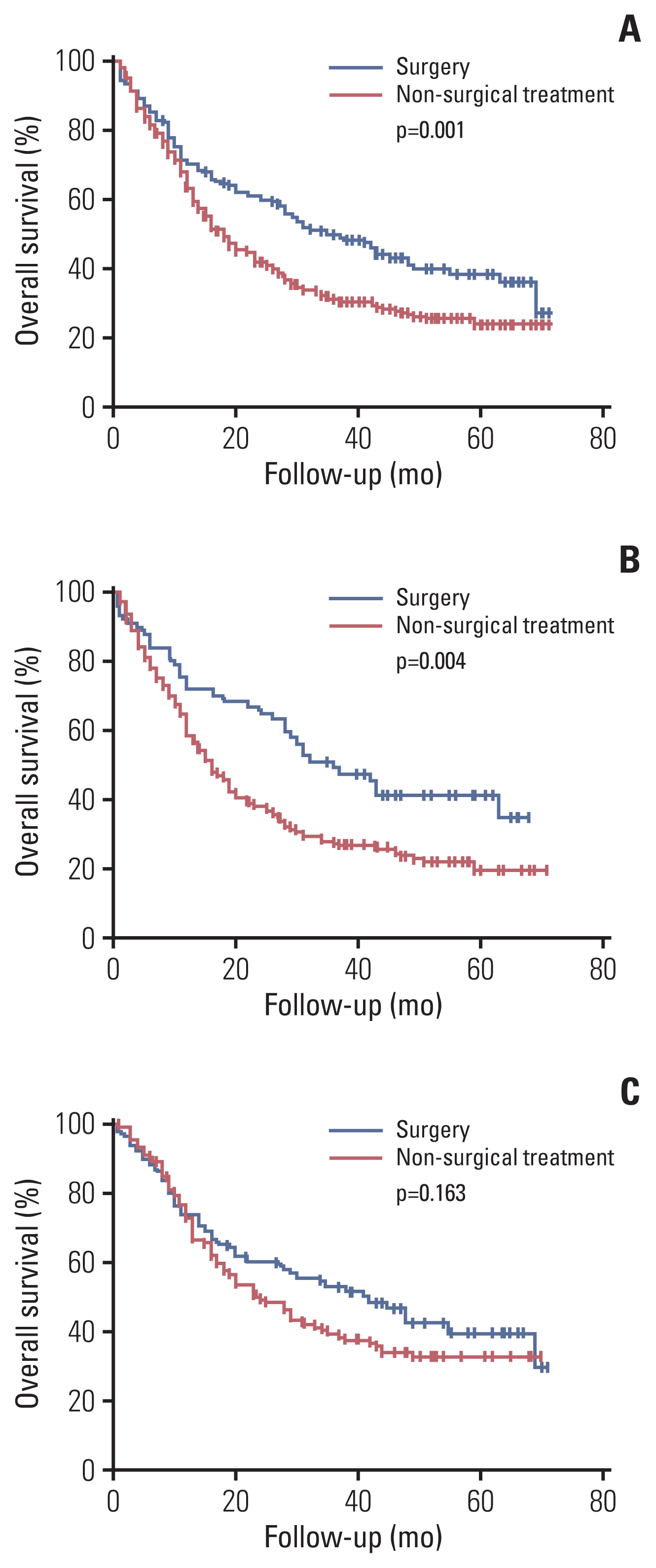
Overall survival of stage IIIA patients according to initial treatment: in total stage IIIA patients (A), in squamous cell carcinoma stage IIIA patients (B), and in adenocarcinoma stage IIIA patients (C).
Discussion
Our study investigated prognostic factors and clinical outcomes in stage III NSCLC. Baseline characteristics such as age, PS, BMI, clinical stage according to the AJCC 8th edition, and treatment modality were associated with the prognosis of the study patients irrespective of histopathology. Moreover, while surgical treatment was associated with better survival outcomes in patients with stage IIIA, CCRT showed clinical benefit in patients with stage IIIB and IIIC. To the best of our knowledge, the present study is the first nationwide-based study in Asia to focus on stage III NSCLC.
Several previous studies reported the clinical characteristics of patients with stage III NSCLC. Morgensztern et al. [12] conducted a study in 12,315 patients with unresectable NSCLC stage III according to the TNM staging 6th edition and showed that tumor size was independently associated with poor prognosis, after adjusting for age, sex, and histopathology. Additionally, Vinod et al. [13] conducted a study in 2,153 patients with stage III NSCLC according to the TNM classification 6th edition and reported that male sex, older age, higher ECOG score, and stage IIIB (vs. stage IIIA) were independently associated with mortality. In addition, Ademuyiwa et al. [14] conducted a study in 203 patients with stage III NSCLC and reported that preserved lung function and higher pretreatment hemoglobin were independent prognostic factors, rather than age and stage (stage IIIA vs. stage IIIB). In the present study, older age, poor PS (in squamous cell carcinoma), and lower BMI (in adenocarcinoma) were independent prognostic factors, a finding that is comparable to those reported in previous studies [15,16].
Several previous studies showed prognostic difference according to the TNM classification system of lung cancer 8th edition [17]. Goldstraw et al. [17] conducted a study on stage III NSCLC and reported that the 5-year survival of patients with clinical stage IIIA, IIIB, and IIIC was 36%, 26%, and 13%, respectively. Chansky et al. [18] conducted a study in 780,294 cases of NSCLC using data from the National Cancer Database, validating the eighth edition of the TNM stage; they showed different median survival time between clinical stages (37.8 months for stage IIIA, 22.2 months for stage IIIB, and 13.8 months for stage IIIC, respectively). However, these previous studies were performed in the Western population, and only few studies validated the TNM classification system eighth edition in the Asian population [19,20]. Although, previous studies showed correlation and prognostic information between the seventh and eighth editions of the TNM classification system in Asia (Korea and Japan) [19,20], these studies focused only on surgical cases. In the present study, although there was no significant prognostic difference between stage IIIA and stage IIIB (median survival period, 20 months vs. 18 months; p=0.080), patients with stage IIIC had worse survival compared to the patients with stage IIIA (median survival period, 12 months vs. 20 months; p < 0.001) or patients with stage IIIB (median survival period, 12 months vs. 18 months; p < 0.001). Future studies with larger populations in Asia will be needed to validate the eighth edition of the TNM classification system and determine prognostic difference.
In the present study, surgical treatment played a beneficial role in patients with stage IIIA NSCLC. Although, surgery group tended to have better survival than the non-surgical treatment group in adenocarcinoma, there was no statistically significant difference. Among patients with stage IIIA adenocarcinoma who did not receive surgery, about 14.1% of patients were treated with EGFR tyrosine kinase inhibitors, which might have induced these results. In patients with T3N1 or T4N1 NSCLC (stage IIIA), surgery might be considered in feasible cases [21]. However, there remains controversy about the role of surgery, especially in patients with N2 involvement. Albain et al. [8] conducted a study in 396 patients with stage T1-3pN2M0 NSCLC and reported that there was no significant survival benefit of surgery after chemoradiation therapy but showed improved progression-free survival in the surgery group. Van Meerbeeck et al. [22] conducted a study in 332 stage IIIA (N2) patients who received induction chemotherapy and reported that surgical treatment did not show any association with better survival outcome than radiation therapy. However, approximately 47% of patients of the surgery group received pneumonectomy in that study, which might have caused a negative clinical outcome in the surgery group. Although currently there are no data available about the definitive benefit of surgery in patients with stage III NSCLC, several studies have suggested the potential role of surgery in these patients. Eberhardt et al. [23] conducted a study in 246 patients who received induction chemotherapy with stage IIIA and IIIB NSCLC and reported that the 5-year overall survival and progression-free survival were not different between the surgery group and the chemoradiation therapy boost group. Yun et al. [24] conducted a study in 706 patients with pathologic N2 disease who underwent upfront surgery and reported that the median survival time of study patients was 52 months, which was comparable to that reported in a previous study. McElnay et al. [25] performed a systematic review and meta-analysis and reported that surgery, as part of trimodality treatment led to better overall survival than chemoradiotherapy alone despite no significant differences. However, there may be selection bias in these results. In the present study, the surgery group had younger age and better PS than the non-surgical treatment group, which might have influenced a better clinical outcome. Considering these findings, surgical treatment can be considered in select patients with stage III NSCLC.
According to the initial treatment methods, the CCRT group showed favorable outcome compared to the chemotherapy or radiation therapy group in patients with stage IIIB and IIIC NSCLC. These findings were consistent with those of previous studies [26–28]. Several randomized phase III trials conducted among patients with stage III NSCLC have shown that the CCRT group had better survival than the sequential treatment group [26,28]. However, there is a lack of data for real-world treatment outcome in patients with stage III NSCLC. Although Ryan et al. [29] showed that most stage III NSCLC patients received CCRT in the clinical setting, the prognosis according to the treatment modality was not shown in their study. Horinouchi et al. [30] conducted a study in 214 patients with unresectable stage III NSCLC who received CCRT and reported that the median overall survival of the study patients from completing CCRT was 36.4 months. Although the CCRT group had younger age and better PS than the chemotherapy alone or radiation therapy alone group, our study showed a beneficial role of CCRT using real-world data. Thus, CCRT should be considered in eligible stage III patients.
There were several limitations in our current study. First, the present study had a retrospective study design and was conducted in Korea. However, the subjects were recruited from 52 centers in Korea, which might have reduced selection bias. Second, although a standardized protocol was used to collect information, missing some clinical information was inevitable. For example, the reason 37 patients with stage IIIC underwent surgery is still unclear. Third, the study population was enrolled between 2014 and November 2016, at which time, the clinical effect of immunotherapy could not be analyzed. Fourth, there are a lot of missing data about exact date of disease progression in each patient, so we did not perform analysis about progression-free survival results. Finally, although this is the largest series of stage III NSCLC cases in Asia reported to date, the numbers are still relatively small to perform a meaningful subgroup analysis. Especially, it is hard to perform meaningful subgroup analysis about N category because there was no accurate information about exact lymph node involvement in many cases. Despite these limitations, we believe that our study will serve as the basis to understand the clinical characteristics of stage III NSCLC patients in the Asian population.
In conclusion, clinical stage according to the eighth edition of TNM staging was associated with the prognosis of stage III NSCLC patients. While surgery might be suitable as an appropriate first-line treatment in select patients with stage IIIA NSCLC, CCRT might be important in patients with stage IIIB or IIIC NSCLC.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study protocol was reviewed and approved by the Institutional Review Board at the National Cancer Center (NCC2018-0193), which waived the requirement for informed consent due to the retrospective study design.
Author Contributions
Conceived and designed the analysis: Kim HC, Ji W, Lee JC, Kim HR, Song SY, Choi CM.
Collected the data: Kim HC, Choi CM.
Contributed data or analysis tools: Kim HC, Ji W, Lee JC, Kim HR, Song SY, Choi CM.
Performed the analysis: Kim HC.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
This study was supported by the Health Promotion Fund, Ministry of Health and Welfare, Republic of Korea (1660680). The data used in this study were provided by the Korean Association for Lung Cancer (KALC) and the Ministry of Health and Welfare, Korea Central Cancer Registry (KCCR).