Clinical Significance of Acute Kidney Injury in Lung Cancer Patients
Article information
Abstract
Purpose
Acute kidney injury (AKI) in cancer patients is associated with increased morbidity and mortality. The incidence of AKI in lung cancer seems to be relatively higher compared with other solid organ malignancies, although its impact on patient outcomes remains unclear.
Materials and Methods
The patients newly diagnosed with lung cancer from 2004 to 2013 were enrolled in this retrospective cohort study. The patients were categorized according to the presence and severity of AKI. We compared all-cause mortality and long-term renal outcome according to AKI stage.
Results
A total of 3,202 patients were included in the final analysis. AKI occurred in 1,783 (55.7%) patients during the follow-up period, with the majority having mild AKI stage 1 (75.8%). During the follow-up of 2.6±2.2 years, total 1,251 patients (53.7%) were died and 5-year survival rate was 46.9%. We found that both AKI development and severity were independent risk factors for all-cause mortality in lung cancer patients, even after adjustment for lung cancer-specific variables including the stage or pathological type. In addition, patients suffered from more severe AKI tend to encounter de novo chronic kidney disease development, worsening kidney function, and end-stage kidney disease progression.
Conclusion
In this study, more than half of the lung cancer patients experienced AKI during their diagnosis and treatment period. Moreover, AKI occurrence and more advanced AKI were associated with a higher mortality risk and adverse kidney outcomes.
Introduction
Innovative advances in the treatment of cancer are beginning a new era in the field of oncology. With such great changes, the overall survival of cancer patients is increasingly prolonged; therefore, it has become necessary to consider and manage the associated medical conditions [1]. Acute kidney injury (AKI) is a common and critical problem in cancer patients [2,3]. The incidence, severity, and causes of AKI in cancer patients depend on the type and stage of cancer, treatment modalities, and comorbidities [2]. In a Danish study on AKI in cancer patients, the 1-year risk of AKI was 17.5%, with 27% risk over 5 years [4]. There are various reported mechanisms of AKI in cancer patients, including: (1) prerenal causes due to volume depletion related to nausea, vomiting, or diarrhea resulting from treatments such as chemotherapy or the cancer itself, (2) intrinsic causes including tumorous infiltration of the kidney, cast nephropathy, and tumor lysis syndrome, and (3) post-renal causes represented by obstructive nephropathy [5–7]. Regardless of the cause of AKI, the presence of AKI in cancer patients is associated with substantial morbidity and mortality and can disturb the dose and timing of appropriate systemic chemotherapy [8].
Previous studies showed that hematologic malignancy, hepatocellular carcinoma, pancreatic cancer, biliary tract cancer, and kidney and urinary tract cancer had a higher incidence of AKI than other types of cancer [4,9]. However, there have been few studies on the incidence and risk factors for AKI in patients with lung cancer [10–12]. Older age, use of radiocontrast, receiving nephrotoxic chemotherapeutic agents, and multiple cycles of chemotherapy are known to be associated the development of AKI in patients with lung cancer, as in other AKI-prone malignancies [10,11]. Only one study exploring the impact of AKI on mortality in lung cancer patients receiving chemotherapy has demonstrated that most AKI developed to a mild degree, thus its prognosis was favorable [10]. Considering the small number of patients included in the analysis, their non-significant result should be supported further by large scale studies.
In our previous studies, we reported an unexpectedly higher incidence of AKI in lung cancer patients than other malignancies [13–15]. Therefore, in the present study, we aimed to determine the clinical significance of AKI in lung cancer patients with a focus on the impact on both patient and kidney prognosis.
Materials and Methods
1. Study population
This retrospective cohort study was conducted at Seoul National University Hospital, which is a tertiary referral hospital in Seoul, South Korea. We used data from electronic medical records and the cancer registry system. In Korea, The Ministry of Health and Welfare generated a nationwide registry called the Korea Central Cancer Registry (KCCR), beginning in 1980, to register cancer patients and provide medical insurance benefits to them. All patients diagnosed with any type of cancer in Korea were registered on the KCCR based on the International Classification of Diseases 10th revision (ICD-10) [14].
We included patients who were diagnosed with cancer between January 1, 2004, and December 31, 2013 based on the KCCR registration in Seoul National University Hospital. From a total of 106,004 patients who were registered on the KCRR, we excluded patients having the following exclusion criteria after electronic medical record review: (1) aged less than 18 years, (2) registered with more than two types of cancer, (3) no measured serum creatinine (sCr) or checked only once, (4) initial estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2 or undergoing dialysis before cancer diagnosis, and (5) no accurate data for death and follow-up duration, as previously reported [14]. Therefore, 67,986 patients were eligible for this study, and among them, 5,855 were patients diagnosed with respiratory tract cancer. We selected the final study subjects who had been diagnosed with lung cancer and by setting additional exclusion criteria: (1) no accurate data for demographic information, laboratory findings, and treatment history, (2) development of AKI within 2 weeks before death, and (3) initial sCr < 0.6 mg/dL.
2. Data collection
We reviewed and collected data from the electronic medical records. Demographic characteristics included the age, sex, body mass index (BMI), and smoking history. Systolic and diastolic blood pressure was measured at the time of the first hospital admission. The data regarding a history of diabetes mellitus (DM) was also gathered. Initial laboratory findings including initial sCr, anemia, and serum level of calcium, phosphorus, sodium, potassium, chloride, total CO2, uric acid, albumin, and alkaline phosphatase (ALP) were collected at the time of cancer diagnosis. The eGFR was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula [16].
Like other solid organ cancers, lung cancer consists of various histological subtypes and the cancer stage determines the treatment strategy. Both histological subtypes and the cancer stage could affect AKI occurrence. Therefore, we investigated cancer-specific factors, such as the pathological subtype, cancer stage, and treatment modality. Pathological subtypes were divided into four groups: adenocarcinoma, squamous cell carcinoma, small cell carcinoma, and others. Most of the “others” group included non-small cell carcinoma, which was difficult to classify into more detailed pathological subtypes such as adenocarcinoma or squamous cell carcinoma. Carcinoid, neuroendocrine tumor, and sarcoma were also included in the “others” group. We used the cancer staging system provided by National Institutes of Health in the United States, which is named the Surveillance, Epidemiology, and End Results (SEER) stage [17]. The SEER stage is the most basic way of categorizing how far a cancer has spread from its point of origin. It consists of nine codes: in situ, localized only, regional by direct extension only, regional lymph nodes involved only, regional by both direct extension and lymph node involvement, regional not otherwise specified, distant site(s)/node(s) involved, and unknown if extension or metastasis (un-staged, unknown, or unspecified) [17]. Treatment modalities were divided into four groups: surgery only, surgery and chemotherapy, chemotherapy only, and neither surgery nor chemotherapy, respectively. Radiation therapy was excluded from our analysis because it was thought that radiation had little effect on the kidney function [18]. We investigated whether the chemotherapeutic agents known to have nephrotoxicity including cisplatin, carboplatin, ifosfamide, gemcitabine, and pemetrexed were administered during the study period [19]. We reviewed the guidelines and chose the drugs based on the chemotherapy regimen of lung cancer used in our institution [20]. A count of radiocontrast-enhanced computed tomography per year was also explored to consider possible contrast-induced AKI.
3. AKI definition
The definition of AKI was applied in the same way as in our previous study [14]. Initial sCr was defined as the first measured sCr within 2 months before or after the KCCR registration date. Considering possible fluctuating kidney function during cancer diagnosis and treatment in lung cancer patients, we defined the baseline sCr as the minimum value of sCr from the current time to the previous 3 weeks by shifting the reference point every 3 weeks based on the KCCR registration date for a more precise analysis.
We defined AKI and the stage of AKI based on the Kidney Disease: Improving Global Outcomes (KDIGO) AKI guidelines [21] using sCr levels. Urine output criteria could not be considered due to lack of complete urine output data. Patients were classified according to the presence and its severity of AKI into three groups as no AKI, AKI stage 1, and AKI stage 2/3.
4. Study outcomes
The primary outcome was to identify the incidence of AKI and its impact on all-cause mortality in patients with lung cancer. In the mortality analysis, we analyzed the patients who survived for > 1-year after lung cancer diagnosis because we intended to determine the direct impact of AKI on mortality. If a patient died within 1 year of the cancer diagnosis, even if they had AKI, we could not presume that the incident AKI affected the death. Subgroup analysis was performed according to age, sex, pathologic subtype, and SEER stage to determine the effect of advanced AKI on mortality.
The secondary outcome was long-term kidney outcomes. The last eGFR was calculated as the mean value of eGFRs based on the last three sCr measurements. The average of the last three eGFRs was categorized in five groups: (1) 60 or more, (2) 45 or more and less than 60, (3) 30 or more and less than 45, (4) 15 or more and less than 30, and (5) less than 15. In the same way, the initial eGFR was also classified, and the change of the stage was examined. We defined a reduction of the eGFR to less than 60 mL/min/1.73 m2 as new-onset chronic kidney disease (CKD). In addition, we explored cases of progression to end-stage kidney disease (ESKD) requiring maintenance renal replacement therapy such as hemodialysis and peritoneal dialysis. To assess long-term outcomes, we divided the proportions of early and last eGFR into quartiles and looked at their distribution.
5. Statistical analysis
We presented continuous variables as mean±standard deviation, and categorical variables as percentage. We used the student t test, one-way analysis of variance (ANOVA), and chi-square test to investigate the differences among the no AKI group, AKI stage 1 group, and AKI stage 2/3 group. A multivariable logistic model was developed to identify the independent risk factors of AKI using the odds ratio (OR) and 95% confidence interval (CI). Cox regression analyses were used to calculate the hazard ratio (HR) and 95% CI for the risk of all-cause mortality. The Kaplan-Meier survival curve was used to compare the cumulative mortality among the no AKI group, AKI stage 1 group, and AKI stage 2/3 group with the p-value calculated from the log-rank method. The SPSS ver. 25 (IBM Corp., Armonk, NY) and R software ver. 3.6.1 (R Software, R Foundation for Statistical Computing, Vienna, Austria) were used to perform the statistical analysis. A p-value < 0.05 was considered statistically significant.
Results
1. Baseline characteristics
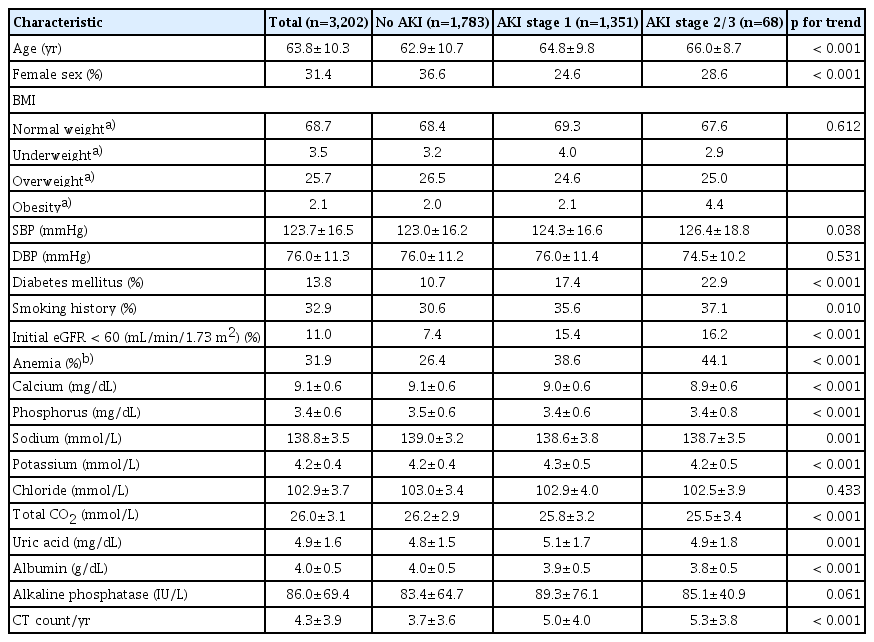
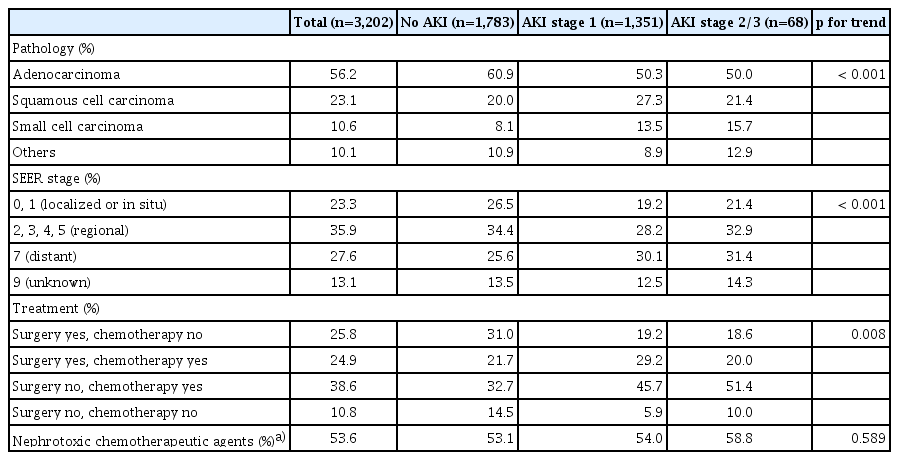
A total of 3,202 patients were enrolled in this study (S1 Fig.), and the mean follow-up duration was 2.6±2.2 years. The mean age was 63.8±10.3 years, and 31.4% of the patients were female as shown in Table 1. Patients with DM and CKD with an initial eGFR < 60 mL/min/1.73 m2 were 13.8% and 11%, respectively. A total of 3,061 patients (95.6%) underwent radiocontrast exposure by computed tomography more than once; the mean annual number of radiocontrast exposures in these patients was 4.3±3.9. Overall, the most common pathological subtype was adenocarcinoma (56%), followed by squamous cell carcinoma (23.1%), small cell carcinoma (10.6%), and others (10.1%). We found that 35.9% of the patients were at the “regional” SEER stage at the initial diagnosis, and “distant” (27.6%), “localized or in situ” (23.3%), and “unknown” (13.1%) SEER stages following that. Chemotherapeutic agents were used in 63.5% of the enrolled patients (Table 2).
2. Clinical characteristics of lung cancer patients with AKI
According to the severity of AKI, three groups were categorized as the no AKI group, AKI stage 1 group, and AKI stage 2/3 group, and accounted for 55.7%, 42.1%, and 2.1%, respectively. After diagnosis of lung cancer, it took 0.64±1.10 years to develop first AKI. The more severe AKI was, the older the patients were. Males were predominant in all AKI groups. The severity of AKI was correlated with higher systolic blood pressure, increased annual radiocontrast exposure, a higher proportion of DM and anemia, and higher proportion of initial eGFR < 60 mL/min/1.73 m2. Among the pathological subtypes, small cell carcinoma was more significantly associated with AKI than other subtypes. Patients receiving chemotherapy tend to develop AKI, although, unexpectedly, the use of nephrotoxic chemotherapeutic agents including cisplatin, carboplatin, ifosfamide, gemcitabine, and pemetrexed seemed not to be associated with AKI risk elevation.
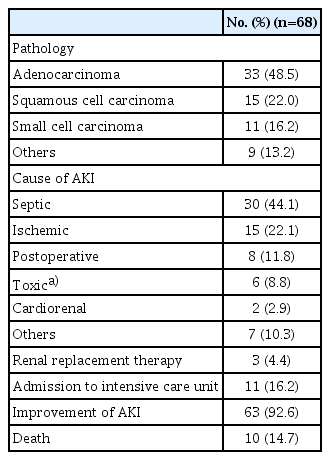
A total of 68 patients experienced severe AKI (stage 2/3); their clinical characteristics are shown in Table 3. The most common cause of severe AKI was sepsis (44.1%), followed by ischemia (11.8%), nephrotoxic drugs (8.8%), cardio-renal syndrome (2.9%), and others (10.3%). The majority of these patients recovered from AKI (92.6%); however, the remaining 4.4% of patients experiencing severe AKI eventually progressed to kidney failure requiring maintenance renal replacement therapy. Ten patients (14.7%) died after the development of AKI. The mean duration from the development of AKI to death was 48.4±50.6 days.
3. Risk factors of incident AKI
We describe the clinical risk factors of AKI occurrence in lung cancer patients during their cancer diagnosis and treatment in S2 Table. Among the clinical characteristics, older age, male sex, higher systolic blood pressure, presence of DM, anemia, and hyponatremia, lower initial eGFR < 60 mL/min/1.73 m2, and lower serum albumin were independent risk factors of AKI development after lung cancer diagnosis. Increased annual radiocontrast exposure and receiving chemotherapy were also correlated with an elevated risk of incident AKI, although SEER stage was not.
Among the pathological subtypes, squamous cell carcinoma (adjusted OR, 1.29; 95% CI, 1.047 to 1.590; p=0.017) and small cell carcinoma (adjusted OR, 1.57; 95% CI, 1.188 to 2.067; p=0.001) were associated with AKI occurrence. When comparing baseline characteristics according to the pathological subtypes, the proportion of patients with risk factors of AKI in small cell carcinoma and squamous cell carcinoma was statistically significantly higher (S3 Table).
4. Impact of AKI on all-cause mortality
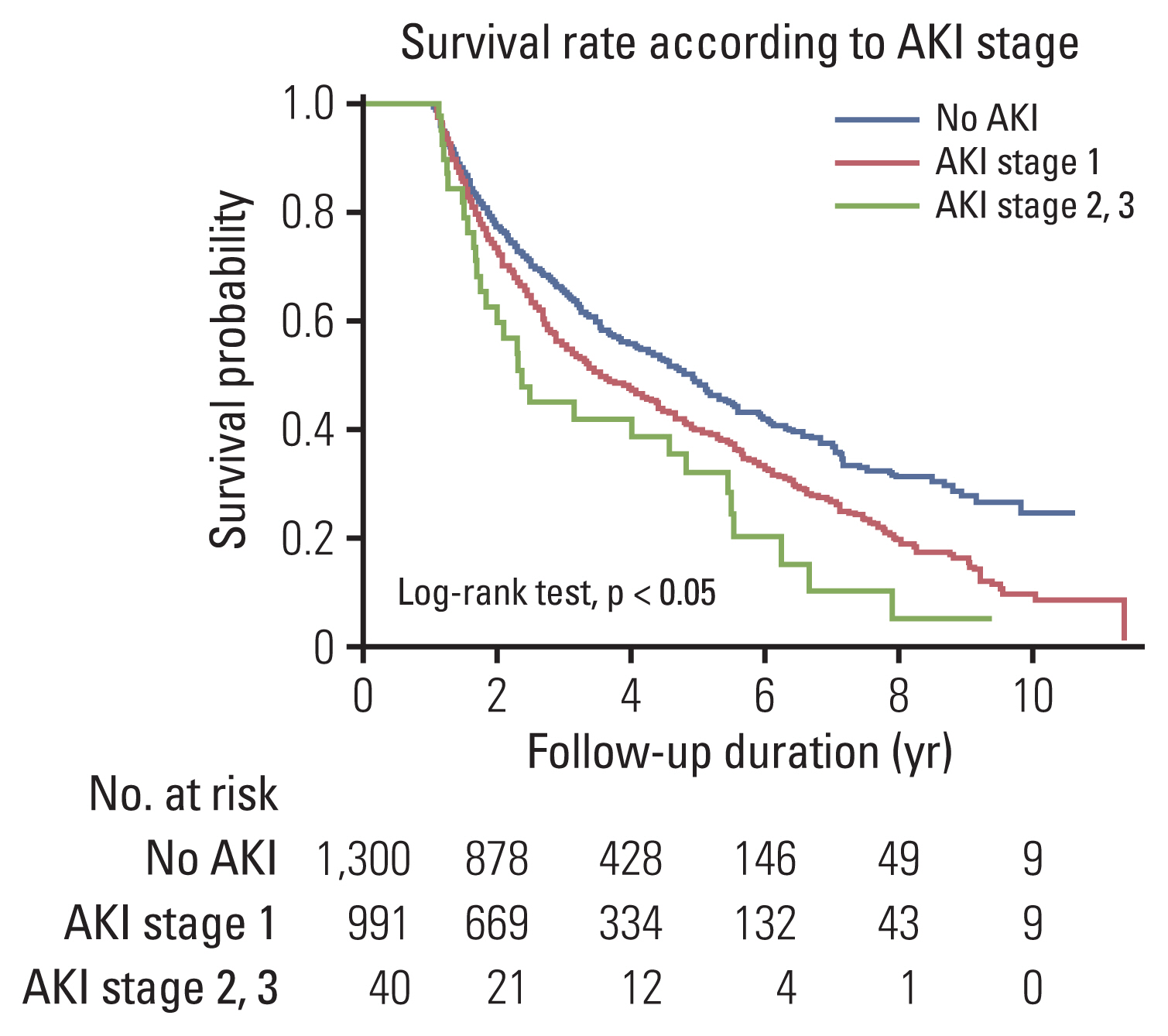
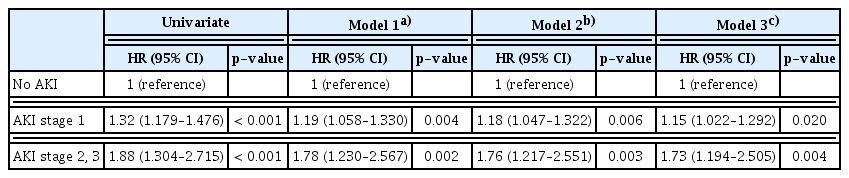
To elucidate whether AKI affected lung cancer patient survival, we analyzed the effect of AKI on mortality only among patients who survived > 1 year after cancer diagnosis. During the study period, 1,251 patients (53.7%) were died, and overall 5-year survival rate was 46.9%. The Kaplan-Meier curve showed that more severe AKI was related to a lower survival rate (Fig. 1). As shown in Table 4, the incident AKI elevated all-cause mortality in a dose-responsive manner (AKI stage 1: adjusted HR, 1.15; 95% CI, 1.022 to 1.292; p=0.020 and AKI stage 2/3: adjusted HR, 1.73; 95% CI, 1.194 to 2.505; p=0.004), even after the adjustment of age, sex, BMI, smoking history, initial eGFR < 60 mL/min/1.73 m2, presence of anemia and hyponatremia, and pathological subtypes.
Subgroup analyses showed that even mild AKI (stage 1) was associated with increased mortality, especially in women and adenocarcinoma cancer type. Severe AKI (stage 2/3) was associated with elevated mortality in every subgroup except women, small cell carcinoma, and distant and unknown SEER stage shown in S4 Fig.
5. Impact of AKI on long-term kidney outcomes
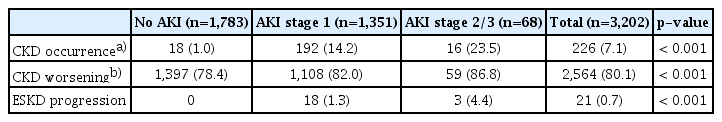
The effect of AKI on long-term kidney outcomes was shown in Table 5 and S5 Table. The incidence rate of the new-onset CKD was 7.1% and it was associated with AKI experiences with dose-responsive manner. When comparing the CKD stage of the last eGFR with the initial eGFR, most lung cancer patients experienced worsening kidney function. Particularly, patients suffered from more severe AKI tended to face more worsening CKD stages. Among the enrolled patients, we found that 21 patients (0.7%) progressed to ESKD requiring maintenance renal replacement therapy after lung cancer diagnosis during the study period, and all of them experienced prior AKI (Table 5). Their median time to ESKD progression was 2.16±2.71 years. Furthermore, the ratio of initial eGFR and the average of the last three eGFRs (last three eGFRs/initial eGFR) was lower in patients with more advanced AKI (stage 2/3) (S5 Table).
Discussion
With the growing importance of kidney function in cancer patients, the field of oncology is gaining attention. Unfortunately, there are no large cohort studies regarding the clinical significance of AKI in lung cancer patients. In this study, we found that incident AKI was more commonly found in lung cancer patients than previously reported and is associated with a high mortality rate. In addition, we showed that both the presence and severity of AKI were associated with higher mortality and worse long-term kidney outcomes in lung cancer patients.
In addition to our previous study, there have been few studies that have aimed to determine the relationship bet-ween incident AKI and mortality in cancer patients. Two studies showed that kidney dysfunction was related to higher mortality in cancer patients admitted to the intensive care unit [22,23]. In our study, we analyzed a total of 2,331 patients who survived > 1 year after lung cancer was diagnosed. We hypothesized that incident AKI will be negatively associated with mortality and long-term kidney outcomes, which was supported by our results. A total of 1,251 deaths were observed; 601 (46.2%), 620 (62.6%), and 30 (75%) deaths were observed in the no AKI, AKI stage 1, and AKI stage 2/3 groups, respectively. Considering the Cox proportional hazard analysis, these results suggest that not only the presence of AKI but also the severity of AKI may affect mortality in lung cancer patients. However, in the subgroup analysis, statistically significant results were not obtained in patients with small cell carcinoma and with SEER stage 7. Even in patients with SEER stage 7, mild AKI was found to be protective against all-cause mortality. Small cell carcinoma in the lung is known to have a poorer prognosis than other subtypes [24]. It is also clear that patients with distant metastasis have a poorer prognosis than those who do not. Further analysis with more subjects may be needed, but these results suggest that other factors beyond the incidence of AKI may have a greater impact on mortality.
In terms of long-term kidney function, we found that 7.1% of lung cancer resulted in de novo CKD. Furthermore, the proportion of the patients who deteriorated their CKD stage and who progressed to ESKD were higher in the more advanced AKI group, although its absolute ESKD incidence was rare. The relationship between cancer and kidney seems to be a circular, but previous studies usually limited to acute kidney damage in cancer patients or cancer development in CKD patients [25,26]. By virtue of the recent advancement in early detection and cancer treatment, the numbers of cancer survivors are increasing. Keeping pace with it, the late sequelae of cancer treatment such as cardiovascular diseases, metabolic syndrome, diabetes, and osteoporosis increases, although it is easy to be overlooked [27–30]. However, there are only rare data on CKD development or worsening kidney function which is one of the important sequelae in cancer patients after treatment. CKD incidence or worsening kidney function raises the nihilistic approach to cancer treatment, leading the patients to frequent under-treatment and sequential loss of opportunity to gain prolonged survival [31]. As shown in our results, the development of AKI in lung cancer patients does not end with the occurrence of simple acute complications but may lead to a decrease in long-term kidney function, eventually leading to CKD. Clinicians should pay attention to this and try to detect AKI earlier and to prevent it.
In order to prevent AKI development in lung cancer patients, it is necessary to evaluate the possibility of occurrence and to adjust the risk factors. We intended to determine what characteristics of lung cancer were involved in a higher than expected rate of incident AKI compared with other types of malignancy. Few studies have identified risk factors for AKI in lung cancer patients. Predictive factors for AKI after lung cancer surgery were presented: hypertension, sCr elevation, low forced vital capacity, high N-terminal pro-B-type natriuretic peptide, pneumonectomy, and blood loss during surgery [11]. In another study, the number of chemotherapy cycles was independently associated with AKI in lung cancer patients with palliative chemotherapy [10]. Several factors including old age, higher systolic blood pressure, presence of DM and anemia, lower initial eGFR < 60 mL/min/1.73 m2, and receiving chemotherapy could be considered predictive risk factors for AKI in this study. Based on these results, we would like to provide a basis for selecting patients with a high probability of AKI and carefully tracking kidney function when treating lung cancer patients. However, among the chemotherapy group, the use of nephrotoxic chemotherapeutic agents was no significantly different among AKI groups. We presumed that these results were due to the tendency of physicians to be less likely to prescribe drugs with nephrotoxicity to patients predicted with impaired kidney function.
There are several limitations in this study. First, this is the retrospective study conducted in a single medical center. Second, despite the revised method for sCr, we only used the sCr to evaluate the kidney function. If an evaluation of proteinuria or urine volume is added, more accurate kidney function can be assessed. Third, we excluded the patients with initial sCr less than 0.6 mg/dL and limited to patients with sCr within the normal range. Since sCr is dependent on muscle mass, in patients with low muscle mass, the eGFR can be overestimated [32,33]. Also, we concerned that low sCr could cause a sharp change in eGFR even with a small change, resulting in severe AKI. In actual clinical settings, it was often difficult to trust the severity of AKI in these cases. Fourth, we did not determine the causes of all AKI that occurred in this study. In practice, AKI in the clinical setting is caused by a combination of multiple factors [34]. Initially, we intended to find out the specific causes of AKI in lung cancer; however, we only identified the causes of severe AKI through chart review, and that findings were also not very different from those in other settings. Fifth, we could not review all medications administered to study subjects; in addition to the chemotherapeutic agents we analyzed, there are drugs such as antibiotics, anti-hypertensive drugs, DM treatments, and new drugs such as immune checkpoint inhibitors that known to affect kidney function [35]. Sufficient analysis of all drugs will help to predict the cause of AKI.
Although there are some limitations, this study aimed to identify the incidence of AKI and the association of AKI and mortality in patients with lung cancer. We found that incident AKI is a risk factor of mortality in patients with lung cancer, and more advanced AKI is related to higher mortality than no AKI and mild AKI. Incident AKI affects not only death but also long-term deterioration of kidney function. Advances in the treatment of cancer will prolong the lifespan of cancer patients, which also require the consideration of kidney function and efforts to preserve it. Therefore, further research is needed to determine which characteristics of cancer patients are linked to kidney function.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki and approved by the institutional review board at SNUH (H-1509-051-702) with waiver of individual patient consent.
Author Contributions
Conceived and designed the analysis: Kang U, Kang HG, Yoon HJ, Lee H.
Collected the data: Cho S, Kang E, Kim JE, Park M, Lee H.
Contributed data or analysis tools: Cho S, Kang E, Park M, Lee H.
Performed the analysis: Cho S, Kang E, Park M, Lee H.
Wrote the paper: Cho S, Lee H.
Editing and revision of manuscript and approval of final version: Kang E, Kim JE, Kang U, Kang HG, Park M, Kim K, Kim DK, Joo KW, Kim YS, Yoon HJ, Lee H.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.