Clinical Prognostic Factors for Locally Advanced Esophageal Squamous Carcinoma Treated after Definitive Chemoradiotherapy
Article information
Abstract
Purpose
Locally advanced esophageal cancers are generally treated with neoadjuvant chemoradiotherapy, followed by surgery in operable candidates. However, even if the patients were diagnosed as operable disease, surgery could not be performed on patients with poor condition or other comorbidity. In this case, definitive chemoradiotherapy (dCRT) is the other option for localized esophageal cancer. Therefore, the purpose of this study was to evaluate the efficacy and clinical prognostic factors for dCRT in locally advanced esophageal cancer.
Materials and Methods
We conducted a review of patients who received dCRT for locally advanced squamous esophageal cancer from 2004 to 2010, focusing on stages III and IVa. All patients received at least two cycles of platinum-based chemotherapy during radiation, and all tumor burdens were included in the radiation field. The treatment results were analyzed for patterns of failure and prognostic factors associated with survival.
Results
In total, 63 patients were enrolled in this study. The overall response rate was 84.1%. Relief from dysphagia after dCRT was achieved in 48 patients. The most frequent failure was local recurrence. The median overall survival (OS) was 23.0 months, and the 2-year survival rate was 45.4%. Similar results were observed for elderly study patients. Significant prognostic factors for OS were duration of smoking, high grade of dysphagia (score of 3 or 4), and shorter duration of progression-free and dysphagia-free survival. Maintenance chemotherapy after dCRT did not influence OS. However, "good risk" patients receiving maintenance chemotherapy showed better OS than those who did not receive maintenance chemotherapy (30.4 months vs. 12.0 months, p=0.002).
Conclusion
dCRT has a major role in improving survival and palliation of dysphagia in inoperable advanced esophageal cancer, even in elderly patients. Maintenance chemotherapy after dCRT may be effective in prolonging survival in "good risk" patients.
Introduction
Esophageal cancer is the seventh most common cause of cancer-related deaths, and the 5-year survival rate was only 19% in 2001-2007 in the United States [1]. Each year, approximately 2,136 persons receive a diagnosis of esophageal cancer, and 1,406 persons died of esophageal cancer in South Korea in 2009 [2]. Surgical treatment should be considered for all localized esophageal cancer; however, because of the tumor site or comorbid disease, surgical resection is possible in only 15-20% of patients. In addition, the outcome of surgery is not satisfactory: the 2-year survival rate is only 15-24%, and 30-40% of these patients suffer from postoperative complications [3]. Thus, management of locally advanced esophageal cancer patients has been challenging, requiring a multimodal approach.
Since the Radiation Therapy Oncology Group (RTOG) 85-01 trial was reported [4], concurrent chemoradiotherapy has been widely used as the standard treatment. To enhance the efficacy, not only the radiation technique but also many chemotherapeutics such as docetaxel, cetuximab, erlotinib, and bevacizumab have been incorporated.
With trials using multimodal treatments, it is important to understand the characteristics of esophageal cancer subpopulations. Particularly in Korea, more than 85% of esophageal cancer patients show squamous cell carcinoma, which is well-known to have an association with smoking or alcohol use. In addition, more than 60% of esophageal cancer patients are diagnosed at ages older than 60 years. These findings suggest that many patients will not be candidates for surgery due to comorbid conditions, which increases occurrence of pre- and post-operative complications. For these reasons, definitive chemoradiotherapy (dCRT) has been the standard treatment of choice for locally advanced esophageal cancer, even in operable cases.
The importance of preoperative chemoradiotherapy was re-emphasized in one recent study [5]. Therefore, we retrospectively examined the efficacy of dCRT in patients with locally advanced esophageal cancer who were not candidates for surgery and explored prognostic factors associated with survival. In addition, we consider the value of maintenance chemotherapy after dCRT.
Materials and Method
1. Eligibility criteria and staging
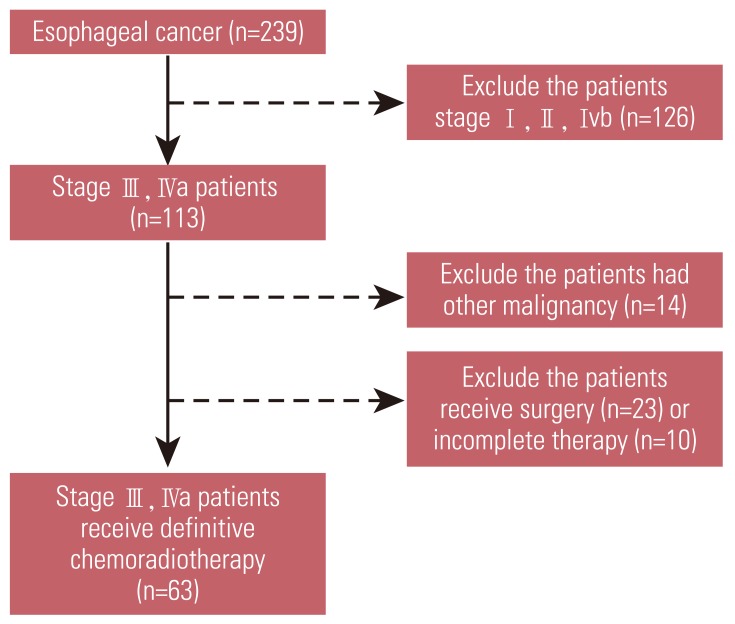
Esophageal cancer patients treated with dCRT at Chonnam National University Hwasun Hospital between January 2004 and December 2010 were retrospectively reviewed. Inclusion criteria for this study were 1) locally advanced esophageal cancer staged as T3 N1, T4 any N, or any T any N M1a using American Joint Committee on Cancer (AJCC) staging (6th edition [6]) with no clinical evidence of distant organ metastasis (stages III, IVa), 2) inoperable condition such as tumor location, comorbid disease, or patient's refusal, 3) histologically confirmed as squamous cell carcinoma, 4) all of the tumor burden should be included in the radiation field. Patients were excluded if they met the following criteria: 1) history of concomitant or previous malignancy, 2) esophageal carcinoma with distant visceral oral metastasis (M1b), 3) medical comorbidities that might compromise the delivery of therapy or that may be exacerbated by the planned treatment. Esophagoscopy, esophageal ultrasonography, computed tomography (CT), and positron emission tomography-computed tomography (PET-CT) scans were used in tumor staging. Qualified subjects had Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2 or lower and adequate bone marrow, renal, and hepatic functions, defined as an absolute neutrophil count ≥1,500 mm3, platelet count ≥100,000 mm3, serum creatinine ≤1.7×upper limit of normal (ULN), total bilirubin ≤1.5×ULN, and serum aspartate aminotransferase and alanine aminotransferase ≤2.5×ULN. The current study was approved by the Institutional Review Board of Chonnam National Univeristy Hwasun Hospital.
2. Treatment
1) Chemotherapy
Chemotherapy was administered concurrently with radiotherapy starting on day 1. Platinum-based chemotherapy combined with 5-fluorouracil (5-FU, n=48) or docetaxel (n=15) was administered. The 5-FU combined regimen, cisplatin 75 mg/m2, was administered as a 4-hour infusion on day 1, and 5-FU 1,000 mg/m2 was administered as a continuous infusion on days 1-4 of each cycle, with standard premedication. With the docetaxel combined regimen, docetaxel 20 mg/m2 was administered as a 1-hour infusion and cisplatin 25 mg/m2 was administered as a 3-hour intravenous infusion on days 1, 15, and 18, with standard premedication. Both regimens were repeated for two cycles during radiotherapy. Patients with a complete, partial response (PR) or stable disease (SD) after dCRT received two to six additional cycles of platinum-based chemotherapy as maintenance treatment according to their condition.
2) Radiotherapy
Radiotherapy was performed using a 3-D conformal technique with LINAC 6- or 10-MV X-rays. Gross tumor volume included the primary tumor and enlarged mediastinal lymph nodes or M1a lymph nodal lesions, such as the celiac area in cases of lower esophageal cancer or the cervical area in upper esophageal cancers. Clinical target volumes were determined as the primary gross tumor with superoinferior 3-cm and lateral 2-cm margins and gross lymph nodes with a radial 2-cm margin. Radiotherapy fields were set using a two- to four-field technique, limiting the dose to the spinal cord, heart, and lung. Total radiotherapy doses ranged from 50.4 Gy in 28 fractions to 64.8 Gy in 36 fractions, depending on tumor bulk, using a 1.8-Gy daily fraction and five fractions per week.
3) Treatment response assessment
The clinical tumor response was assessed 6-8 weeks after dCRT according to the Response Evaluation Criteria in Solid Tumors (RECIST ver. 1.0). A physical examination, CT scan of the chest and upper abdomen, and esophagoscopy were performed for evaluation of tumor responses. A complete response (CR) was defined by pathological CR on esophagoscopy and no remnant disease on a CT scan. A CR was confirmed four weeks later by PET-CT. Locoregional recurrence was defined as recurrence in the esophagus or lymph node within the radiation field. Distant lymph node recurrence was defined as recurrence in a lymph node out of the radiation field. Dysphagia [7] was scored at the initial diagnosis and 6-8 weeks after chemoradiotherapy, as follows: 1, asymptomatic; 2, could eat a solid diet with some dysphagia; 3, could drink a liquid diet; and 4, complete dysphagia.
3. Statistical analyses
Overall survival (OS) was calculated from the start date of dCRT to the date of death or the date of last follow-up in survivors. Progression-free survival (PFS) was calculated from the start date of dCRT to the date of progression or death from any cause. Of the patients who had aggravated dysphagia during dCRT, dysphagia-free survival (DFS) was calculated from the date of diagnosis to the date when dysphagia symptoms of more than grade 3 occurred. OS and PFS were calculated using the Kaplan-Meier method. Factors subjected to univariate analysis were age, smoking status, TNM staging according to the 6th AJCC TNM classification of malignant tumors, histological grade, dysphagia grade, ECOG PS, body weight loss at initial diagnosis, PFS, DFS, receiving second-line chemotherapy, and laboratory findings, including hemoglobin, albumin, and C-reactive protein (CRP). In univariate analysis, a prognostic model was established by searching all variables that significantly influenced OS at p<0.05. Multivariate analysis for prognostic factors was then performed using Cox regression. All statistical tests were two-sided, and p<0.05 were taken to indicate statistical significance. Analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL).
Results
1. Patients and tumor characteristic
Among 239 esophageal cancer patients treated at Chonnam National University Hwasun Hospital from January 2004 to December 2010, 63 patients met these criteria and were included in this analysis (Fig. 1). Baseline characteristics of the patients and tumors are shown in Table 1. The median age of patients was 67 years (range, 51 to 81 years). There were 62 males and one female. Forty patients (63.5%) were ECOG PS 0, and 19 patients (30.2%) were ECOG PS 1. Underlying comorbidities were hyperftension in nine patients, diabetes mellitus in five patients, previous tuberculosis in three patients, and liver cirrhosis in four patients. Twenty-six patients (38.1%) were current or ex-smokers of more than 20 pack-years, and 38 patients (60.3%) were current alcohol drinkers.
2. Treatment response and patterns of failure
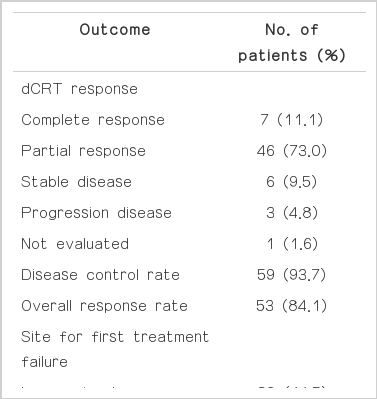
Forty-eight patients (76.2%) received chemotherapy with 5-FU and cisplatin in combination, and 15 patients (23.8%) received docetaxel plus cisplatin chemotherapy. The median dose of radiation was 54.0±0.48 Gy (range, 50.4 to 66 Gy). Seven patients (11.1%) achieved CR, 46 patients (73.0%) achieved PR, and six patients (9.5%) achieved SD. No significant difference in response rate was observed between platinum based 5-FU and docetaxel. Fifty-two patients (82.5%) complained of dysphagia of more than grade 1 at the time of diagnosis, and 20 patients (31.7%) required endoscopic or surgical intervention for relief from dysphagia during dCRT or follow-up. Relief from dysphagia after dCRT was achieved in 48 patients (68.3%). Fifty-one patients (81.0%) had progressive disease after dCRT. The most common site of recurrence was the locoregional area (n=28), followed by distant lymph node metastasis (n=12). Thirty-three patients had distant organ metastasis (Table 2).
3. Survival outcomes
The median follow-up duration was 18.4 months (range, 10 to 103.6 months). At the time of analysis, 41 patients (65.1%) had died, and three (4.8%) were lost to follow-up. The most common cause of death was disease progression, in 36 patients (85.7%). Treatment-related death due to aspiration occurred in five patients (14.3%); three of these patients had trachea-esophageal fistulas. The median OS time was 23.0 months (95% confidence interval [CI], 17.3 to 28.7), and the 2-year survival rate was 45.4±6.7%. The median PFS was 10.9 months (95% CI, 8.5 to 13.3), and the 2-year PFS rate was 20.8±5.4% (Fig. 2).
4. Clinical prognostic factors for survival
A summary of the results of univariate analyses for survival is shown in Table 3. The following factors were identified as having prognostic significance: smoking (p=0.031), dysphagia grade (p=0.001), ECOG PS (p=0.002), body weight loss at initial diagnosis (p=0.026), PFS (p=0.001), DFS (p=0.001), and CRP (p=0.004). Based on these results, a multivariate analysis was performed. Independent poor prognostic factors for OS were smoking, dysphagia (grade 3-4), PFS (less than five months), and DFS (less than 10 months). Regarding PFS, weight loss at initial diagnosis and CRP showed statistical significance (Table 4).
5. Role of maintenance chemotherapy
Among responders (CR+PR+SD) after dCRT, we evaluated the efficacy of maintenance chemotherapy. We divided the responders into two groups: those who received maintenance chemotherapy (n=43) and those who did not receive maintenance chemotherapy (n=16). The median OS times were 25.5 and 12.3 months (p=0.114), and the median PFS times were 13.3 and 7.4 months (p=0.138), respectively. For risk stratification to evaluate the benefit of maintenance chemotherapy, we divided patients who received maintenance chemotherapy into two risk groups based on the following prognostic factors showing a significant association with OS: 1) smoking>20 pack-years, 2) dysphagia score >2, 3) PFS≤5 months, 4) DFS≤10 months. Patients with none or one prognostic factor were categorized as the "good risk" group (n=30), and those with more than two prognostic factors were categorized as the "poor risk" group (n=13). The median OS times for the good and poor risk groups were 30.4 and 14.0 months (p=0.001), respectively. In comparison with patients who did not receive maintenance chemotherapy, the good risk group showed better OS (30.4 months vs. 12.0 months, p=0.002), where as the poor risk group did not show a benefit of maintenance chemotherapy compared with patients who did not receive maintenance chemotherapy (14.0 months vs. 12.0 months, p=0.828) (Fig. 3).
6. Role of dCRT in elderly patients
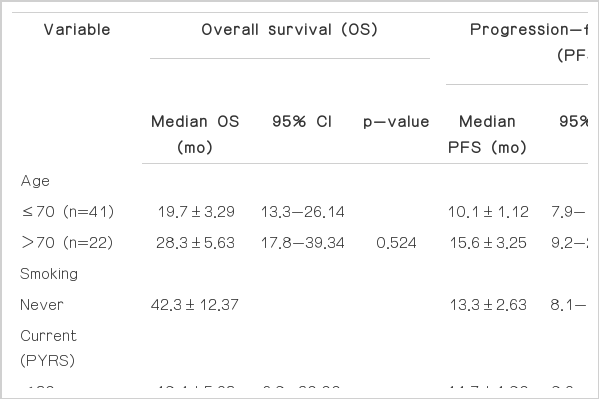
Twenty three patients were older than 70 years. Among them, 16 patients (69.6%) were ECOG PS 0, and their median radiation dose was 5.4 Gy. Fifteen patients (65.2%) showed improved dysphagia symptoms during and after treatment. The median OS was 28.3 months (95% CI, 16.9 to 39.7), and the 2-year survival rate was 51.5±11.1%. The median PFS was 14.5 months (95% CI, 7.7 to 21.3).
Discussion
Treatment for locally advanced esophageal cancer is still challenging and requires a multimodal approach. Using radiation therapy alone, the 5-year survival rate was only 0-10% [8]. In the RTOG 85-01 randomized trial comparing radiation therapy alone (64 Gy) with definitive radiotherapy combined with concurrent chemotherapy, the combined therapy arm showed a 5-year survival benefit (0% vs. 27%) [9]. Based on those studies, dCRT with 5-FU and cisplatin has become standard therapy for patients with inoperable esophageal cancer, with a 16-43% pathological CR rate [10], median OS of 17-26 months [11], and manageable toxicity.
Although the importance of dCRT is well known, analysis of outcomes for esophageal cancer according to stages and cell types has been difficult because most reported studies have included cases with various stages or histology. A Kaplan-Meier plot showed overlapping curves for stages III and IV based on the AJCC 6th TNM classification, because not all stage IV disease involved distant lymph node metastasis. According to the AJCC 7th TNM classification, only patients with distant metastasis should be categorized as having stage IV disease. Thus, our patients with stage IVa disease were reclassified as having stage IIB, IIIA, IIIB, or IIIC disease according to the 7th edition criteria. We included stage III and IVa esophageal cancer patients according to the AJCC 6th TNM classification in the current analysis because the tumor burden could be covered by radiation.
Up to present, in Korea, squamous histology has been more common than adenocarcinoma and a recent study reported a better response after chemoradiotherapy in squamous cell carcinoma than in adenocarcinoma [5]. In our study, the disease control rate was 93.7%, and the overall response rate was 84.1%. Although the pathological CR rate (11%) was relatively low compared with previously reported rates, this might have been due to limitations of the confirmation method without surgical resection or inadequate time for evaluation after chemoradiation. Nevertheless, the median OS (23.0 months; 95% CI, 17.3 to 28.7) and 2-year survival rate (45.4±6.7%) were comparable to those of the surgery-only group in the CROSS study [5].
Considering the median patient age in our study (67 years), these results are promising for management of inoperable elderly patients. Studies evaluating the efficacy of dCRT in elderly patients have reported median survival rates of more than 30% at two years and treatment-related death rates of 10-18% [12]. In this study, the analysis of the 23 patients older than 70 years showed a median OS of 28.3 months (95% CI, 16.9 to 39.7) and a 2-year survival rate of 50%.
Even though dCRT is the treatment of choice for inoperable locally advanced esophageal cancer, treatment failure occurs in approximately 37-85% of patients, the median PFS times are 10.8-26.0 months, and the 2-year survival rates are only 22-50% [13-17]. In the RTOG 85-01 trial, the locoregional failure rate was 53%, and the rate of distant metastasis was 15% [4]. In a retrospective study, Di Fiore et al. [18] found that 88 of 145 patients showed evidence of relapse, and 85% of these patients had in-field relapses. Thus, National Comprehensive Cancer Network guidelines recommend that, if possible, salvage esophagectomy should be performed after dCRT. However, postoperative complications after esophagectomy are common, with a rate of 40-80% [19,20].
Many studies have attempted to enhance the efficacy of chemoradiotherapy in order to overcome local failure after dCRT. The RTOG 94-05 study used a radiation dose higher than the standard dose (64.8 Gy vs. 50.4 Gy) with concurrent chemotherapy, however, no significant difference in the locoregional failure rate was observed (56% vs. 52%), and there were increased grade 3-4 toxicities [18]. In other approaches, various chemotherapeutic agents such as docetaxel [21], paclitaxel [5], and cetuximab [22,23] have been evaluated in clinical trials, and their efficacies compared with those of 5-FU will be determined in the near future.
Alternatively, maintenance chemotherapy may be considered after dCRT in order to reduce the locoregional or distant failure, and we sought to assess its benefit in patients who showed complete or PRs or SD. Of 59 patients, 43 who received 2-6 additional cycles of chemotherapy as maintenance chemotherapy tended to have prolonged PFS (13.3 months vs. 7.4 months, p=0.138) and OS (25.5 months vs. 12.3 months, p=0.114) compared with the no maintenance group. The lack of statistical significance may be attributable to the low number of patients in the no maintenance group (n=16).
Recently, researchers have made efforts to identify prognostic factors for patients with esophageal cancer [24,25]. ECOG PS, initial weight loss, lymph node stage, serum CRP level, cigarette smoking, and dysphagia grade have been reported as prognostic factors for esophageal cancer. According to multivariate analysis in this retrospective study, smoking, dysphagia grade, loss of body weight, CRP, PFS, and DFS were known as independent prognostic factors for OS. In fact, PFS and DFS are not prognostic factors at presentation. However, evaluation of these variables on OS, which could be associated with results from maintenance chemotherapy, would be meaningful. Using the prognostic factors identified in the multivariate analysis of OS, we divided the maintenance chemotherapy group into "good risk" patients and "poor risk" patients. The good risk group (<2 factors, n=30) showed significant improvement in OS with maintenance chemotherapy compared with the no maintenance group (30.4 months vs. 12.0 months, p=0.002). On the other hand, no benefit was observed in the poor risk group (≥2 prognostic factors, n=13) (14.0 months vs. 12.0 months, p=0.828). Although the tolerability of maintenance chemotherapy would be the main problem [25], the RTOG 85-01 trial demonstrated the possible benefit of maintenance chemotherapy in view of locoregional failure, adjuvant chemotherapy after dCRT could be beneficial for locally advanced esophageal cancer in patients with good PS. To the best of our knowledge, no previous report has evaluated the efficacy of maintenance chemotherapy. Our results suggest that maintenance chemotherapy after dCRT may be beneficial in improving survival in "good risk" subjects with locally advanced esophageal cancer.
Conclusion
Despite its retrospective nature and small number of patients, the current study demonstrated that dCRT had an efficacy comparable to that of surgery in inoperable locally advanced esophageal cancer, including elderly patients. Maintenance chemotherapy may be helpful in prolonging survival, particularly in "good risk" patients, and conduct of further studies is warranted in order to define patients who are likely to benefit from maintenance chemotherapy. This approach may be important for improving outcomes for patients with inoperable esophageal cancer.
Notes
Conflict of interest relevant to this article was not reported.