The Bone Morphogenesis Protein-2 (BMP-2) is Associated with Progression to Metastatic Disease in Gastric Cancer
Article information
Abstract
Purpose
Bone Morphogenetic Proteins (BMPs) are members of the TGF-β superfamily and it has been demonstrated that BMPs enhance migration, invasion and metastasis. The purpose of this study was to identify the association between the serum BMP-2 level and the progression status of gastric cancer.
Materials and Methods
Fifty-five patients with metastatic gastric cancer (metastatic disease group), six patients with early gastric cancer without lymph node metastasis (the EGC group), and ten healthy control subjects were enrolled in this study. The serum BMP-2 level was quantified by use of a commercially available ELISA kit. In EGC group patients and patients with metastatic disease, whole blood was obtained before endoscopic mucosal resection and before the commencement of a scheduled cycle of systemic chemotherapy, respectively.
Results
No significant difference in the mean serum BMP-2 levels was observed between the control subjects and the EGC group patients (87.95 pg/ml for the control subjects and 84.50 pg/ml for the EGC group, p=1.0). However, the metastatic disease group patients had a significantly higher level of serum BMP (179.61 pg/ml) than the control subjects and EGC group patients (87.95 pg/ml for the control subjects and 84.50 pg/ml for the EGC group, p<0.0001). Moreover, the mean serum BMP-2 level from patients with a bone metastasis was significantly higher than the mean serum BMP-2 level from patients without a bone metastasis (204.73 pg/ml versus 173.33 pg/ml, p=0.021).
Conclusions
BMP-2 seems to have a role in progression to metastatic disease in gastric cancer, especially in the late stage of tumorigenesis, including invasion and metastasis. BMP-2 may facilitate bone metastasis in gastric cancer. To confirm these findings, further studies are required with tissue specimens and the use of a cancer cell line.
INTRODUCTION
Bone Morphogenetic Proteins (BMPs) are members of the TGF-β superfamily, and more than 20 different BMP isoforms have been identified in mammals and Drosophila. BMPs were originally described as osteoinductive cytokines that enhance bone and cartilage formation when injected into mice (1). Many studies have demonstrated that BMPs also normally function in the regulation of embryonic development and cellular homeostasis as well, including the regulation of proliferation, differentiation, apoptosis and remodeling of the extracellular matrix (2). BMPs mediate their biological effects by binding to heteromeric type I/II receptor complexes that contain serine/threonine kinase domains. Upon BMP binding, the receptor complex mediates intracellular signaling via phosphorylation of Smad 1/5/8. Subsequently, the phosphorylated Smads associate with Smad4 and translocate to the nucleus to activate the transcription of downstream targets (3).
In addition to the physiological roles of BMPs, dysregulation of BMPs has been documented in several types of cancers. BMPs contribute to the numerous aspects of tumorigenesis. For example, BMPs increase the motility and invasiveness of prostate (4) and colon(5) cancer cells, stimulate the proliferation of pancreatic (6), prostate and bladder cancer cells (7) and inhibit the apoptosis of colon cancer cells (5). Among the many isoforms of BMPs, BMP-2 and BMP-4 have been especially shown to be associated with invasion and metastasis based on studies with the use of cell lines and animals. BMP-2/4 were demonstrated to enhance migration and invasion of a highly aggressive prostate cancer cell line, PC-3, both in vitro and in vivo (8). BMP4 has been shown to promote invasion and migration of melanoma cells (9), and BMP-2 has been shown to enhance migration and invasion of non-small cell lung cancer and metastasis in nude mice (10). The mRNA levels of BMP-2 and BMP receptors (BMPRs) were reported to be higher in metastatic human breast cancer cells than in cancer cells with a smaller metastatic potential (11). Furthermore, BMP-2 expression was shown to be associated with bone metastasis, including osteoblastic and osteolytic metastasis of prostate cancer (8), and increased levels of BMP-6 and BMP-7 expression have been shown to correlate with bone metastasis (12).
For gastric cancer, however, there have been no reports on the association of BMP-2/4 with invasion and metastasis. Recently, sonic hedgehog (Shh), another key molecule in the developmental process, has been reported to enhance the invasion of gastric cancer cells (13). As Shh and BMP share a similar action in respect to the control of embryogenesis, cell proliferation, differentiation, and apoptosis, BMP may have a role in invasion and metastasis of gastric cancer.
In the present study, we investigated whether the serum BMP-2 level from patients had any association or tendency with the progression status of gastric cancer. The sera of normal subjects and patients with early gastric cancer without lymph node metastasis (the EGC group) and metastatic gastric cancer (the metastatic group) were assayed by use of the enzymelinked immunosorbent assay (ELISA) sandwich method to determine significant differences in the BMP-2 levels among the groups. Moreover, we analyzed the difference in the serum BMP-2 levels between patients with and without bone metastasis, and the differences among the pathological types of gastric cancer.
MATERIALS AND METHODS
1) Patients
Sixty-one patients suffering from early gastric cancer (EGC) and metastatic gastric cancer who were treated at the Departments of Gastroenterology and Hemato-Oncology, Guro Hospital, Korea University Medical Center, were included in this study. There were six patients with EGC and 55 patients with metastatic disease. EGC patients were treated with endoscopic mucosal resection (EMR) or with endoscopic submucosal dissection (ESD). All EGC patients showed no presence of significant lymphadenopathy based on the findings of pretreatment imaging studies such as CT, MRI and PET-CT. Patients with metastatic disease were treated with systemic chemotherapy. The presence of a distant metastasis was determined by the use of CT, MRI or PET-CT. Regional lymphadenopathy was not considered as a distant metastasis but other lymph node involvement beyond the extent of regional lymph nodes, such as the retroperitoneal lymph nodes or lymph nodes outside of the abdomen, was considered as a distant metastasis. In EGC patients and patients with metastatic disease, whole blood was obtained before EMR or ESD and before commencement of a scheduled cycle of systemic chemotherapy, respectively. Serum samples were obtained after centrifugation (1,000×g) at 4℃ and were then cryopreserved at -80℃. Sera from ten healthy (control) subjects were obtained from volunteers and were cryopreserved at -80℃.
2) Specific BMP-2 ELISA
The level of BMP-2 was quantified using a commercially available ELISA kit (Quantikine, BMP-2 Immunoassay, R&D Systems, Minneapolis, MN USA). The detailed procedures of this 4.5 hour solid-phase ELISA were as follows. Assay diluents (100 µl) (provided by the manufacturer) were added to each well that had been pre-coated with a monoclonal antibody specific for BMP-2 2. This step was followed by the addition of 50 µl of control standard or sample per well and incubation for two hours at room temperature on a horizontal orbital microplate shaker at 500 rpm. Each well was aspirated and subsequently washed with wash buffer (provided by the manufacturer) by use of an autowasher; the preceding step was repeated three times for a total of four washes. After removing any remaining wash buffer, 200 µl of BMP-2 conjugate (provided by the manufacturer) was added to each well and plates were incubated for two hours at room temperature on a shaker. Aspiration and washes were repeated as described above. Substrate solution (200 µl) was added to each well and the plates were incubated for 30 minutes at room temperature and were protected from light. Stop Solution (50 µl) (provided by the manufacturer) was added to each well. The optical density of each well was determined using a microplate reader at 450 nm. BMP-2 concentrations of samples were determined from the optical densities in relation to standard experimental curves. No interference and no cross reactivity was expected based on the manufacturer's instructions. All of the serum samples were measured twice and the mean level of each measurement was used for analysis.
3) Statistical analysis
The statistical tests used included the Wilcoxon rank sum test, Mann-Whitney U test and Kruskal-Wallis test. A vaule of p<0.05 was considered statistically significant. All of the statistical analyses were performed with the use of SPSS 12.0 K software (SPSS, Chicago, IL).
RESULTS
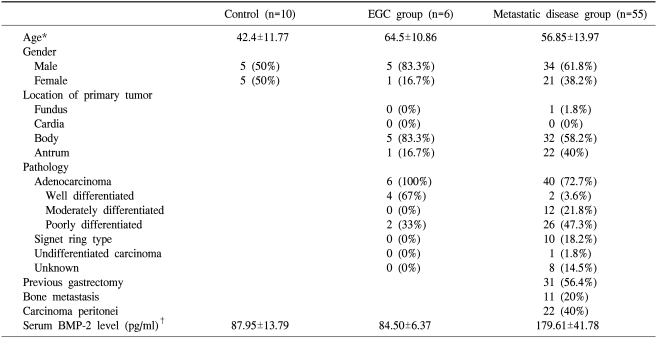
The characteristics of patients at the time of blood collection are summarized in Table 1. In 55 patients with metastatic disease, bone metastasis was reported in 11 patients (20%). The pathological type was identifiable in 53 patients (80%) as an adenocarcinoma. A poorly differentiated type of adenocarcinoma was the most frequent lesion (22 patients, 53%) and signet ring type adenocarcinoma was seen in 10 patients (19%).
The BMP-2 serum levels were measured in 55 patients with metastatic disease and five EGC patients, and the levels were compared with ten control subjects. The mean BMP-2 level in healthy (control) subjects was 87.95 pg/ml (95% CI, 78.09~97.81 pg/ml). When an increased BMP-2 level threshold was defined as the mean of control serum level+2SD (>115.52 pg/ml), no increased level was observed in all of EGC patients. The mean BMP-2 serum level for the EGC group was 84.50 pg/ml (95% CI, 77.81~91.19 pg/ml). A significantly increased mean BMP-2 serum level of 179.61 pg/ml was observed in all patients with metastatic disease (95% CI, 168.31~190.9 pg/ml, p<0.0001).
When the mean level of each group was compared, there was no significant difference between the control subjects and EGC group (87.95 pg/ml and 84.50 pg/ml, respectively, p=1.0). However, a significant difference was observed between the control subjects and the metastatic group of patients (87.95 pg/ml and 179.61 pg/ml, respectively, p<0.0001). A significant difference was also observed between the EGC group and metastatic disease group (84.05 pg/ml and 179.61 pg/ml, respectively, p<0.0001). These differences are depicted in Fig. 1.
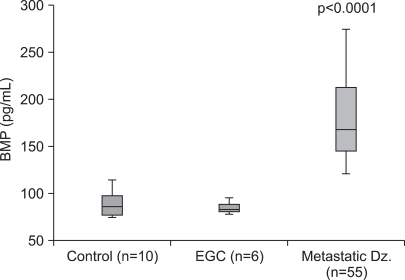
Serum BMP-2 level in EGC patients and metastatic disease group patients as compared with control subjects. The upper and lower limits of each box represent the interquantile range (25th~75th percentile) and the horizontal line within each box shows the median value. The BMP-2 level was significantly increased in all patients with metastatic disease according to the use of the Wilcoxon rank sum test (p<0.0001). The BMP-2 level in the metastatic disease group shows a statistically significant difference as compared with the control subjects and EGC patients according to the use of the Mann-Whitney U test (p<0.0001, respectively).
We divided patients with metastatic disease into two subgroups according to the presence or absence of a bone metastasis. The mean BMP-2 level of patients with a bone metastasis was 204.73 pg/ml (95% CI, 178.46~231.00 pg/ml) and the mean BMP-2 level of patients without a bone metastasis was 173.33 pg/ml (95% CI, 161.04~185.62 pg/ml); a significant difference was demonstrated between the two subgroups (p=0.021). The mean serum BMP-2 level from patients with a bone metastasis was significantly higher than the mean serum BMP-2 level from patients without a bone metastasis (Fig. 2).
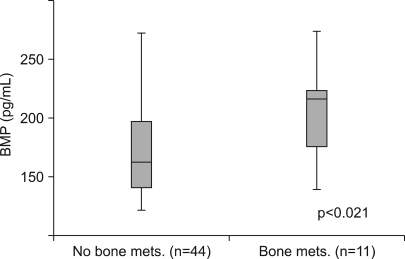
Comparison of the serum BMP-2 level in patients without a bone metastasis and patients with a bone metastasis. The upper and lower limits of each box represent the interquantile range (25th~75th percentile), and the horizontal line within each box shows the median value. The mean serum BMP-2 level from patients with a bone metastasis was significantly higher than the mean serum BMP-2 level from patients without a bone metastasis according to the use of the Mann-Whitney U test.
BMP-2 serum levels were analyzed according to the tumor histology in 53 identifiable patients. All patients presented with an adenocarcinoma. As only one case presented with an undifferentiated adenocarcinoma, it was excluded from the analysis. The mean BMP-2 serum levels of each subgroup were as follows: for a well-differentiated adenocarcinoma, 156.25 pg/ml (95% CI, 70.48~242.02 pg/ml); for a moderately differentiated adenocarcinoma, 185.33 pg/ml (95% CI, 159.38~211.29 pg/ml); for a poorly-differentiated adenocarcinomas, 180.21 pg/ml (95% CI, 162.42~198.00 pg/ml); for a signet ring cell, 171.90 pg/ml (95% CI, 143.25~171.90 pg/ml). There were no significant differences between the groups and no significant tendency according to the grade of differentiation (Fig. 3).
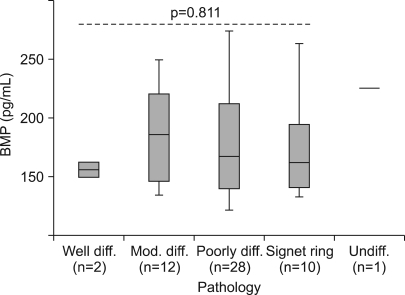
A comparison of the serum BMP-2 level according to the tumor histology. The upper and lower limits of each box represent the interquantile range (25th~75th percentile), and the horizontal line within each box shows the median value. The mean level of serum BMP-2 among each group showed neither a difference nor tendency with differentiation according to the use of the Kruskal-Wallis test.
DISCUSSION
BMPs are ligands secreted from tumor cells, and affect cell proliferation and differentiation via an autocrine or paracrine route. The role of BMPs in the development of cancer from a normal cell is diverse, and presently remains a subject of debate. Some studies have shown the oncogenic potential of BMPs; however, several other studies have shown a tumor suppressor effect. For example, BMP2 inhibits colonic epithelial cell growth in vitro by promoting apoptosis and differentiation (14). BMP-4 has been reported to reduce tumorinitiating precursors of human glioblastomas (15), and BMP-2 and BMP-6 have been shown to induce apoptosis in medulloblastoma (16), multiple myeloma (17) and renal cell carcinoma cells (18). In gastric cancer, BMP-2 has been reported to be associated with dose-dependent inhibition of the growth of human gastric cancer cells (19). However, BMP-2 has been reported to be highly expressed in approximately 98% of human lung carcinomas, whereas little expression has been seen in normal lung tissues (20). Recently, BMP-4 overexpression was shown to occur in advanced stages of colorectal cancer as well as in highly invasive colonic mucosa, but expression was absent in the normal colonic mucosa (5). BMP-2 was reported to promote the proliferation of breast cancer cells in vivo (21). Although the exact role of BMPs in early tumorigenesis is unclear, several studies have consistently shown that BMP-2 and BMP-4 enhance the migration and invasion in established cancer cell lines. This effect has been well shown in prostate cancer (8), breast cancer (22), malignant melanoma (9) and non-small cell lung cancer (10).
The present study showed no difference in BMP-2 serum levels between the control subjects and EGC patients whereas a significantly higher serum level of BMP-2 in patients with metastatic disease was found. As it is quite reasonable to consider that metastatic disease has more migrating activity and invasiveness than early-localized disease, this finding is compatible with previous studies performed with cell lines of different types of cancer cells that have shown that BMP-2 enhances the migration and invasion. Therefore, in gastric cancer, BMP-2 may play a role in the progression of earlylocalized disease to advanced and metastatic disease.
In order for epithelial cancer to become a more aggressive phenotype, it is necessary for cancer cells to acquire migratory and invasive capabilities, the so-called epithelial-mesenchymal trasition (EMT). The EMT is a complex process that leads to loss of epithelial morphology and gain of an invasive fibroblast-like mesenchymal phenotype. Originally, the EMT was reported as a crucial process in the organogenesis of an embryo. In various types of cancer cells including gastric cancer, the EMT has been identified to lead to the degradation of the basement membranes and the extracellular matrix (ECM) at the primary tumor site and to the establishment of metastatic colonies at distant organs. Therefore, the EMT is one of the earliest steps for cancer to invade around the surrounding tissue and to metastasize at distant organs. Furthermore, it is reasonable to consider that the same molecule that led to induce the EMT during embryogenesis also leads to the EMT in cancer, and that BMPs may have a role in the EMT during tumorigenesis. In cardiac cushion development, BMP-2 has been shown previously to be associated with the EMT (23). Moreover, a recent study reported that BMP-4 induced the EMT in human ovarian cancer cells (24). Therefore, a high BMP-2 serum level in patients with metastatic disease as compared to patients with EGC and as compared to healthy subjects may partially be explained by the possibility of the BMP-2-mediated EMT of gastric cancer cells. Further studies to examine the role of BMP-2 and the EMT are needed.
We analyzed the mean levels of serum BMP-2 according to the presence or absence of a bone metastasis. The mean levels of serum BMP-2 in patients with a bone metastasis were significantly higher than in mean levels of serum BMP-2 in patients without a bone metastasis. To the best of our knowledge, there has been no study about the role of BMP-2 in bone metastasis of gastric cancer. Therefore, we suggest a possible mechanism for the present findings. BMPs, which are secreted from tumor cells, exert their effect via autocrine or paracrine mechanisms. With increased secretion of BMPs there is an increase of BMP receptor expression. As endogenous BMPs are constitutively expressed in the normal bone and are stored in large concentrations in the ECM, circulating tumor cells with highly expressed BMPR are guided to the bone. A chemoattractant property of BMPs in BMPR-expressing cell types other than cancer cells has been suggested by several studies (25). Further studies are required to confirm these possible mechanisms of BMPs.
In the present study, we failed to demonstrate a correlation between tumor histology and the serum level of BMP-2. However, samples were unevenly distributed (well differentiated adenocarcinoma, n=2; moderately differentiated adenocarcinoma, n=12; poorly differentiated adenocarcinoma, n=28; signet ring, n=10) and the total sample size was insufficient. Consequently, we could not conclude if serum BMP-2 levels were different or had any particular tendency according to the pathological subtype of gastric cancer. In order to demonstrate whether a correlation exists between the tumor histology and BMP-2, a further study will be required.
The findings of this study suggest that BMP-2 is associated with progression to metastatic disease of gastric cancer, especially in late tumorigenesis involving invasion and metastasis. These findings are in accordance with previous studies that have shown that BMP-2 enhances migration and invasion of many types of cancer cells. Moreover, there is a possibility that BMP-2 facilitates bone metastasis. However, because of the relatively limited number of patients with EGC (n=6), a further study with a large number will be required. Moreover, to exclude the effect of systemic chemotherapy on the serum BMP-2 level, a future study should be performed with pretreatment samples in patients with metastatic disease. To confirm the correlation between the elevated serum BMP-2 level of patients with metastatic disease and tissue expression, the expression of BMP-2 in biopsy specimens and in cancer cell lines should be examined.
CONCLUSION
BMP-2 seems to have a role in progression to metastatic disease in gastric cancer, especially in late tumorigenesis involving invasion and metastasis, and BMP-2 may facilitate bone metastasis in gastric cancer. To confirm these findings, further studies are required with tissue specimens and cancer cell lines.