High-Dose-Rate Brachytherapy for the Treatment of Vaginal Intraepithelial Neoplasia
Article information
Abstract
Purpose
Vaginal intraepithelial neoplasia (VAIN), a rare premalignant condition, is difficult to eradicate. We assess the effectiveness of high-dose rate intracavitary brachytherapy (HDR-ICR) in patients with VAIN or carcinoma in situ (CIS) of the vagina after hysterectomy.
Materials and Methods
We reviewed 34 patients treated for posthysterectomy VAIN or CIS of the vagina by brachytherapy as the sole treatment. All patients underwent a coloposcopic-directed punch biopsy or had abnormal cytology, at least 3 consecutive times. All patients were treated with a vaginal cylinder applicator. The total radiation dose was mainly 40 Gy in 8 fractions during the periods of 4 weeks at a prescription point of the median 0.2 cm (range, 0 to 0.5 cm) depth from the surface of the vaginal mucosa.
Results
Acute toxicity was minimal. Seven patients had grade 1/2 acute urinary and rectal complications. There were 15 cases of late toxicity, predominantly vaginal mucosal reaction in 12 patients. Of these patients, two patients suffered from grade 3 vaginal stricture and dyspareunia continuously. After a median follow-up time of 48 months (range, 4 to 122 months), there were 2 recurrences and 2 persistent diseases, in which a second-line therapy was needed. The success rate was 88.2%. The average prescription point in failure patients was 1.1 mm from the surface of the vagina compared to an average of 2.6 mm in non-recurrent patients (p=0.097).
Conclusion
HDR-ICR is an effective treatment method in VAIN patients. In spite of high cure rates, we should consider issues regarding vaginal toxicity and radiation techniques to reduce the occurrence of failure and toxicity.
Introduction
Vaginal intraepithelial neoplasia (VAIN) or carcinoma in situ (CIS) of the vagina is an uncommon premalignant condition of the vaginal epithelium that occurs most commonly in patients who had undergone hysterectomy for cervical neoplasia [1,2]. This premalignant condition was first described by Hummer in 1933 [2]. VAIN is a clinically unique and rare condition, but has become much more recognized with improved colposcopic training and widespread cytologic screening [3].
The most important risk factor to consider for VAIN is a history of cervical neoplasia, which is also caused by an human papillomavirus infection [4]. The incidence of VAIN has been reported as 0.6 per 100,000 women, but can increase to 0.91% in women with a history of hysterectomy due to cervical intraepithelial neoplasia grade 3 [5].
Although the best treatment option for VAIN is uncertain, there are various choices, including partial or total colpectomy, laser ablation, cavitational ultrasonic surgical application, vaginectomy, topical application of 5-fluorouracil, and brachytherapy [3]. Several previous studies have described the effectiveness of brachytherapy in VAIN [6-11]. However, the results are difficult to interpret due to small sample sizes and various radiation techniques. Although it seems clear that brachytherapy can achieve high cure rates among several treatment options, long-term side effects should be considered since VAIN patients are thought to have a long life expectancy.
Here, we report a single institution study of the application of high-dose-rate intracavitary brachytherapy (HDR-ICR) for the treatment of VAIN.
Materials and Methods
We retrospectively reviewed the medical charts to evaluate the clinical usefulness of HDR-ICR for the treatment of VAIN. Between December 1998 and January 2011, 34 patients were treated for post-hysterectomy VAIN at Seoul St. Mary's Hospital. All patients had previous history of total hysterectomy, developed VAIN or CIS of the vagina, and were treated with brachytherapy as the sole treatment.
The diagnosis was based on repeated vaginal smears or on vaginal biopsies performed during a colposcopic procedure. All included patients who had not undergone a colposcopic-directed punch biopsy had no definite visible lesion that could be biopsied and had abnormal cytology results at least 3 consecutive times.
All patients were treated with HDR-ICR delivered by a remotely controlled afterloading system (micro Selectron) with a train of iridium-192 source. In all patients, a cylinder applicator was used to irradiate most parts of the whole vagina. The exact length of the vagina included in the treatment field was decided by the site and the number of lesions. The median prescription point was 0.2 cm (range, 0 to 0.5 cm) in depth to the surface of the vaginal mucosa. The radiation dose was mainly 40 Gy in 8 fractions during the periods of 4 weeks. Five cases were treated with other dose schedules (Table 1).
The radiation plan was accepted when the dose to the rectum and bladder was below 80% of the prescription dose. The bladder point was marked at the center of the radio-opaque contrast filled Foley catheter balloon on the frontal radiograph and at the posterior surface of the balloon on the lateral radiograph following the Internaltional Committee on Radiation Units and Measurements (ICRU) report No. 38. The rectal reference was defined at the closest point from the applicator on the anterior wall of the rectum, defined by a barium contrast radiograph (Fig. 1). The plan was modified to acquire adequate coverage of the vaginal lesions at risk (especially the upper vagina).

Antero-posterior and lateral simulation films of cylinder insertion. The rectal point and bladder point is marked on the films.
All patients underwent radiation therapy on an outpatient basis, by the benefit of good performance status. Subsequent follow-up included clinical, cytological, and colposcopic assessments at intervals of 3 to 6 months. Toxicity grade was recorded according to the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0.
Results
1. Patient characteristics
The clinical and treatment features are summarized in Table 1. The median age of the patients was 53 years (range, 33 to 71 years). Of the 34 patients, 22 patients had undergone hysterectomy due to cervical dysplasia (13 patients) or cervical cancer (9 patients). Rest of the patients received hysterectomy due to benign disease, mostly uterine myoma. The duration between hysterectomy and diagnosis of VAIN was 12.1 months for cervical dysplasia or cervical cancer patients compared to 137.1 months for benign disease patients.
Three patients had a history of previous external radiation therapy for cervical cancer treatment. They received an external radiation therapy as an adjuvant purpose, and one of these patients received concurrent chemotherapy. The radiation dose was 50.4 Gy in 28 fractions for two patients and 49.3 Gy in 29 fractions for one patient. The time interval between external radiation and brachytherapy was 17.5, 75.2, and 139.5 months in each of the three patients.
2. Complications of brachytherapy
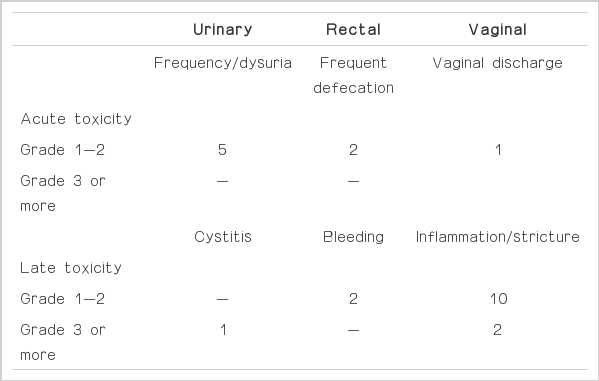
All patients tolerated well, and treatment interruption was not necessary for any patient. Complications are summarized in Table 2. Acute toxicity was minimal. Five patients had grade 1 or 2 urinary complication, and two patients complained of frequent defecation. One patient had grade 2 vaginal discharge. All toxicity disappeared a third month follow-up.
There were 15 cases of late toxicity, predominantly vaginal mucosa reaction in 12 patients. Of these patients, 10 patients received hormone replacement and the symptom subsided. Two patients continuously complained of grade 3 vaginal stricture and dyspareunia, and needed consistent special gynecologic care. Rectal complication occurred in 2 patients, 6-9 months after treatment that were both grade 2 rectal bleeding. The symptom was mild, did not require any further treatment and subsided within 3 months. One patient had grade 3 cystitis and was hospitalized three times.
3. Treatment outcome
After a median follow-up time of 48 months (range, 4 to 122 months), all patients were alive at the last follow up. However, there were 2 recurrences and 2 persistent diseases. The success rate was 88.2%. Both recurred patients relapsed in low grade VAIN, 2 years and 7 months after the brachytherapy respectively. Two persistent disease patients had continuous abnormal cytology. One patient was successfully treated with second-line brachytherapy. The other patient did not receive any further treatment at the last follow-up. There were no patients who developed invasive vaginal carcinoma. During the follow-up, there were 6 cases of repeated abnormal cytology, which was confirmed as benign inflammation from a biopsy. All these patients showed some symptoms of vaginal complication.
4. Risk factor for treatment failure and toxicity
There was no definite risk factor of recurrence. The prescription point in failure patients was on average 1.1 mm from the surface of the vagina compared to an average of 2.6 mm in non-recurrent patients, but this was not statistically significant (p=0.097).
The patient who suffered from grade 3 cystitis had a history of radiation (50.4 Gy of external radiation and 30 Gy of brachytherapy) 12 years ago in the course of treatment for cervical cancer. However, we failed to define a dose-toxicity relationship in these patients with vaginal or rectal complications.
Discussion
There are many controversies regarding the management of VAIN due to the rarity of the disease. There exists a debate of whether it is appropriate to treat VAIN patients aggressively. The main risk of VAIN is the possibility of progression to invasive vaginal carcinoma. Aho et al. [12] reported a study of 23 untreated VAIN patients. The lifetime risk of malignant carcinoma was 9% (2 cases). Persistence of VAIN also occurred in 3 cases (13%). They also demonstrated that VAIN lesions associated with cervical lesions have a lower spontaneous regression rate (67% vs. 91%). In our study, grade 1 VAIN patients were also included. All of these patients had a history of cervical intraepthelial neoplasia or cervical cancer; they were closely followed up initially, but their cytologic results were constantly positive, thus they were decided to undergo active treatment.
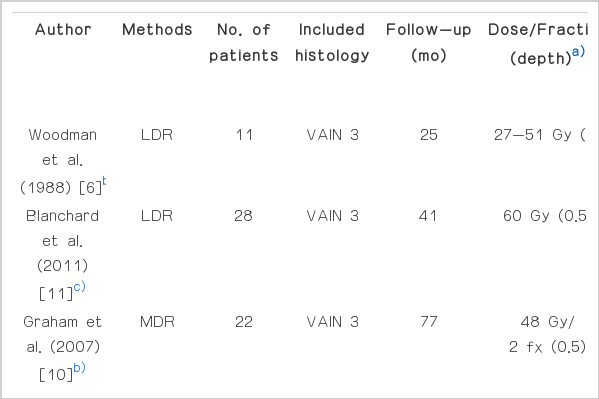
The second issue is which treatment option is the best. There yet exists a randomized control study comparing the different treatment methods. Surgical excision, laser ablation, ultrasonic surgical aspiration, vaginectomy, radiation therapy and topical 5-fluorouracil (5-FU) are the options that are widely used at present [3,4]. All treatment options have both advantages and limitations, which are well documented in some reviews. A number of factors, such as age and comorbidities of the patient, the site and number of lesions, the need of preservation of sexual function, and physician's experience, need to be considered before deciding on the treatment method [3]. Brachytherapy is also known as an effective treatment option with high cure rates (Table 3) [6-11]. Although there are various radiation techniques, the success rate is over 85%. The variability in the success rate might be explained by the differences in radiation techniques, population (only VAIN 3 or all grades VAIN), or duration of follow-up.
In spite of the high cure rate, the toxicity of brachytherapy should always be considered. It is difficult to draw a conclusion from only several studies due to various radiation doses, different toxicity reporting systems, and limitation in the retrospective nature of the studies. However, it seems that a clear acute toxicity is minimal and almost always self-limiting. In all studies, the treatment was well tolerated and no limiting toxicities were encountered. The major late toxicity is vaginal toxicity, such as stenosis and ulcer. Graham et al. [10] reported that all patients developed at least Radiation Therapy Oncology Group (RTOG) grade 1 or 2 vaginal toxicity. Five patients developed grade 3 toxicity with severe stenosis, and one patient developed grade 4 vaginal ulcer 2 years after the treatment. These patients were treated with the medium-dose-rate technique, with typically 24 Gy per fraction, over 15 to 16 hours with ovoids, and 2 fractions with a one week separation. Ogino et al. [8] also reported two cases of moderate to severe vaginal complications, whose treatment had included the entire vagina. Teruya et al. [7] reported that all patients had evidence of vaginal mucosal radiation reactions with 2 cases of grade 3. In our study, there were 12 patients who needed to receive hormone replacement therapy because of vaginal mucosal reactions. However, two patients had no improvement, and they continuously complained of vaginal stricture and dyspareunia. Compared to vaginal toxicity, gastrointestinal and urinary toxicity was usually minimal. In a few cases with moderate to severe toxicity, including our case, patients had a history of external radiation. When excluding patients who received previous external radiation therapy, one grade 2 vaginal late toxicity and one grade 3 urinary late toxicity can be excluded.
Analyzing their simulation films, they received the whole pelvis irradiation with the lower border at the bottom line of the obturator foramen level. This suggests that those previous external radiations had not included the entire vagina because of the patient's young age. In patients whose lower border was the tuberosity of the ischium, treatment options, other than brachytherapy, were selected. Another point to consider with regard to them is that we prescribed the tumor dose at the vaginal surface (0 mm depth from vaginal cylinder) rather than 2 to 5 mm in depth to the surface of the vaginal mucosa at the time of brachytherapy planning. In spite of careful patient selection and the efforts to reduce the toxicities in these patients, we should keep in mind that there can be a potential risk of complication in patients who had previous external pelvic irradiation.
Considering the toxicities of brachytherapy, this treatment option for low grade (grade 1 or 2) VAIN can be an aggressive and inconvenient approach. Although topical 5-FU, laser ablation or even observation can be a treatment option for low grade VAIN [3,4]. We consider that VAIN itself is not a localized focal disease, but an entire organ disease. Therefore, once VAIN is confirmed from a pathologic diagnosis, we need to be concerned whether prophylactic treatment of the remaining vagina should be included or not. Therefore, we did not select patients who are sexually active young women as a brachytherapy candidate. Recently, most treatment decision making was made by the gyne-oncology tumor board consisting of gynecologists, medical oncologists, pathologists, diagnostic radiologists and radiation oncologists.
Lastly, we should consider a radiation technique. It seems that both low-dose-rate (LDR) and HDR brachytherapy show a similar high successful rates in VAIN (Table 3). Despite the fact that HDR systems are widely used in cervical cancer with similar outcomes and toxicity compared to LDR [13], some authors suggest the potential inferiority of HDR in biological terms. The use of increased dose per fraction can produce more late normal tissue toxicity than increase the tumor cell kill; thereby, decreasing the therapeutic ratio [9]. Although it looks like HDR study series show a somewhat higher grade 3 toxicity (Table 3), it is difficult to draw a conclusion due to variable treatment techniques.
The second issue to consider in radiation technique is which intracavitary brachytherapy (ICR) device should be used. There are a number of ICR devices designed for treating the vagina. Although ovoid has an advantage to concentrate the dose on the upper vagina, there is a risk of underdosing the dysplastic changes that can be involved with the entire vagina. In contrast, cylinder has an advantage of treating the entire vagina uniformly. However, treating the entire vagina is correlated with high rates of late toxicity, especially the urethral necrosis [10]. Our patients were treated uniformly with a cylinder applicator, with an attempt to not include the urethra and the entire vagina, except in patients whose dysplastic changes were definite in the lower vagina. However, when using the cylinder, physicians should carefully design a plan so that the upper vagina is covered adequately. After hysterectomy, the vault is difficult to assess and abnormal epithelium can be sequestered above the suture line, which can be missing when treating patients with a cylinder applicator [9,10].
Third, there can be an argument of prescription point and dose. MacLeod et al. [9] recommended that the whole residual vagina should be treated using a total dose of 42.5 Gy in 8.5 Gy per fraction, which is prescribed at the vaginal surface on the walls and at a depth of 0.5 to 1.0 cm of the vault. Ogino et al. [8] treated patients using 25 or 30 Gy in 5 Gy per fraction, prescribed at a depth of 1.0 cm superior to the vaginal apex. Teruya et al. [7] suggested a total dose of 30 Gy with 5 Gy per fraction prescribed at 0.5 cm depth to the vaginal surface is sufficient to control the disease with no complications. We treated patients usually with 40 Gy in 5 Gy per fraction, prescribed at various depths in consideration of the rectal dose. Although there is no statistical significance, the prescription point in failure patients was shallower (mean, 0.11 cm) from the vaginal surface compared to non-recurrent patients (mean, 0.26 cm). It seems that while prescribing 40 Gy in 8 fractions, the prescription point should be at least 0.2 cm from the vaginal surface if the doses to normal structures are acceptable. Even though we could not draw a definite conclusion from these data, we should keep in mind that the depth for a prescription is one of the most important issues for balancing tumor control and complications.
Conclusion
The outcome of this study shows that HDR-ICR is an effective treatment method in VAIN patients. In spite of high cure rates, we should consider issues regarding vaginal toxicity and radiation techniques to reduce the occurrence of failure and toxicity. More basically, because no national or international guidelines exist for the management of VAIN, the choice of treatment modality should be decided carefully.
Notes
Conflict of interest relevant to this article was not reported.