A 21-day Schedule of Gemcitabine and Cisplatin Administration in the Treatment of Advanced Non-Small Cell Lung Carcinoma: a Phase II Study
Article information
Abstract
Purpose
To evaluate the efficacy and toxicity of gemcitabine and cisplatin combination chemotherapy, we conducted a phase II study of this regimen in patients with advanced non-small cell lung carcinoma (NSCLC).
Materials and Methods
From June 2001 to August 2003, 36 chemotherapy-naive patients with stage IIIB or IV NSCLC were enrolled. The median age was 59 years (range, 42 to 75 years), and performance status was 0 or 1. Eleven patients had stage IIIB disease, and 25 patients had stage IV disease. 1,000 mg/m2 of gemcitabine was administered on day 1 & 8, and 60 mg/m2 of cisplatin was administered on day 1. Each cycle was repeated every 21 days.
Results
Everyone subject who participated were assessable. A total of 160 cycles of chemotherapy were delivered, and the median number of chemotherapy courses was 3.5 (range, 2 to 9). Two patients (5.6%) achieved a complete response, and 14 patients (38.9%) achieved a partial response. The overall response rate was 44.5% (95% confidence interval [CI], 32.5 to 56.5%). The median follow-up duration was 9.3 months. The median time to disease progression was 8.6 months (95% CI 7.4 to 9.9 months), and median survival time was 12.2 months (95% CI, 10.5 to 12.9 months). Grade 3/4 neutropenia occurred in 9 patients (25.0%), neutropenic fever occurred in 3 patients (8.3%), and grade 3/4 thrombocytopenia occurred in 7 patients (19.5%). Mild forms of non-hematologic toxicities, such as nausea, vomiting or skin reactions, were observed.
Conclusion
The combination of gemcitabine and cisplatin in a 21-day schedule is an effective regimen for patients with NSCLC in its advanced stages.
INTRODUCTION
In 1995, there was a meta-analysis of data from 52 randomized clinical trials that compared chemotherapy to the best supportive care at the time for stage IV NSCLC. The results showed that chemotherapies improved the median survival time of 2 months and the 1-year survival probability of 10% (1). Many clinical studies attempted various kinds of cisplatin-based regimens to improve the survival of patients with NSCLC. In the 1990s, a number of new drugs, including paclitaxel, docetaxel, gemcitabine, irinotecan, and vinorelbine, were introduced to use with chemotherapy. These agents were proven, either as a single agent or as a doublet with cisplatin (2~6). Most of the phase II and III studies showed that combination therapy of new drugs improved response rate, survival, and quality of life with a less toxic profile compared to the old cisplatin-based regimens. Based on these findings, the combination of cisplatin with one of these new drugs was recently accepted as the standard treatment for an advanced stage of NSCLC.
Among the new drugs, a nucleotide analog, gemcitabine, showed high activity against solid tumors, such as lung, breast, colon, and pancreatic cancers (7,8). Gemcitabine has demonstrated similar or superior activity both as a single agent or as a doublet with cisplatin compared to the old cisplatin-based regimens for NSCLC (6,9~11). Its good safety profile, in vitro synergism with cisplatin, and lack of overlapping toxicities with cisplatin made the combination of gemcitabine and cisplatin popular and widely used (12). Early phase I studies demonstrated clinically significant and schedule dependent differences in the toxicity profile and antitumor activity. Side effects such as flu-like symptoms, idiosyncratic reactions with severe hypotension, or thrombocytopenia were reported daily or twice a week, which signifies a high incidence (7,13). To reduce these toxicities, schedules for weekly gemcitabine administration were adopted in late phase I studies and showed an acceptable toxicity profile, which included thrombocytopenia as the dose limiting toxicity at doses of 790 to 1,500 mg/m2 (8). In the phase II studies with gemcitabine as a single agent, gemcitabine was administered weekly at doses of 800~1,250 mg/m2, and an overall response rate of 20~26% was achieved (9~11). In most of the phase II studies with the gemcitabine and cisplatin combination, gemcitabine was usually given three times per week followed by one week of rest in a 28-day schedule, but cisplatin was given on days 1 or 2 or 15, or 1, 8, 15 according to the different schedules. In these phase II studies, grade 3/4 hematologic toxicities during the third week were reported as major causes of gemcitabine dose reduction or omission of day 15 (14~17). In retrospective analyses of these phase II studies, it was known that the major hematologic toxicities and survival results were different depending on the schedule of cisplatin administration (18,19). A high incidence of day 15 gemcitabine dose omission due to hematologic toxicities allowed for the recent phase III studies to adopt a 21-day schedule, in which gemcitabine was given on day 1 and day 8 and cisplatin on day 1 or 2 (20,21). Although the results of this 21-day schedule cannot be directly compared with the previous 28-day schedule, it showed consistent activity and toxicity profile without any significant clinical differences. In this study, we used the 21-day schedule for the administration of gemcitabine and cisplatin in order to evaluate the hematologic toxicity and efficacy in patients with NSCLC.
MATERIALS AND METHODS
1) Eligibility criteria
Patients with the histologically proven stage IIIB/IV of NSCLC were enrolled. Patients with post-operative or recurrent NSCLC were also included. Patients should not have received any previous chemotherapy or radiation therapy involving main chest lesions. Previous palliative radiation therapies to symptomatic metastatic lesions were allowed, but these lesions were not considered in the tumor response evaluation. All patients had radiological chest lesions that were measurable in two dimensions. All patients were given written informed consent.
Other inclusion criteria that determined eligibility included the following: age between 20 and 80 years old; ECOG performance status 0 or 1; adequate bone marrow reservoir; 4,000/mm3≤WBC≤12,000/mm3, ANC≥2,000/mm3, hemoglobin≥9.0 g/dL, and platelet count≥100,000/mm3.
Exclusion criteria included the following: inadequate liver function test; serum total bilirubin level>1.5 mg/dL, serum ALT and AST level being three times above the normal reference range, abnormal prothrombin time and activated partial thromboplastin time both above the normal reference range, inadequate renal function; serum creatinine level>1.5 mg/dL, and creatinine clearance<60 mL/min. Serious preexisting medical or surgical conditions, such as severe infection, uncompensated liver or heart disease, a recent history of a major operation unrelated to NSCLC, pregnancy, or lactation, were also excluded.
2) Pretreatment evaluation
Pretreatment evaluation included a complete history taking and physical examination, CBC, chemistry and electrolyte profile, urine analysis, electrocardiogram, chest X-ray, chest CT, CT and, if necessary, magnetic resonance imaging of the brain, CT and ultrasonography of the abdomen, and whole body radioisotope bone scanning. A physical examination and CT were repeated before the start of each cycle of chemotherapy. CBC and chemistry were repeated weekly.
3) Drug administration
Gemcitabine 1,000 mg/m2 was administered on day 1 & 8 for 2 weeks followed by one week of rest in a 21-day cycle. Cisplatin 60 mg/m2 was administered on the first day of every 3 weeks. Enough hydration and diuretic agents were given through cisplatin infusion. Prophylactic antiemetics including 5HT3-receptor antagonist, dexamethasone, and metoclopropamide were administered prior to the cisplatin infusion.
4) Toxicity assessment and dose modification
Toxicities were graded according to the NCI-CTC. In cases of grade IV neutropenia or thrombocytopenia being present, the next cycle was delayed until the ANC and platelet count (ANC>1,000/mm3, platelet count>50,000/mm3) was recovered. The doses of gemcitabine and cisplatin were reduced by 75% in the next cycle also. Granulocyte colony stimulating factor (G-CSF) and systemic antibiotics were used in patients with neutropenic fever. Platelet transfusion was performed only when the platelet count was below 20,000/mm3 or when thrombocytopenia related bleeding was present.
5) Response assessment
The treatment response was recorded using the criteria of the World Health Organization (WHO). Two-dimensional lesions of the tumor were assessed by chest X-ray after each cycle of chemotherapy. A regular chest CT scan was performed after completing two cycles of chemotherapy. If there were any signs of change in the chest X-ray, an additional CT scan was performed. A physician and a radiologist reviewed the chest X-ray and CT films, independently. Complete response (CR) was defined as the state in which no evidence of the tumor remained. Partial response (PR) was defined as the decrease of ≥50% of well-outlined lesions within at least 4 weeks. Stable disease (SD) was defined as a decrease of <50% or an increase of <25% in well-outlined lesions within at least 4 weeks. Progressive disease (PD) was defined as an increase of ≥25% in the cross-sectional area of one or more lesions or the development of new lesions. If the patients with CR or PR responses were able to tolerate the chemotherapy, we performed three additional cycles of chemotherapy after completing the original six cycles. For patients with SD or PD (non-responders), three additional cycles with paclitaxel and cisplatin were performed as a second line chemotherapy.
6) Statistical analysis
We used the SPSS program for statistical analysis. The differences in response rates were compared according to the prognostic factors by Fischer's exact test. Survival data was evaluated by the Kaplan-Meier survival analysis method and the log-rank test.
RESULTS
1) Patients' characteristics
From June 2001 to August 2003, the 36 consecutive chemotherapy-naive patients with NSCLC who met the selection criteria were enrolled. The 36 patients consisted of 22 men (61.1%) and 14 women (38.9%). The median age of all the patients was 59 years (range, 42 to 75 years). The cancer type distribution was as follows: twenty patients (55.6%) had squamous cell carcinoma, eleven patients (30.6%) had adenocarcinoma, and five patients (13.8%) had large cell carcinoma. Eleven patients (30.6%) had inoperable stage IIIB disease, and 25 patients (69.4%) had stage IV. All patients had an ECOG performance status of 0 or 1.
2) Response and survival results
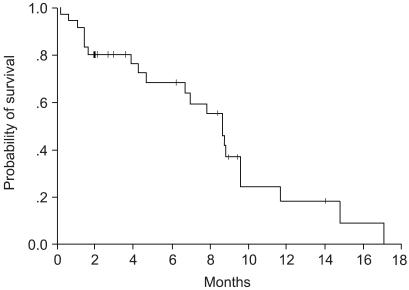
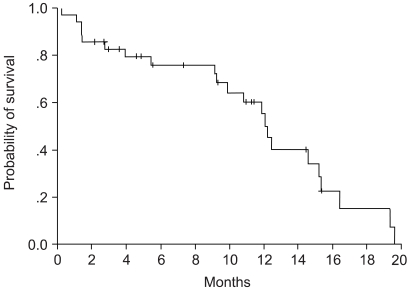
Survival and responses were evaluated according to the intention-to-treat principle in all of the enrolled patients, where the median follow-up duration was 9.3 months. Of the 36 assessable patients, two patients (5.6%) achieved a CR, and 14 patients (38.9%) achieved a PR. The overall response rate was 44.5% (95% CI, 32.5 to 56.5%). Twelve patients (33.3%) had SD, and eight patients (13.8%) had PD. Of the two patients who obtained a CR, there was a recurrence in one of them after six months, and the other patient remained free of disease evidence for nine months from the time of analysis. Response analysis using the prognostic factors, such as age, sex, pathology, stage, and performance status, showed no statistically significant correlation. Survival analysis also using the same prognostic factors again showed no statistically significant differences. There were no response and survival differences between those that achieved some sort of response and those that did not achieve a response at all. The median time to disease progression was 8.6 months (95% CI, 7.4 to 9.9 months), and the median survival time was 12.2 months (95% CI, 10.5 to 12.9 months). 1-year survival probability was 50.6%, and the Kaplan-Meier survival curves can be seen in (Fig. 1~2).
3) Dose modification
A total of 160 cycles of chemotherapy were delivered, and the median number of chemotherapy courses was 3.5 (range: 2 to 9). There were no cases of gemcitabine dose omission on day 8 that was related to the hematologic toxicities. There was only one case of dose omission on day 8 due to an acute myocardial infarction. The patient completed six cycles of chemotherapy after the gemcitabine dose reduction. Of the entire 160 cycles, 122 (76.3%) cycles were administered without dose omission or reduction. Relative dose intensity of gemcitabine was 83.5% (557.5 mg/m2/week), and for cisplatin, it was 81.5% (18.3 mg/m2/week).
4) Toxicities
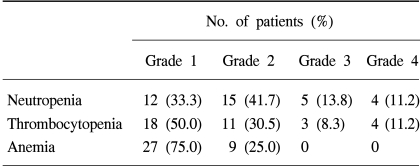
The most common hematologic toxicity was neutropenia. Grade 3/4 neutropenia occurred in 9 patients (25.0%), and cases of neutropenic fever occurred in 3 patients (8.3%). Because neutropenic fever was controlled with empirical antibiotics therapy and G-CSF administration, there was no neutropenic fever-related death. As for grade 3/4 thrombocytopenia, it occurred in 7 patients (19.5%), but there was no clinically significant thrombocytopenia-related bleeding. Hematologic toxicities are summarized in Table 1.
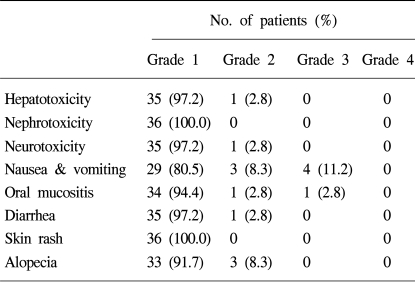
Seven patients (19.5%) experienced grade 2 or 3 nausea and vomiting. Four patients (11.2%) experienced various skin reactions, but they were mild and were determined to be of grade 1. There was neither hepatotoxicity, nephrotoxicity, nor neurotoxicity that were clinically significant. Other toxicities were rarely observed and were generally of grade 1 or 2. Non-hematologic toxicities are summarized in Table 2.
DISCUSSION
Recently, a combination of cisplatin with one of the new drugs, such as gemcitabine, has been accepted as an effective regimen for the treatment of an advanced stage NSCLC. Among these combinations, the gemcitabine and cisplatin combination illustrated a good response and good survival data compared to other combinations, such as taxanes and cisplatin or vinorelbine and cisplatin (20~22).
Gemcitabine (2'-2' difluorodeoxycytidine) is a deoxycytidine analog that is structurally related to cytosine arabinoside (ara-C) and also functions as a pyrimidine antimetabolite. Gemcitabine mimics the structure of the natural nucleoside, deoxycytidine, and is incorporated into DNA. After its incorporation into the end of the elongating DNA strand, an additional deoxynucleotide is added, and gemcitabine acts as the masked chain terminator in DNA synthesis (23,24). Cisplatin binds to DNA and produces intrastrand and interstrand DNA-DNA crosslinks or DNA-protein crosslinks. When the DNA-cisplatin adducts are excised by the DNA repair system of the tumor cell, gemcitabine is used as a substitute for the damaged DNA and eventually terminates the process of DNA synthesis. Gemcitabine also depletes the deoxyribonucleotide pool and the ribonucleotide pool that are essential for DNA repair and even facilitates the formation of DNA-cisplatin adducts. Synergistic and additive effects of these two agents were shown in vivo and in vitro. Sequential administration of either cisplatin followed by gemcitabine or gemcitabine followed by cisplatin showed synergistic anti-tumor effects, but the toxicities were markedly enhanced (14).
In order to increase synergism and decrease toxicities, various administration schedules of gemcitabine with cisplatin were attempted in many clinical studies. The most commonly adopted schedule in phase II studies was weekly administration of gemcitabine for three weeks followed by one week of rest and then followed by the monthly administration of cisplatin. Cisplatin was given on different days in the studies. Through retrospective analyses of early phase II studies, it was known that cumulative hematologic toxicities and survival results varied depending on the day that the monthly cisplatin was administered. The response rate and toxicity profile were more desirable when cisplatin was given on day 2 or 15 than when it was given on day 1 or 1, 8, and 15 (18,19). In a study by Crino et al, 1,000 mg/m2 of gemcitabine was administered weekly (on day 1, 8, 15) followed by one week of rest, and 100 mg/m2 of cisplatin was administered on day 2 of each 28-day cycle. The 52% of patients experienced WHO standard grade 3/4 of thrombocytopenia, which was the reason for the gemcitabine dose omission of day 15 in 50% of chemotherapy courses (15). In other phase II studies, there were also many cases of gemcitabine dose omission or reduction related to the hematologic toxicities (14~17). Because of this, recent phase III studies adopted the 21-day schedule and have illustrated good response rates and tolerable toxicity profiles compared to the previous 28-day schedule (20,21).
It will be more informative to specify what type of studies are indicated here. We planned this 21-day schedule in order to reduce cumulative hematologic toxicities and to maintain gemcitabine dose intensities that were initially planned by administrating the drugs in the lowest doses possible. Gemcitabine, at a dose of 1,000 mg/m2, was given for one hour after the infusion of cisplatin at a dose of 60 mg/m2 for 15 minutes. In many phase II or III studies, cisplatin was usually given at doses of 75~100 mg/m2. In our study, the overall response rate was 44.5% (95% CI, 32.5 to 56.5%), and the 1-year survival probability was 50.6%. The median time to disease progression was 8.6 months, and median survival time was 12.2 months. Although the dose of cisplatin was relatively lower than other studies, we were able to get good response and survival results. Thus, we assume that 60 mg/m2/cycle of cisplatin is a dose that allows for good chemotherapy response. In a Korean study with gemcitabine and carboplatin, Jae Wan Park et al reported similar results including an overall response rate of 44%, time to disease progression an average 6.5 months of time to disease progression, and an average 12.5 months of the survival time (25). The survival and response results of our study were relatively better than that of other studies, but they must be interpreted with caution. Because the pool of enrolled patients was not very large and because the patients of good performance status without underlying severe medical conditions were selected, there is a possibility for a patient selection bias. Therefore in future studies, more patients should be evaluated to clarify the response and survival of this regimen. In our study, grade 3/4 hematologic toxicities occurred frequently compared with recent studies that adopted the 21-day schedules. The occurrence of grade 3/4 neutropenia occurred in 9 patients (25.0%) and grade 3/4 thrombocytopenia in 7 patients (19.5 is due to the close administration of cisplatin and gemcitabine on the same day without wide enough time intervals. It was known that sequential administration of these two drugs not only enhanced the response rate but also enhanced the hematologic toxicities (15). In phase II studies with 28-day gemcitabine schedules, hematologic toxicities were more frequent when cisplatin was given on day 1 or 1, 8, 15 rather than when given on day 2 or 15 (14~17). Recently, cisplatin has been administered on day 2 for newly diagnosed NSCLC patients in order to reduce hematologic toxicities.
CONCLUSIONS
The combination of gemcitabine and cisplatin of the 21-day schedule was an effective regimen for in-patients with advanced stage NSCLC.