RASSF1A Suppresses Cell Migration through Inactivation of HDAC6 and Increase of Acetylated α-Tubulin
Article information
Abstract
Purpose
The RAS association domain family protein 1 (RASSF1) has been implicated in a tumor-suppressive function through the induction of acetylated α-tubulin and modulation of cell migration. However, the mechanisms of how RASSF1A is associated with acetylation of α-tubulin for controlling cell migration have not yet been elucidated. In this study, we found that RASSF1A regulated cell migration through the regulation of histon deacetylase 6 (HDAC6), which functions as a tubulin deacetylase.
Materials and Methods
The cell migration was assessed using wound-healing and transwell assays. The role of RASSF1A on cell migration was examined by immunofluorescence staining, HDAC activity assay and western blot analysis.
Results
Cell migration was inhibited and cell morphology was changed in RASSF1A-transfected H1299 cells, compared with controls, whereas HDAC6 protein expression was not changed by RASSF1A transfection in these cells. However, RASSF1A inhibited deacetylating activity of HDAC6 protein and induced acetylated α-tubulin expression. Furthermore, acetylated α-tubulin and HDAC6 protein were co-localized in the cytoplasm in RASSF1A-transfected H1299 cells. Conversely, when the endogenous RASSF1A expression in HeLa cells was blocked with RASSF1A siRNA treatment, acetylated α-tubulin was co-localized with HDAC6 protein throughout the whole cells, including the nucleus, compared with scramble siRNA-treated HeLa cells. The restoration of RASSF1A by 5-Aza-dC treatment also induced acetylated α-tubulin through inhibition of HDAC6 activity that finally resulted in suppressing cell migration in H1299 cells. To further confirm the role of HDAC6 in RASSF1A-mediated cell migration, the HDAC6 expression in H1299 cells was suppressed by using HDAC6 siRNA, and cell motility was found to be decreased through enhanced acetylated α-tubulin.
Conclusion
The results of this study suggest that the inactivation of HDAC6 by RASSF1A regulates cell migration through increased acetylated α-tubulin protein.
Introduction
The RAS association domain family protein 1 (RASSF1) gene is implicated as a tumor suppressor, and its two major isoforms, A and C, are produced by the human RASSF1 gene on chromosome 3p21 [1,2]. RASSF1A has frequently been found to be inactivated in the lung, breast, and other cancer cells as a result of hypermethylation of a CpG island in its promoter [3], and the restoration of the RASSF1A expression by demethylating agents is shown to suppress tumor cell growth in vivo and in vitro [4,5]. RASSF1A plays an important role in the regulation of cell cycle and apoptosis. RASSF1A regulates the cell cycle by inhibiting the anaphase-promoting complex/Cdc20 complex [6] and inhibits cell cycle progression at the G1-S transition by preventing the accumulation of cyclin D1 [7] and interacting with the transcription factor p120E4F [8]. Moreover, RASSF1A induces apoptosis by regulating apoptotic protein such as NORE1, MST1, MST2, and BAX [9-11].
RASSF1A has also been associated with stabilization of microtubules and has been shown to influence cell motility and genomic stability [12]. Furthermore, it displays tumor suppressive functions through its interaction with microtubules and modulation of microtubule dynamics [13]. A recent study suggests the functional importance of RASSF1A in microtubule localization as the demonstration that the loss of microtubule localization of RASSF1A results in its enhanced tumorigenic potential [14]. Moreover, the overexpression of RASSF1A causes the formation of stable circular and acetylated α-tubulin for the suppression of cell migration and a deletion of RASSF1A increases phosphatidylinositol 3-kinase (PI3K)-dependent cellular motility [15].
Histon deacetylase 6 (HDAC6) is a member of the histone deacetylase family and is predominantly localized in the cytoplasm [16]. It has been reported to be associated with microtubules and localizes the microtubule motor complex, including p150glued, a subunit of dynactin, in the cytoplasm [17]. HDAC6 is known to function as a potent α-tubulin deacetylase: it deacetylates α-tubulin in assembled microtubules, whereas a decrease of HDAC6 increases α-tubulin acetylation [18]. Decreasing tubuin acetylation enhaces cell motility through the reduction of microtubule stability [19]. Several studies have also found that HDAC6 enhances cell motility through the deacetylation of α-tubulin, indicating that the inhibition of HDAC6-mediated α-tubulin deacetylation suppresses cell migration [20].
Inhibition of HDAC6 catalytic activity promotes α-tubulin acetylation, and acetylated tubulin enhances microtubule stability and suppresses cell migration. Furthermore, RASSF1A has also been implicated in cell motility by stabilization of the microtubule. However, it still remains to be determined how the RASSF1A induces α-tubulin acetylation and controls cell migration. We therefore investigated the putative mechanism of RASSF1A-mediated cell migration suppression, focusing on the possibility that RASSF1A might regulate HDAC6 as the α-tubulin deacetylase. We found that RASSF1A inhibited cell migration through the suppression of the HDAC6 activity and induction of acetylated α-tubulin expression in H1299 cells.
Materials and Methods
1. Cell cultures
NCI-H1299 and HeLa cells obtained from American Type Culture Collection (ATCC, Manassas, VA) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 units/mL penicillin and 100 µg/mL streptomycin) at 37℃ in a humidified 5% CO2 atmosphere. The cells were subcultured routinely with trypsin (0.25%)/ethylenediaminetetraacetic acid (1 mM) upon reaching 80-90% confluence.
2. Transient and stable transfection
The day before transfection, H1299 cells (2×105) were plated in 60-mm plates. On the next day, the cells were transiently transfected with 1 µg of RASSF1A DNA (kindly provided by Dr. S. Tommasi, Beckman Research Institute, Duarte, CA) or pcDNA3 using Lipofectamine reagent (Invitrogen, Carlsbad, CA). Forty-eight hours after the transfection, the cells were harvested for western blot analysis. To generate cells stably expressing RASSF1A, H1299 cells were transfected with 1 µg DNA of RASSF1A using Lipofectamine reagent, and colonies were selected from the culture by treating them with G418 (1 mg/mL). The expression of RASSF1A was confirmed by western blotting with RASSF1A antibody (Abcam, Cambridge, UK). Selected colonies were maintained in a medium containing G418 (1 mg/mL); only low-passage cells (p<10) were used for experiments.
3. RNA isolation and reverse transcription-polymerase chain reaction (PCR) assay
Total cellular RNA was isolated using TriReagent-RNA isolation reagent (Life Technologies Inc., Gaithersburg, MD) according to the manufacturer's instructions. Aliquots of total RNA (1 µg) were used to produce cDNA using Moloney murine leukemia virus reverse transcriptase (RT) (Life Technologies Inc.) and oligo-dT 15 primer (Roche, Indianapolis, IN) in a final volume of 20 µL. The cDNA products (1 µL) obtained were used for the PCR amplification of RASSF1A and GAPDH. The sense and antisense primers used for RASSF1A were 5'-TCT GGG GCG TCG TGC GCA AA-3' and 5'-GAA CCT TGA TGA AGC CTG TG-3', respectively, and a 256 bp PCR-amplified DNA fragment product was obtained. The sense and antisense primers for GAPDH, which was used as an internal control, were 5'-ACC CAG ATC ATG TTT GAG ACC-3' and 5'-GGA GTT GAA GGT AGT TTC GTG-3', respectively, and the amplified PCR product was a 486 bp DNA fragment. The conditions used for PCR were 95℃ for 5 minutes, 30 amplification cycles (95℃ for 30 seconds, 60℃ for 30 seconds, and 72℃ for 1 minute), and a final extension at 72℃ for 5 minutes. The PCR products from each sample were subjected to electrophoresis in 2% agarose gels, stained with ethidium bromide, and then photographed.
4. Western blot analysis
H1299 cells were stably transfected with pcDNA3 or RASSF1A, harvested, and washed with ice-cold phosphate-buffered saline (PBS). The cells were lysed with RIPA buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% sodium deoxychlolate, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride, 5 µg/mL aprotinin, and 2 µg/mL leupeptin), and then centrifuged at 12,000 rpm and 4℃ for 30 minutes. The total cell lysate (50 µg) was resolved by 8% SDS-polyacrylamide gel electrophoresis gel and transferred to polyvinylidene difluoride membranes membrane (Amersham Pharmacia Biotech, Little Chalfont, UK). Non-specific binding of the membrane was blocked by PBS containing 0.1% Tween 20 and 5% non-fat milk for 60 minutes, and then incubated overnight, with primary antibody (1:1,000) at 4℃. The membranes were washed three times for 10 minutes each, with PBS containing 0.1% Tween 20 and then incubated for 1 hour with horseradish peroxidase-labeled secondary antibodies (1:5,000) at room temperature. After washing, the signal was detected by using an ECL-plus reagent (Amersham Pharmacia Biotech). Blots were probed with primary anti-RASSF1A monoclonal antibody (Abcam), anti-HDAC6 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), anti-acetylated α-tubulin monoclonal antibody (Sigma, St Louis, MO), and anti-β-actin monoclonal antibody (Sigma). The secondary antibodies were anti-rabbit or anti-mouse IgG antibody.
5. Wound-healing and transwell assays
For the wound-healing assays, cells were grown to confluence, and the wound was induced by scraping the cell monolayer with a pipette tip and left for 24-72 hours before photographing. The transwell assay was carried out. In brief, serum-starved cells were trypsinized and plated at 2×104 cells per upper chamber, together with serum-free RPMI 1640 medium (8 µm, Costar, Birmingham, UK) and 10% FBS placed in the low chamber. The cells were then allowed to migrate for 48 hours at 37℃. Membrane inserts (8.0 µm) were coated on the lower side with collagen I (Sigma). After the incubation for 48 hours at 37℃, non-migrating cells were removed from the upper chamber with a cotton swap and the insert was stained with crystal violet solution. Motility was quantified by counting the cells that had migrated to the undersurface in the serum medium. Each well was counted under a light microscope at a magnification of 100× and then photographed.
6. HDAC colorimetric activity assay
HDAC6 activity assay was performed using the HDAC Colorimetric Activity Assay Drug Discovery Kits (BIOMOL Research Laboratories, Plymouth Meeting, PA) according to the manufacturer's recommendations. Briefly, H1299 cells were stably transfected with pcDNA3 and RASSF1A. The total cell lysates were then extracted in NP40-based lysis buffer (0.1% NP40, 150 mM NaCl, 50 mM Tris-HCl), and 200 µg of total cell lysate was immunoprecipitated with the HDAC6 antibody. The immunocomplexes were then collected in protein G PLUS-Agarose (Santa Cruz Biotechnology), washed, and incubated overnight with 0.5 mM colorimetric histone deacetylase lysyl substrate at 37℃ in a total volume of 100 µL. After incubation, developer (100 µL) was added to the sample and incubated for 15 minutes at 37℃. Absorbance was measured at 405 nm using a microplate reader.
7. Immunofluorescence staining
For studying the subcellular localization of RASSF1A, HDAC6, and α-tubulin, H1299 cells were placed on 18 mm coverslips and incubated overnight. After the overnight incubation, the cells were washed in PBS and fixed with PBS containing 3.7% formaldehyde for 10 minutes at 37℃. Coverslips were then rinsed three times with PBS, and the cells were permeabilized with PBS containing 0.1% Triton X-100 for 30 minutes at room temperature. Coverslips were then incubated in blocking solution (PBS containing 5% bovine serum albumin) for 1 hour at room temperature to reduce non-specific binding of the antibody. Incubation with appropriate primary antibodies was carried out for 1 hour at room temperature, and the coverslips were then washed three times with PBS and incubated for 30 minutes at room temperature with Alexa-Fluor 564-labeled goat anti-mouse or Alexa-Fluor 564-labelled goat anti-rabbit IgG (Molecular Probes Inc., Eugene, OR). After washing three times with PBS, the slides were mounted on VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA), and immunofluorescence was detected by fluorescence microscopy.
8. Subcellular fractionation
Cytoplasmic and nuclear extracts from cells were isolated using a nuclear and cytoplasmic extract reagents kit in accordance with the manufacturer's instructions (Pierce, Rockford, IL). Briefly, RASSF1A-transfected cells were harvested by centrifugation at 12,000 rpm and 4℃ for 10 minutes. The cellular membrane was then lysed by adding cytoplasmic extraction reagent buffer and incubating on ice for 5 minutes to separate cytoplasm proteins. After centrifugation at 13,000 rpm for 5 minutes, pellets were resuspended in nuclear extraction reagent buffer and incubated for 1 hour at 4℃ to release the nuclear proteins and then centrifuged at 13,000 rpm for 10 minutes. The expression of RASSF1A, acetylated α-tubulin, and HDAC6 was confirmed by western blot analysis.
9. Small interfering RNA assay
The double-stranded small interfering RNA (siRNA) oligonucleotide used for targeting RASSF1A was synthesized by Ambion Inc. (Austin, TX). The sequences used for RNA interference (RNAi) were as follows: sense 5'-GACCUCUGUGGCGACUUCATT-3' and antisense 5'-UGAAGUCGCCACAGAGGUCTT-3'. Two 21-nucleotide, double-stranded siRNA were used for targeting HDAC6 and were synthesized by Dharmacon (Dharma on Research, Lafayette, CO). Cells were transected with siRNA or scramble siRNA using Lipofectamine reagent (Invitrogen) according to the manufacturer's instructions.
10. 5-Aza-dC treatments
The H1299 cells were seeded at 2×105/60 mm plate with 10% FBS-maintaining medium before treatment. Cells were then treated with 10 µM 5-aza-2'-deoxycytidine (Sigma). After incubation for 48 to 72 hours, the cells were harvested for RT-PCR and western blot analysis.
11. Statistical analysis
All experiments were repeated at least three times. Data were calculated with Microsoft Excel software (Microsoft Co., Seattle, WA). Differences between experimental groups were determined by a Student's t-test, using Microsoft Excel. Every error bar indicates standard error (SE), and p-values were also calculated. A p-values of <0.05 were considered statistically significant.
Results
1. RASSF1A changes cell morphology and suppresses cell migration
H1299 non-small cell lung cancer (NSCLC) cell line was selected for the experiment because it has inactivated RASSF1A gene by promoter methylation. Thus, pcDNA3 and RASSF1A-transfected H1299 cells were constructed, and each transfectant was confirmed by western blot analysis and RT-PCR (Fig. 1A). Differences in morphology between the pcDNA3 and the RASSF1A-transfected H1299 cells were noted (Fig. 1B). The control cells seem more scattered and refractive, and had more cellular protrusions. In contrast, however, the RASSF1A-transfected cells seem less refractive and show less cellular protrusions than the control cells. This morphological change represents the hallmark of migrating cells. To investigate whether RASSF1A plays a role in cell migration, wound healing and transwell assays were performed with the RASSF1A-stable H1299 transfectant cells. RASSF1A significantly suppressed cell migration compared to the control cells (Fig. 1C). As shown in Fig. 1D, the transwell assay revealed that more control cells could pass through the membrane than the RASSF1A-transfected cells. Chemotactic migration toward the serum was also significantly decreased in the RASSF1A-transfected cells compared to the control cells, showing at least two-fold reduction in migration ability.

The effects of RASSF1A on cell morphology and migration. (A) H1299 cells stably transfected with RASSF1A or pcDNA3. RASSF1A expression was analyzed by reverse transcription polymerase chain reaction (RT-PCR) and western bolt analysis. GAPDH and β-actin served as a loading control. (B) RASSF1A stable transfected H1299 cells and control cells were photographed before the use for the migration assay at 20× magnification. (C) The results of wound healing assay. Cells were grown to confluence, and the wound was made by scraping the cell monolayer with a pipette tip and left for 24 to 72 hours before photographing (D) the results of transwell assay. Cells were plated at 2×104 cells per upper chamber together, along with serum free medium and 10% fetal bovine serum placed in the low chamber. The cells were then allowed to migrate for 48 hours. The membrane inserts were stained with crystal violet stain and photographed at 10× magnification. Quantification of relative migration of RASSF1A stably transected H1299 cells in relation to vector control cells (p<0.05).
2. RASSF1A increases acetylation of α-tubulin and suppresses deacetylating activity of HDAC6
To investigate the possible mechanism of RASSF1A-mediated suppression of cell migration, we examined whether RASSF1A regulated the protein expressions of HDAC6 and acetylated α-tubulin by using western blot analysis. In RASSF1A-transfected H1299 cells, the level of acetylated α-tubulin protein was greatly increased, whereas that of HDAC6 protein was unaltered (Fig. 2A). Next, to investigate whether RASSF1A regulates the activity of HDAC6, we performed the HDAC colorimetric activity assay. As shown in Fig. 2B, HDAC6 enzymatic activity was found to be inhibited in RASSF1A-transfected H1299 cells.
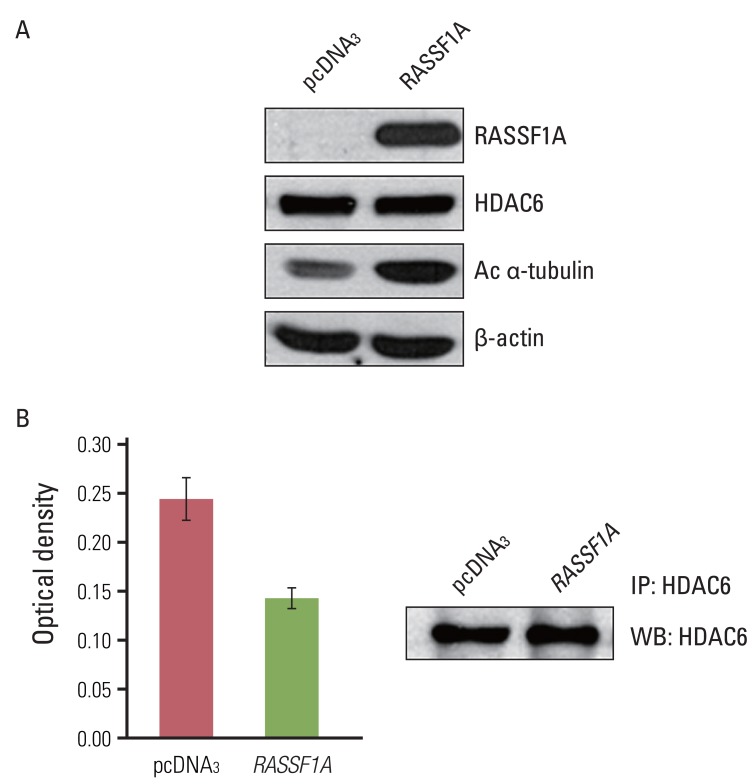
The effects of RASSF1A on acetylated α-tubulin and histon deacetylase 6 (HDAC6) protein. (A) The expression of RASSF1A, HDAC6 and acetylated α-tubulin (Ac α-tubulin) protein, analyzed by western blot analysis. (B) The result of deacetylating activity of HDAC6 protein. Total cell lysates (200 µg) were immunoprecipitated with HDAC6 antibody, and it was then subjected to HDAC activity assay. Columns, mean absorbance value of three independent experiments; bars, standard error; p<0.05.
3. RASSF1A induces cytosolic co-localization of acetylated α-tubulin and HDAC6 protein
To investigate whether the regulation of HDAC6 activity and acetylated α-tubulin expression by RASSF1A is associated with cellular localization of these proteins, the localization of HDAC6 and acetylated α-tubulin protein in the presence of RASSF1A was examined by immunostaining. Fig. 3A shows that subcellular localization of acetylated α-tubulin protein overlapped with HDAC6 protein in the cytoplasm only in RASSF1A-transfected H1299 cells. On the other hand, acetylated α-tubulin protein was colocalized with HDAC6 protein throughout the whole cells, including the nucleus, in the control cells. Then, to monitor the subcellular localization of RASSF1A and HDAC6 protein, we generated an expression construct carrying the GFP-tagged RASSF1A. As shown in Fig. 3B, GFP-alone was diffusely distributed throughout the cells, including the nucleus, and co-localized with HDAC6 protein in the whole cells, including the nucleus and cytoplasm. In contrast, however, the GFP-RASSF1A was localized only in the cytoplasm and co-localized with HDAC6 in the cytoplasm. Moreover, overexpression of RASSF1A led to the accumulation of acetylated α-tubulin and HDAC6 protein in the cytoplasm (Fig. 3C). To confirm these findings, RASSF1A siRNA was transiently transfected into HeLa cells, which were known to display an endogenous RASSF1A expression. The suppression of RASSF1A protein by siRNA RASSF1A decreased the level of acetylated α-tubulin (Fig. 4A). As shown in Fig. 4B, the acetylated α-tubulin protein in RASSF1A siRNA-treated cells was colocalized with HDAC6 protein throughout the cells, including the nucleus, whereas the acetylated α-tubulin protein was co-localized with HDAC6 protein in the cytoplasm of scramble siRNA-treated cells.

The effects of RASSF1A on cellular localization of histon deacetylase 6 (HDAC6) and acetylated α-tubulin (Ac α-tubulin) protein. (A) The results of co-localization of acetylated α-tubulin and HDAC6 protein. Double-immunostaining with an anti-acetylated α-tubulin antibody (green) and anti-HDAC6 antibody (red) in RASSF1A-transfected H1299 cells and control H1299 cells. (B) Co-localization of RASSF1A with HDAC6 protein in cytoplasm. H1299 cells were transiently transfected with expression vectors carrying GFP-alone or GFP-tagged RASSF1A and immunostained with an anti-HDAC6 primary antibody followed by red fluorescence dye conjugated anti-rabbit secondary antibody. (C) The results on cytoplasmic and nuclear extract from RASSF1A-transfected H1299 cells and control cells. Cells were prepared and the proteins indicated were analyzed by western blot analysis.
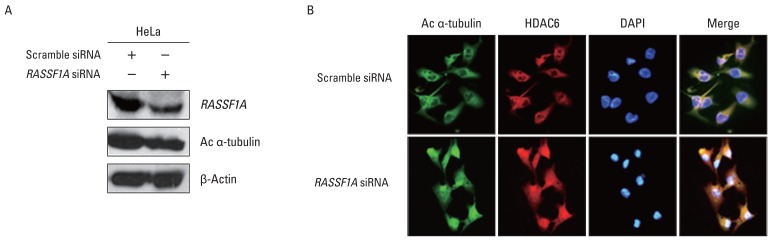
The effects of RASSF1A siRNA on cellular localization of histon deacetylase 6 (HDAC6) and acetylated α-tubulin (Ac α-tubulin). (A) HeLa cells, which display endogenous RASSF1A expression, were transiently transfected with RASSF1A siRNA. RASSF1A and Ac α-tubulin expressions were analyzed by western blot analysis. β-Actin served as a loading control. (B) The effects of RASSF1A knockdown on co-localization of acetylated α-tubulin and HDAC6 protein in HeLa cells.
4. Restoration of RASSF1A by 5-Aza-dC treatment inhibits deacetylating activity of HDAC6 and cell migration
To further confirm the above results, we studied whether the restoration of RASSF1A expression in H1299 cells by 5-aza-2'-deoxycytidine (5-Aza-dC) treatment inhibits cell migration. Thus, we analyzed RASSF1A expression by RT-PCR and western blot analysis after 5-Aza-dC treatment. RASSF1A mRNA and protein expressions were restored by 5-Aza-dC-treatment for 48 and 72 hours (Fig. 5A and B). As shown in Fig. 5C, the restoration of the expression of RASSF1A protein inhibited the HDAC6 activity by approximately 50% of untreated 5-Aza-dC H1299 cells. Likewise, the restoration of RASSF1A expression suppressed cell migration compared with the untreated 5-Aza-dC H1299 cells (Fig. 5D).

The effects of restoration of RASSF1A on deacetylating activity of histon deacetylase 6 (HDAC6) and cell migration in parent H1299 cells. (A) The restoration of RASSF1AmRNA expression in H1299 cells by 5-Aza-dC treatment was analyzed by reverse transcription polymerase chain reaction. GAPDH served as a loading control. (B) After H1299 cells were treated with 5-Aza-dC, the expressions of RASSF1A, HDAC6, and acetylated α-tubulin (Ac α-tubulin) protein were analyzed by western blot analysis. β-Actin served as a loading control. (C) The results of deacetylating activity of HDAC6 protein. Columns, mean absorbance value of three independent experiments; bars, standard error; p<0.05. (D) The results of wound-healing assay after the restoration of RASSF1A expression by 5-Aza-dC treatment. Cells were grown to confluence, and the wound was made by scraping the cell monolayer with a pipette tip and left for 48 to 72 hours before photographing.
5. Down-regulation of HDAC6 protein by HDAC6 siRNA increases acetylated α-tubulin and suppresses cell migration
To confirm whether RASSF1A-mediated suppression of cell migration depends on HDAC6, we examined whether down-regulation of HDAC6 activity suppresses cell migration in RASSF1A-tranfected H1299 cells. Therefore, we transfected control H1299 cells with HDAC6 siRNA. As shown in Fig. 6A and B, HDAC6 siRNA increased the expression of acetylated α-tubulin and inhibited HDAC6 activity, similar to RASSF1A-transfected H1299 cells. Moreover, the suppression of HDAC6 expression decreased cell migration, similar to scramble siRNA in RASSF1A-transfected H1299 cells, compared with scramble siRNA in control H1299 cells (Fig. 6C).
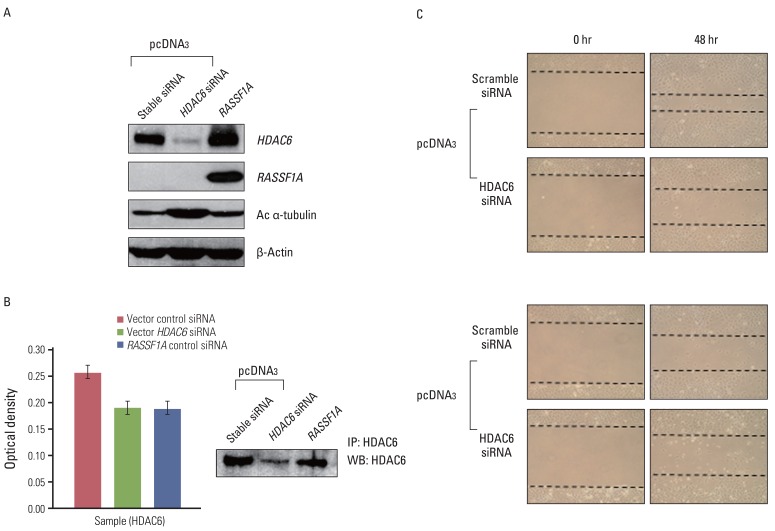
The effects of histon deacetylase 6 (HDAC6) siRNA on cell migration. (A) Control H1299 cells were treated with HDAC6 siRNA. The expressions of HDAC6, acetylated α-tubulin (Ac α-tubulin), and RASSF1A were analyzed by western blot analysis. β-Actin served as a loading control. (B) The results of deacetylating activity of HDAC6 protein. Columns, mean absorbance value of three independent experiments; bars, standard error; p<0.05. (C) The results of wound-healing assay. RASSF1A-transfected H1299 cells and control cells were transiently transfected with HDAC6 siRNA. Cells were grown to confluence, and the wound was made by scraping the cell monolayer with a pipette tip and left for 48 hours before photographing.
Discussion
Frequent inactivation of RASSF1A gene in human cancers suggests that it play a pivotal role in tumor prevention, and indeed RASSF1A functions as a tumor suppressor gene by playing a modulating role in apoptosis and cell cycle. An another feature of RASSF1A function is its effect on the microtubules [21]. RASSF1A induces microtubular structures radiating from the centrosome to become hyperstabilized circular structures and also protects the cells from nocodazole, a microtubule-destabilizing drug [12,22]. Furthermore, RASSF1A combines with microtubule and can modulate its polymerization, as indicated by changes in their structure and increased acetylation [13,23]. RASSF1A-induced microtubule stability suggests the possibility that RASSF1A regulates cell migration. However, little research has so far been focused on the role of RASSF1A in microtubule-mediated cell migration. Dallol et al. [15] reported that RASSF1A modulates cell migration in non-small cell lung cancer cells, A549, and Agathanggelou et al. [24] showed that genes for cell adhesion and motility, such as tropomyosin I and CDH2 (NCAD), were up-regulated in A549 cells stably expressing RASSF1A. Our present findings are in line with those by Dallol et al. [15] and Agathanggelou et al. [24], suggesting that RASSF1A plays a critical role in cell migration in lung cancer. In the present study, we found that RASSF1A inhibited cell migration and changed cell morphology. Furthermore, in agreement with the study by Dallol et al. [13], our data indicated that RASSF1A induced the acetylation of microtubules. Therefore, our data together with the above study suggest that RASSF1A regulates cell migration through enhanced microtubule stability in NSCLC cells. Nevertheless, it still remains to be determined how the RASSF1A induces acetylated α-tubulin expression.
The signaling mechanism by which RASSF1A controls cell migration has not been completely understood. Dallol et al. [15] suggested that RASSF1A regulates PI3K-dependent cellular motility in A549 cells, and that RASSF1A controls focal adhesion dynamics though inactivation of Rac1 GTPase. Tran et al. [25] suggested the possibility that deacetylation of tubulin by HDAC6 regulates dynamics of cellular adhesion via modulating Rac and Rho. In addition, several studies suggested that HDAC6 reduces acetylated α-tubulin and increases cell migration [19]. On the basis of these studies, this study proposes that RASSF1A might regulate the deacetylating activity of HDAC6. Indeed, the results of this study suggest that RASSF1A suppressed the activity of HDAC6 in RASSF1A-transfected H1299 cells; however, it did not change the expression of HDAC6 protein. Furthermore, down-regulation of HDAC6 protein by using siRNA technology induced the suppression of cell migration via increasing acetylated α-tubulin in control H1299 cells. To the best of our knowledge, this report is the first to describe that RASSF1A inhibits cell migration by inactivation of HDAC6 activity.
Several studies have focused their attention on subcellular localization of HDAC6. Hubbert et al. [19] reported that endogenous HDAC6 is co-localized with α-tubulin protein in the cytoplasm, and co-localization of HDAC6 with acetylated α-tubulin protein is increased after treatment with HDAC6 inhibitor, trichostatin A, in vivo and in vitro [18,20], demonstrating that HDAC6 and acetylated α-tubulin protein are co-localized in the cytoplasm. Our present result that acetylated α-tubulin and HDAC6 protein were co-localized in the cytoplasm of RASSF1A-transfected H1299 cells is consistent with that earlier report. Recent studies have shown the interaction of RASSF1A with microtubules and co-localization with endogenous α-tubulin in the cytoplasm [12,22,23], although earlier studies did not attempt to study subcellular localization of RASSF1A and HDAC6 protein. We found in the present study that exogenously expressed RASSF1A co-localizes with HDAC6 protein in the cytoplasm, leading to accumulation of HDAC6 and acetylated α-tubulin proteins in the cytoplasm. Conversely, knockdown of RASSF1A in HeLa cells, which display an endogenous RASSF1A expression, by siRNA technology resulted in acetylated α-tubulin protein co-localized with HDAC6 protein throughout the whole cells, including the nucleus. This finding indicates that endogenous RASSF1A is co-localized with acetylated α-tubulin and HDAC6 proteins into the cytoplasm, suggesting that RASSF1A may induce translocation of acetylated α-tubulin and HDAC6 protein into cytoplasm, and that RASSF1A suppresses cell migration through the accumulation of cytosolic HDAC6 and acetylated α-tubulin protein in RASSF1A-transfected H1299 cells. Exact mechanisms of RASSF1A-mediated α-tubulin and HDAC6 protein translocation into cytoplasm needs to be elucidated.
Conclusion
We investigated whether the interaction between RASSF1A, HDAC6, and acetylated α-tubulin induced the suppression of cell migration in H1299 cells, and found that RASSF1A suppressed cell migration through the inhibition of HDAC6 activity and the induction of acetylated α-tubulin protein expression in H1299 cells. RASSF1A caused co-localization with acetylated α-tubulin and HDAC6 in the cytoplasm. Our findings led us to conclude that RASSF1A increased the expression of acetylated α-tubulin protein through suppressing the HDAC6 activity, subsequently inhibiting cell migration. To our best knowledge, this study is the first to provide evidence that RASSF1A regulates the deacetylating activity and cellular localization of HDAC6 protein. Although further studies on the mechanism involved in the suppression of HDAC6 activity by RASSF1A are needed, these findings nevertheless provide an important new alternative mechanism by which RASSF1A controls its effects on cell migration.
Acknowledgments
This study was supported by grants of the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (no. A010250).
Notes
Conflict of interest relevant to this article was not reported.