Retrospective Analysis of the Treatment Results for Patients with Squamous Cell Carcinoma of Tonsil
Article information
Abstract
Purpose
There has been no definitive randomized study to identify the optimal therapeutic regimen for treating squamous cell carcinoma of tonsil. The purpose of this study was to retrospectively evaluate the treatment outcome according to various combinations of surgery, radiation therapy and chemotherapy.
Materials and Methods
Fifty-six patients with tonsillar carcinoma, who were treated at Seoul National University Hospital from March 1985 to August 2001, were the subjects of this study. Twenty-one patients received surgery followed by radiation therapy (SRT), 16 patients underwent radiation therapy alone (RT), and 19 patients received neoadjuvant chemotherapy and radiation therapy (CRT). The median radiation dose was 66.6 Gy for the SRT group and 70.2 Gy for the RT and CRT groups. Surgery comprised extended tonsillectomy and modified radical neck dissection of the involved neck. Cisplatin and 5-fluorouracil were used every three weeks for 3 cycles in the SRT group. The median follow-up was 73.2 months.
Results
The distribution of T-stage was 4 cases of T1, 14 cases of T2, 1 case of T3 and 2 cases of T4 staging in the SRT group, 2 cases of T1, 6 cases of T2, 5 cases of T3 and 3 cases of T4 staging in the RT group and 0 cases of T1, 7 cases of T2, 9 cases of T3 and 3 cases of T4 staging in the CRT group. The distribution of N-stage was 5 cases of N0, 2 cases of N1, 13 cases of N2 and 1 case of N3 staging in the SRT group, 6 cases of N0, 5 cases of N1, 5 cases of N2 and 0 cases of N3 staging in the RT group, and 2 cases of N0, and 7 cases of N1, 9 cases of N2 and 1 case of N3 staging in the CRT group. The five-year overall survival rate (OSR) for all patients was 78%. The five-year OSR was 80% for the SRT group, 71% for the RT group, and 80% for the CRT group (p=ns). The five-year disease-free survival rate was 93% for the CRT group and 71% for the RT group (p=0.017). Four patients developed local failure and one patient failed at a regional site in the RT group, and one patient failed at a primary site in the CRT group. The five-year DFS was 84% for patients who had undergone neck dissection and 76% for patients who had not undergone neck dissection (p=ns). Treatment-related complications of grade 3 or 4 occurred in 15 patients, and the incidence of complication was not different between each of the treatment methods.
Conclusion
Although the patients with more advanced T stage were included in the RT and CRT groups, the OSR was not statistically different according to the treatment methods. In the radical radiation therapy group, the addition of neoadjuvant chemotherapy showed an improvement in the disease-free survival. Because of the retrospective nature of our study and the small number of patients, this study cannot draw any definite conclusions, but it suggests that radiation therapy with chemotherapy can be a good alternative option for squamous cell carcinoma of tonsil. Controlled randomized study is necessary to confirm this hypothesis.
INTRODUCTION
Carcinoma of the tonsil is one of the most common malignancies experienced in the head and neck region, and it is second in frequency only to squamous cell carcinomas of the larynx. Carcinoma arising from the tonsil is usually squamous in origin and is strongly related to smoking and, to a lesser degree, to alcohol ingestion (1). Usually, this carcinoma affects patients from the fifth through the seventh decades of life, and its incidence is two to five times greater in men than in women.
The management of patients with squamous cell carcinoma of tonsil is controversial (2~5). It is often stated that radiation therapy and surgery are equally effective for the treatment of patients with early-stage disease, whereas a combination of the two modalities is the standard care for patients with advanced disease, with the presumption being that the combined modalities are a more effective treatment. There is insufficient randomized data for adequately comparing radiation therapy alone with the combined modality treatment. The only randomized trial has been a small study that was conducted by the Radiation Therapy Oncology Group (6). Of the seventy patients with oropharyngeal carcinoma, 24 patients received definitive radiation therapy, 23 patients received preoperative radiation therapy 23 patients received surgery or surgery along with postoperative radiation therapy. There was no significant difference noted in the end results among the different treatment groups. In the absence of any definitive randomized trials, treatment decisions have been based on retrospective data. The purpose of this study was to analyze treatment outcome when using surgery with adjuvant radiation therapy (SRT), radiation therapy alone (RT) and neoadjuvant chemotherapy followed by radiation therapy (CRT) for the patients with squamous cell carcinoma of tonsil who were treated at Seoul National University Hospital.
MATERIALS AND METHODS
1) Patients
From March 1985 to August 2001, 56 patients received radiation therapy for squamous cell carcinoma of the tonsil at the Department of Radiation Oncology, Seoul National University Hospital. Twenty-one patients were treated with SRT and 16 patients underwent RT. Nineteen patients received CRT. There were no specific criteria for deciding upon the use of neoadjuvant chemotherapy or radiation alone, but the patients who had more advanced disease tended to be chosen to receive CRT or RT.
Forty-nine of the patients were male. The median age was 57 years (range: 27 to 83 years). The patients were staged according to the 2002 American Joint Committee on Cancer TNM staging system (7). The distribution of the T-staging was 6 T1 cases, 27 T2 cases, 15 T3 cases and 8 T4 cases; the distribution of N-staging was 13 N0 cases, 14 N1 cases, 27 N2 cases and 2 N3 cases.
2) Treatment
All the patients were treated with a continuous course of once-daily radiation therapy using a 4-megavoltage photon beam. Most of the patients were treated with parallel opposed lateral fields with a matched anterior lower neck portal that was delivered using midline shielding. Electron boosts to the posterior neck nodes were occasionally used after the cord-off cone-down. Radiation therapy was delivered at a median dose of 66.6 Gy in 37 fractions for the SRT group and 70.2 Gy in 39 fractions for both of the RT group and the CRT group.
The distribution of the surgical method in the SRT group was as follows: tonsillectomy (2), extended tonsillectomy (2), tonsillectomy with neck dissection (5), extended tonsillectomy with neck dissection (11), and neck dissection only (1). Seventeen patients underwent neck dissection as part of their treatment. The neck dissection preceded radiation therapy for 16 patients and 1 patient underwent only neck dissection as their curative surgery. At Seoul National University hospital, the patients who require more aggressive surgery are usually were treated with radiation therapy to avoid surgical morbidity.
All of the patients in the CRT group received 5-FU 1,200 mg/m2 on days 1 to 5, and they received cisplatin 40 mg/m2 on day 1 for 3 cycles every three weeks, which was followed by radiation therapy alone.
3) Statistics
Survival analysis was done using the Kaplan Meier method (8) on SAS software, and the confidence intervals for survival were calculated (9). Comparison of survival curves was done by the log rank test and the confidence intervals were constructed as described by Gardner and Altman (10). Cox's proportional hazard model was used for the multivariate analysis (9). Survival was further analyzed using Cox's proportional hazards model for multivariate analysis. We scored complications by using Radiation Therapy Oncology Group morbidity scoring system (11).
RESULTS
1) Patient characteristics
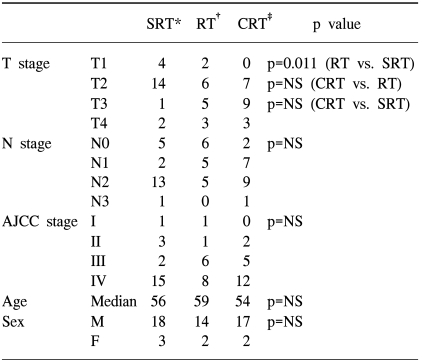
Table 1 shows the relative proportions of the T stage, N stage and AJCC stage distribution according to the treatment group. The distribution of T-stage was 4 T1 cases, 14 T2 cases, 1 T3 case and 2 T4 cases in the SRT group; 2 T1 cases, 6 T2 cases, 5 T3 cases and 3 T4 cases in the RT group, and 0 T1 cases, 7 T2 cases, 9 T3 cases and 3 T4 cases in the CRT group. The distribution of N-stage was 5 N0 cases, 2 N1 cases, 13 N2 cases and 1 N3 case in the SRT group; 6 N0 cases, 5 N1 cases, 5 N2 cases and 0 N3 cases in the RT group, and 2 N0 cases, 7 N1 cases, 9 N2 cases and 1 N3 case in the CRT group. Although the distribution of patients across treatment groups was not statistically different by the N-stage, the SRT group had significantly fewer T3 and T4 cases than did the non-surgery group (both of the RT and CRT group) (p=0.005).
2) Response to chemotherapy and RT
In the CRT group, 2 patients showed complete responses (CRs) and 17 patients showed a partial response (PR) after 3 cycles of chemotherapy. One patient with a CR was followed as having no evidence of disease (NED) status and the other one died of tumor recurrence at the primary site. In the 17 patients with PR, 3 patients who showed PR or stable disease (SD) after radiation therapy died of squamous cell carcinoma of tonsil. Thirteen patients out of the 14 CR patients after radiation therapy were followed as having NED status and 1 patient died of another disease. One CR patient who was followed as having NED status underwent planned neck dissection.
In the RT group, 14 patients showed CR and 2 showed PR after radiation therapy. Eleven patients with CR were followed as having NED status and 2 patients' disease recurred at the primary site. One out of 14 CR patients had disease recurrence at the ipsilateral neck. All of the patients with recurrent disease died of squamous cell carcinoma of tonsil. Two patients who showed PR had recurrent disease at the primary site and they died of their disease.
3) Overall survival and disease-free survival
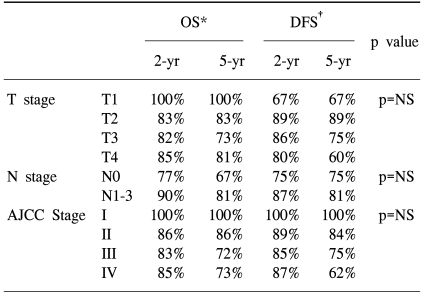
The overall survival rates (OS) were 100%, 83%, 73% and 81% for patients with T1,T2, T3 and T4 disease respectively, and 67%, 64%, 84% and 50% for patients with N0, N1, N2 and N3 disease. The OS rates were 100%, 86%, 72% and 73% for patients with AJCC stage I, II, III and IV disease, respectively. Disease free survival rates (DFS) were 67%, 89%, 75% and 60% for the T1, T2, T3 and T4 classifications, respectively, and 75% and 81% for the N0 and N1- N3 classification. The OS results and DFS results were broken down according to treatment, T stage, N stage and AJCC stage, and this is shown in Table 2. Using a log rank test, we found no significant differences for both the OS and DFS across the T stage, N stage and AJCC stage; however, with using a Cox proportional hazard model, patients with a higher T stage experienced significant higher mortality rates (p=0.03).
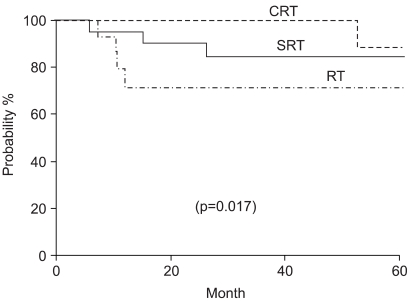
There was no significant difference in OS across the treatment groups, as is shown in Table 2. Fig. 1 plots the Kaplan-Meier curves for OS by the treatment groups. The five-year DFS was 71% in the SRT group, 71% in the RT group and 93% in the CRT group. There were statistically significant differences between the RT and CRT groups (p=0.017), as is shown in Fig. 2.

Overall survival accordilng to treatment modality. SRT: surgery +RT; CRT: chemotherapy +RT; RT: radiation therapy.
4) Patterns of failure
There was no significant difference in the failure pattern among the treatment groups. In the SRT group, 5 patients failed at the primary site, 1 patient failed at both of the primary site and the ipsilateral neck and 1 patient failed at a distant site. In the RT group, four patients failed at the primary site and 1 patient failed at the ipsilateral neck. In the CRT group, 1 primary site failure occurred.
5) Neck Dissection
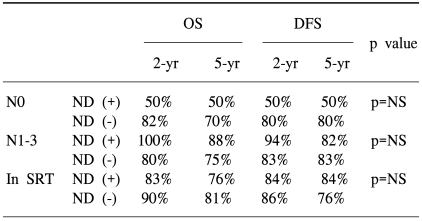
The rates of OS and DFS according to neck dissection (ND) and the N stage are shown in Table 3. We found no significant differences in both the N0 and N1-N3 groups according to ND. In the subgroup analysis, the OS was 76% in the ND group and 81% in the no ND group. For the SRT group, the DFS was 84% in the ND group and 76% in the no ND group (p=0.64).
6) Complications of treatment
The acute grade III and IV complication rate among all the patients was 27%. The Cox proportional hazard model analysis predicted no difference among the 3 treatment groups. Seven (33%) patients in the SRT group, 2 (10%) patients in the RT group and 6 (38%) patients in the CRT group experienced grade III or IV complications. Radiation complications were most commonly mucositis, taste alteration, xerostomia, voice change and dysphasia, and nausea and/or vomiting and desquamation were also reported.
DISCUSSION
The reality for patients with carcinoma of tonsil is that treatment selection often depends on what the physician has at his disposal. Patients who are referred to the department of radiation oncology usually receive radical radiation therapy first, whereas patients who are referred to the department of otorhinolaryngology usually undergo an operation first rather than radiation therapy. There is no adequate randomized data on the treatment for patients with squamous cell carcinoma of tonsil. Although this study is a retrospective, nonrandomized analysis of treatment results for 56 patients who were treated with SRT, RT and CRT for squamous cell carcinoma of tonsil, we believe that this may present a useful information for physicians who make decisions on the treatment modality.
The most remarkable findings in this review were 1) the similarities in the rates of overall survival among the three treatment modalities, 2) the similarities in the rates of severe or fatal complications and 3) in the radical RT group, the addition of neoadjuvant chemotherapy showed an improvement in the disease-free survival.
The individual and collective morbidities that result from surgery and radiation therapy are difficult to categorize and they transcend any single definition. Approximately 15~20% of the patients with tonsillar carcinoma who undergo surgery require segmental mandibulectomy, and this procedure causes severe complications (12). For example, if the patient who has undergone extended tonsillectomy, bilateral neck dissection and postoperative radiation therapy heals uneventfully and they experience no post-irradiation complications, then that patient is scored statistically as a complication free case despite that the patient has suffered considerable physiologic, psychologicaand socioeconomic injury. Conversely, a patient with a T1 or superficial T2 tonsillar region tumor with minimal or absent lymph node metastasis may have a better functional outcome after undergoing surgery plus ipsilateral neck dissection than the similarly staged patient who has undergone high-dose radiation therapy, and thus, the former patient avoids the expected sequelae of xerostomia.
In an analysis of treatment results for carcinoma of the tonsil, Wang et al found that for stage 3 and 4 carcinoma of tonsil, surgery plus radiation therapy yielded significantly better local control rates than did the unimodal therapy (13). Perez et al reported on 384 patients with carcinoma of the tonsil: 154 were treated with radical radiation therapy (55~70 Gy), 144 were treated with preoperative radiation therapy (20~40 Gy), and 86 were treated with postoperative radiation therapy (50~60 Gy). They suggested that T1 or T2 carcinoma of tonsil should also be treated by radiation therapy, whereas for patients with T3 or T4 tumors who are in a good general condition, combined surgery with postoperative radiation therapy gives a better prognosis (14).
In this study, the SRT group had a survival rate of 71%, the RT group had a disease free 5-year survival rate of 71%, and the CRT group had a survival rate of 93%. In the CRT group, the disease free survival rate was higher than the overall survival rate. This was because in the CRT group, there were many patients who died of diseases other than carcinoma of tonsil. The CRT group showed a better DFS than did the RT group, and this difference was statistically significant. Neoadjuvant chemotherapy before radiation therapy is an effective organ-sparing strategy for patients with squamous cell carcinoma of the larynx and hypopharynx (15), and this procedure showed promise as an organ-preserving tool upon retrospective analysis of patients with T4 oropharyngeal squamous cell carcinoma at the University of Florida (16). Concurrent chemoradiation therapy has consistently produced higher rates of both local-regional control and survival compared with the rates produced by radiation therapy alone in the randomized trial studies (17~23).
In our study, in the SRT group, 5 patients failed at the primary site, 1 patient failed at both of the primary site and the ipsilateral neck and 1 patient failed at a distant site. Four patients failed at the primary site and 1 patient failed at the ipsilateral neck in the RT group. In the CRT group, only 1 primary site failure occurred. With these results, it may be suggested that radiation therapy with chemotherapy is a good alternative option for squamous cell carcinoma of tonsil with good loco-regional control, and this treatment was related to the best disease free survival rate in 3 treatment groups. To confirm this hypothesis, controlled randomized study would be necessary.
It must be remembered that the patients in this study received different treatment for their primary tumors and their treatment of the neck also varied. Patients with neck nodes staged up to N1 had their necks treated by irradiation and surgical salvage if necessary. Patients with more extensive neck node disease received radical irradiation to the neck, irrespective of the treatment modality used for the primary tumor. All patients undergoing a radical neck dissection in this series also received irradiation to the neck. Thus, although there was no planned postoperative radiation therapy given to the surgery group at the primary site, the primary site will, by necessity, be treated while treating the neck.
This study found that radiation therapy is at least as good as surgery at curing primary disease, and we recommend that improvements in this treatment modality are the way to move forward. Just as surgical options and ingenious reconstructive techniques have evolved over the years, so have the radiotherapeutic options. Patients with early-stage carcinoma of tonsil are increasingly being treated with ipsilateral radiation therapy techniques that limit the morbidity, and especially the xerostomia, by using mixed-beam, wedge pair, three-dimensional conformal or intensity-modulated radiation therapy techniques (24). In addition, the chemoradiation protocols should be investigated and refined, particularly from the view that we achieved some apparent success with concomitant cisplatin, 5-fluorouracil and other agents.
CONCLUSIONS
We found that in the radical RT group, the addition of neoadjuvant chemotherapy showed an improvement in the disease-free survival and there was a good cure rate of primary tumor. With this finding, radiation therapy with or without neoadjuvant chemotherapy as a neck treatment modality showed very good results particularly in view of the severity of the complications. Because of the retrospective nature of the study and the small number of patients, this study cannot draw definite conclusions. Yet radiation therapy with chemotherapy could be recommended as a good alternative option for squamous cell carcinoma of tonsil, and this treatment holds promise for continued improvement in both tumor control and the functional outcome.