Hepatitis B Virus Reactivation in a Chronic Lymphocytic Leukemia Patient Treated with Ibrutinib
Article information
The risk of hepatitis B virus (HBV) reactivation during rituximab treatment in patients with occult HBV infection (hepatitis B virus surface antigen [HBsAg](−) and hepatitis B core antibody [HBcAb](+)) is well known, and antiviral prophylaxis is strongly recommended. Ibrutinib, a selective Bruton tyrosine kinase inhibitor, has been widely used as an effective treatment for chronic lymphocytic leukemia (CLL). It is presumed that HBV reactivation during ibrutinib treatment is possible because it induces strong immune suppression by inhibiting the B-cell receptor signaling pathway. Several reports have been published regarding HBV reactivation in patients with CLL receiving ibrutinib [1–3]. However, most reactivated patients already have received rituximab and/or hematopoietic transplantation before ibrutinib and there has been no reported case in Korea. Here we describe a case of HBV reactivation during ibrutinib in the absence of exposure to these therapies.
A 81-year-old woman was diagnosed with CLL (Rai stage III, TP53 fluorescence in situ hybridization(−)). Hepatitis serologic markers at the time of diagnosis were HBsAg (−) and hepatitis B virus surface antibody (HBsAb) (−). Chlorambucil was started as initial treatment and the best response was partial response (PR). However, after 16 months of chlorambucil treatment, the disease progressed. Ibrutinib was started 6 weeks after discontinuation of chlorambucil. At the time of ibrutinib initiation, HBV serum markers were HBsAg (−), HBsAb (+), and HBcAb (+), but HBV DNA test was not performed. Although 420 mg once daily is standard dose of ibrutinib, 280 mg was administered because of low body surface area (1.35 m2) and old age. PR was reached after 3 months, however, she was treated with ibrutinib intermittently due to recurrent infection and diarrhea. After 12 months of ibrutinib therapy, she developed HBV reactivation (reverse seroconversion) with increased liver enzyme (Fig. 1). HBV serology showed HBsAg/Ab (+/−), hepatitis Be antigen/Ab (+/−), and HBV DNA > 9×108 copies/mL. Ibrutinib was stopped and tenofovir 25 mg was started. After three months of tenofovir treatment, HBV DNA decreased by 5,418 copies/mL, and ibrutinib was restarted. The patient has continued to take ibrutinib and tenofovir with undetectable HBV DNA level.
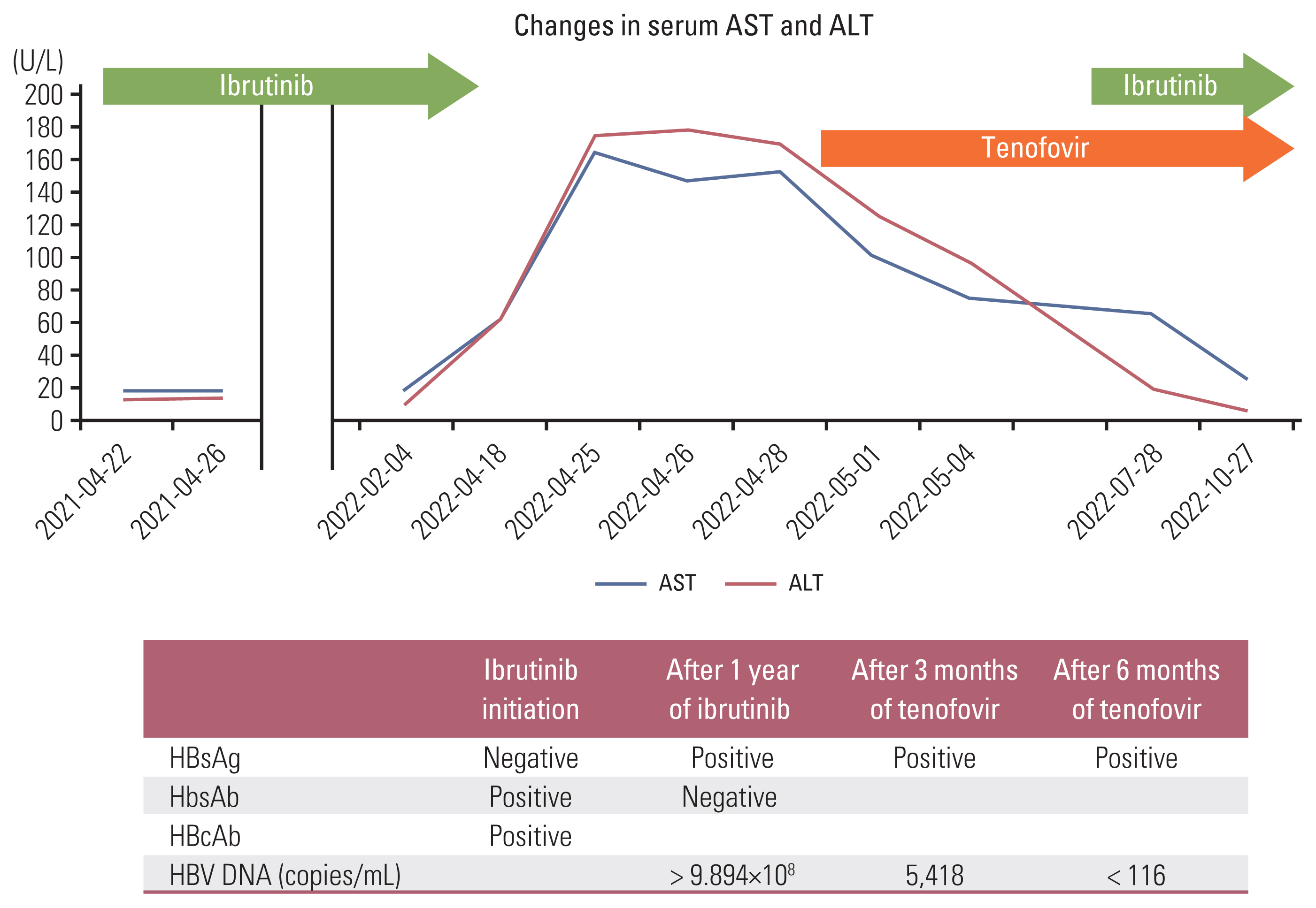
Hepatitis B virus serum markers during course of treatment. ALT, alanine transaminase; AST, aspartate aminotransferase; HBcAb, hepatitis B core antibody; HBsAb, hepatitis B virus surface antibody; HbsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus.
Guidelines for HBV monitoring and prophylaxis during ibrutinib treatment have not been established yet. Although there is not enough reports, the incidence of HBV reactivation was low in CLL receiving ibrutinib [2,4], and ibrutinib could be safely administered after antiviral control of HBV reactivation [5]. Antiviral prophylaxis is unlikely to be necessary; however, regular monitoring of HBV DNA and attention are required
Notes
Ethical Statement
This study was approved by the Institutional Review Board of Hanyang University Guri Hospital with a waiver of informed consent (IRB No. GURI 2022-11-024) and performed in accordance with the principles of the Declaration of Helsinki.
Author Contributions
Conceived and designed the analysis: Choi JH.
Collected the data: Choi JH.
Contributed data or analysis tools: Choi JH, Hur JY, Won YW.
Performed the analysis: Choi JH.
Wrote the paper: Choi JH, Hur JY, Won YW.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
