Androgen Receptor as a Predictive Marker for Pathologic Complete Response in Hormone Receptor–Positive and HER-2–Negative Breast Cancer with Neoadjuvant Chemotherapy
Article information
Abstract
Purpose
This study investigated pathological complete response (pCR) according to androgen receptor (AR) in breast cancer patients undergoing neoadjuvant chemotherapy and estimated the relationship between AR expression and clinicopathological factors.
Materials and Methods
We identified 624 breast cancer patients who underwent surgery after neoadjuvant chemotherapy at the National Cancer Center in Goyang, Korea from April 2016 to October 2019. We retrospectively collected the clinicopathologic information and AR expression results and analyzed the data according to cancer stage, hormonal receptor (HR) status, human epidermal growth factor receptor 2 (HER2) status, tumor subtype, and pCR.
Results
Among the 624 breast cancer patients, 529 (84.8%) were AR-positive (AR+) patients and 95 (15.2%) were AR-negative (AR−) patients. AR+ patients showed more estrogen receptor (ER) positivity, progesterone receptor (PR) positivity, HER2-positivity, and HR-positive and HER2-negative (HR+/HER2−) subtype. The rate of pCR was 31.4% (196/624). AR− patients had a significantly higher rate of pCR than AR+ patients (AR− 43.2% vs. AR+ 29.3%, p=0.007). The tumor factors associated with pCR were early stage, histologic grade 3, ER-negative, PR-negative, AR-negative, HER2-positive, and high Ki-67 values. In univariable analysis, AR+ significantly decreased the state of pCR (odds ratio, 0.546; 95% confidence interval, 0.349 to 0.853; p=0.008). According to tumor subtype, AR− tumor showed higher pCR rate in HR+/HER2− subtype (AR− 28.6% vs. AR+ 7.3%, p=0.022).
Conclusion
AR expression is predominant in the HR+/HER2− subtype. AR− is significantly associated with the pCR rate in breast cancer patients, especially within HR+/HER2− subtype. When determining neoadjuvant chemotherapy for the HR+/HER2− subtype, AR expression can be considered as a pCR predictive marker.
Introduction
Neoadjuvant chemotherapy has become the standard treatment for patients with locally advanced breast cancer [1]. Studies have reported a more favorable prognosis for patients with pathologic complete response (pCR) after neoadjuvant chemotherapy [2]. The rate of pCR by neoadjuvant chemotherapy differs according to the subtypes of breast cancer classified with estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) [3]. Neoadjuvant chemotherapy has shown to be more effective with triple-negative breast cancer (TNBC) or HER2-positive breast cancer than ER-positive breast cancer [1].
The androgen receptor (AR) is a steroid hormonal receptor expressed in about 70%–90% of breast cancers [4,5]. AR is known to be associated with ER in breast cancer and various studies report the different roles of AR according to ER. The expression of AR was reported at about 50%–90% in hormonal receptor (HR)–positive subtypes and its expression varied from 10%–50% in TNBC [5–7]. Well-differentiated types of the histologic grade were associated with high expression of AR [5]. Previous reports found that AR expression is associated with the recurrence rate and survival rate of breast cancer in each subtype [8–10]. However, studies regarding AR as a predictor of pCR rate after neoadjuvant chemotherapy according to cancer subtype are insufficient.
The purpose of this study was to investigate pCR rates of androgen receptors in breast cancer patients undergoing prior chemotherapy, and to confirm the significance of AR as a predictor. Additionally, we evaluated the correlation between pCR and AR by breast cancer subtype.
Materials and Methods
1. Study population and data collection
We included breast cancer patients diagnosed with stage I–III that were receiving neoadjuvant chemotherapy and undergoing surgery at the National Cancer Center in Goyang, Korea. Also, patients with hormone receptor (ER/PR/AR) test results prior to chemotherapy were enrolled. We excluded patients with stage IV breast cancer, those who were treated with neoadjuvant chemotherapy but did not undergo surgery, and those without hormone receptor (ER/PR/AR) test results.
From April 2016 to October 2019, we enrolled 624 patients who underwent surgery after neoadjuvant chemotherapy. We reviewed the patients’ clinicopathological characteristics including age, body mass index, menopause status, hormone status, Ki67 level, cancer subtype, clinical stage, and treatment method through electronic medical records.
We classified breast cancers into four subtypes: HR-positive and HER2-negative (HR+/HER2−), HR-positive and HER2-positive (HR+/HER2+), HR-negative and HER2-positive (HR−/HER2+), HR-negative and HER2-negative (HR−/HER2−). HR-positive was defined as ER and/or PR positive.
This study was approved by the Institutional Review Board of the National Cancer Center (IRB number NCC 2020-0224). The requirement for informed consent was waived as the study was a retrospective medical record review and caused minimal harm to the subjects.
2. Pathologic evaluation
The status of the hormone receptor was analyzed by core needle biopsy at the time of initial diagnosis. Hormone receptor expression including ER, PR, and AR was defined by the Allred score [11]. Scores from 0 to +2 were negative, and scores from +3 to +8 were regarded as positive (Fig. 1).
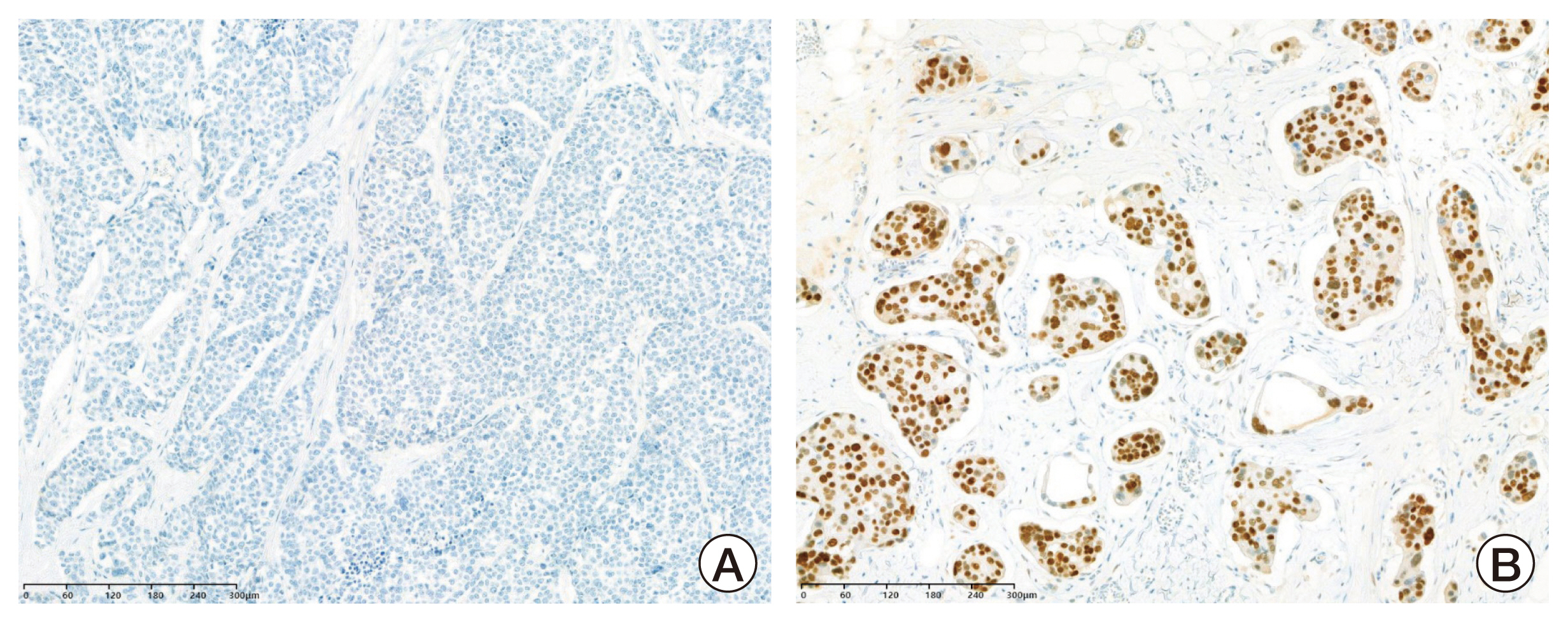
Immunohistochemistry images of androgen receptor expression. Cores were graded as negative (Allred scores 0–2, A) and positive (Allred scores 3–8, B).
Immunohistochemistry (IHC) was performed using the following primary antibodies: prediluted anti-ER rabbit monoclonal antibody (SP1, Ventana-Diapath, Tucson, AZ); prediluted anti-PgR rabbit monoclonal antibody (1E2, Ventana-Diapath); anti-AR rabbit monoclonal antibody (SP107, diluted 1:50, Cell Marque, Rocklin, CA); anti-Ki67 monoclonal antibody (MIB-1, diluted 1:100, Dako, Glostrup, Denmark) and c-erbB2 (4B5, prediluted, Ventana Medical, Tucson, AZ). Sliver in situ hybridization (SISH) assays for assessing HER2 gene amplification were performed for IHC equivocal (score 2+) cases. A positive HER2 SISH result was a HER2/chromosome enumeration probe 17 (CEP17) ratio ≥ 2.0 regardless of the average HER2 copy number or a HER2/CEP17 ratio < 2.0 with an average HER2 copy number ≥ 6.0 signal/cell [12]. IHC results were reviewed by one pathologist. pCR was defined as ypT0N0 or ypTisN0.
3. Statistical analyses
Patient characteristics were summarized by the mean and standard deviation for continuous variables, and frequency counts with percentages for categorical variables. For clinical factors according to pretreatment AR and pCR, depending on the types of variables, the chi-square test or Fisher exact test was used for categorical variables, and the t test was used for continuous variables. Clinical factors affecting pCR were identified using a multivariable logistic regression model. After including all clinical factors, the backward elimination method with criterion p-value < 0.05 was used to identify significant factors. In addition, after correcting for significant factors, to confirm the effect of pretreatment AR on pCR, the distribution of pretreatment AR and pCR in subgroups by subtype was summarized using percentages, and the relationship was confirmed using the chi-square test or Fisher exact test. All statistical analyzes were performed using R ver. 4.1.2 (R Core Team, Vienna, Austria), and a p-value < 0.05 was considered statistically significant.
Results
1. Characteristics of the study population
A total of 624 breast cancer patients who underwent neoadjuvant chemotherapy were included in the analysis. Table 1 summarized the clinicopathologic characteristics of the patients. Five hundred twenty-nine patients (84.8%) were AR-positive and 95 patients (15.2%) were AR-negative. The mean age (±standard deviation) at diagnosis was 50.0±9.6 years. Regarding the AR receptor status, the AR-positive group had significantly higher clinical T stages 3, and 4 than the AR-negative group (32.3% vs. 16.8%, p=0.002). The AR-positive group had significantly higher ER positivity and PR positivity, and HER2 positivity than the AR-negative group. Ki-67 levels were significantly lower in the AR-positive group in comparison to the AR-negative group (38.6±20.1 vs. 62.8±20.6, p < 0.001). Regarding the subtypes, HR+/HER2−, HR+/HER2+, HR−/HER2+, and HR−/HER2− subtypes comprised 46.9%, 23.6%, 20.4%, and 9.1% of the AR-positive group, respectively. In the AR-negative group, the HR−/HER2− subtype was most frequent (72.6%), followed by the HR+/HER2− subtype (14.7%). There was a significant difference between the AR-positive group and AR-negative group regarding subtypes (p < 0.001). The rates of pCR were 29.3% in the AR-positive group and 43.2% in the AR-negative group (p=0.007).
2. Tumor factors and pCR outcomes of patients
The associations between clinicopathologic tumor factors and pCR outcomes are summarized in Table 2. Clinical stage, histologic grade, ER, PR, HER2, AR, subtype, and Ki-67 levels had significant correlations with pCR rates. Patients that achieved pCR showed significantly lower AR-positive rates than non-pCR patients (79.1% vs. 87.4%, p=0.007). Regarding pCR patients, the HR−/HER2+ subtype was most frequent (41.8%), followed by the HR−/HER2− subtype (24%). On the other hand, in non-pCR patients, the HR+/HER2− subtype was most frequent (56.1%) and the HR−/HER2+ subtype was least frequent (8.6%). Ki-67 levels of pCR patients were significantly higher than non-pCR patients (50.8±20.2 vs. 38.5±21.7, p < 0.001).
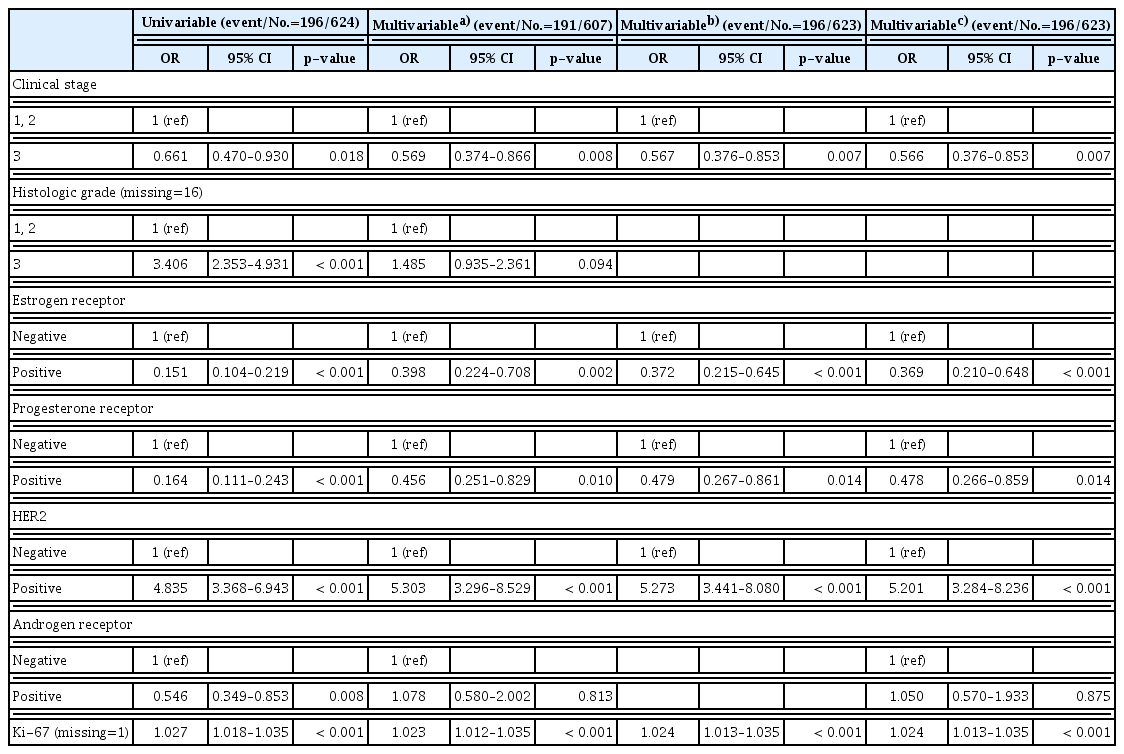
In the univariable analysis, clinical stage, ER, PR, HER2, and Ki-67 were significantly associated with pCR. AR-positive significantly decreased the state of pCR (odds ratio [OR], 0.546; 95% confidence interval [CI], 0.349 to 0.853; p=0.008). However, multivariable analysis showed no associations between AR and pCR (Table 3).
Also, pCR outcomes differed according to AR status in the breast cancer subtype (Fig. 2). In HR+/HER2− subtype, AR negativity showed high correlation with the pCR rate (AR-negative 28.6% vs. AR-positive 7.3%, p=0.022). There were no significant correlations between AR and pCR in other subtypes (S1 Table).
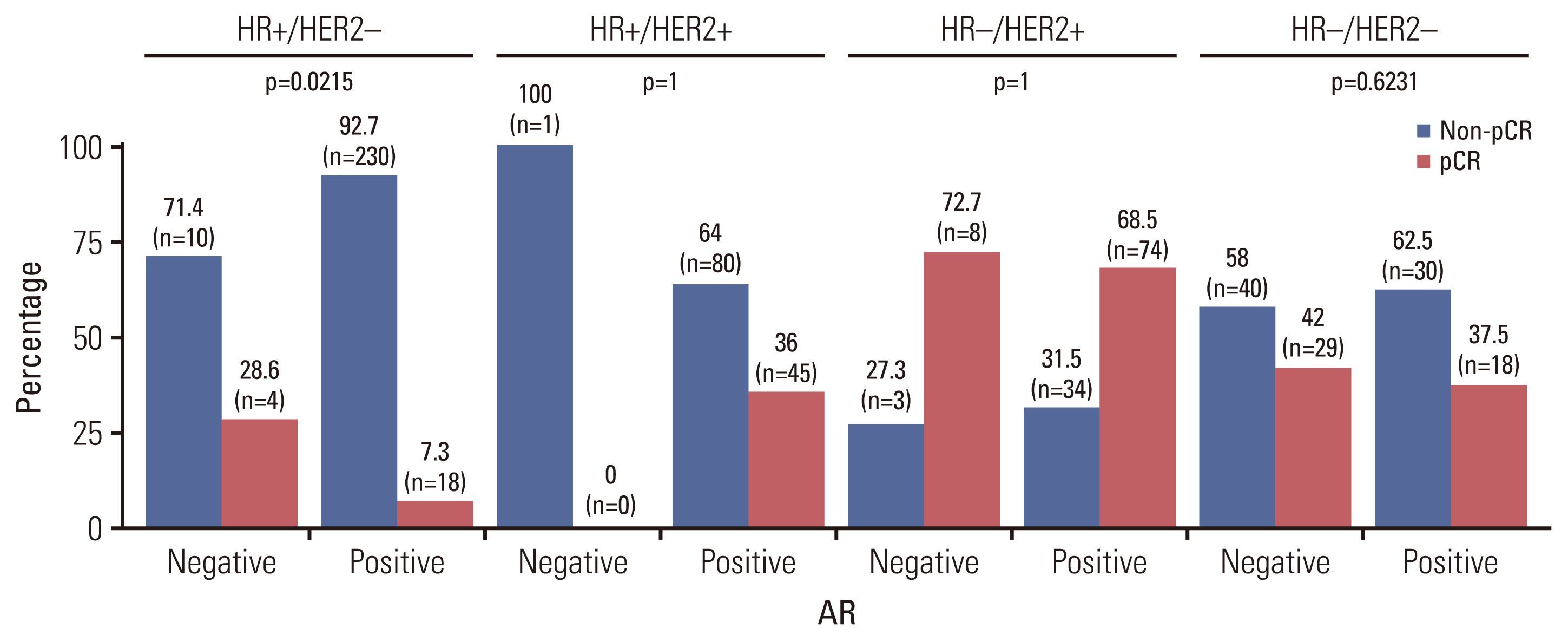
Distribution of pathological complete response according to androgen receptor (AR) expression by subtypes: hormone receptor (HR)–positive and human epidermal growth factor receptor 2 (HER2)–negative (HR+/HER2−), HR-positive and HER2-positive (HR+/HER2+), HR-negative and HER2-positive (HR−/HER2+), HR-negative and HER2-negative (HR−/HER2−).
3. Downstaging of tumors after neoadjuvant chemotherapy
Regarding the effect of neoadjuvant chemotherapy, it was confirmed that 470 patients (75.3%) were downstaging of tumors after neoadjuvant chemotherapy. Among these patients, the subtype distribution was 32.3% in HR+/HER2− subtype, 23.2% in HR+/HER2+ subtype, 23.6% in HR−/HER2+ subtype, and 20.9% in HR−/HER2− subtype. The downstaging effect of tumor after neoadjuvant chemotherapy by subtypes was 58% in HR+/HER2− subtype, 86.5% in HR+/HER2+ subtype, 93.3% in HR−/HER2+ subtype, and 83.8% in HR−/HER2− subtype. The median size of the residual tumor was 1.3 cm (0–11.6) and the median number of metastatic lymph nodes was 3 (0–23). In HR+/HER2− subtype, the median size of the residual tumor was 1.6 cm (0–11.6) and the median number of metastatic lymph nodes was 1 (0–16).
Discussion
In the current study, we reported AR as a predictive factor in breast cancer with neoadjuvant chemotherapy. We showed that HR+/HER2− or HR+/HER2+ subtypes are more frequently associated with AR-positivity. On the other hand, the HR−/HER2− subtype showed higher AR-negative rates.
The distribution of subtypes according to AR in early breast cancer showed that AR positivity was the most common in luminal A (87.6%), followed by luminal B (about 76%), HER2 (56.2%), and TNBC (20.6%) [13]. In our study, although the rates of positivity were different, the patterns of positivity by subtypes were similar.
In TNBC patients, one study reported a 30% positive AR expression percentage. Among patients who received neoadjuvant chemotherapy, the proportion of AR-positive was 25% and AR-negative was 75% [14], which was similar to our results. Our study showed the proportion of AR-positive was 41% and AR-negative was 59%.
AR has been reported as an independent factor in determining prognosis, and other previous studies have shown similar results. In the HER2 subtype, AR expression was associated with favorable clinicopathological characters and prognosis [15]. Previous observational studies have shown results for AR as predictors for anti-hormonal therapy in HR-positive breast cancers. Regarding postmenopausal women with early stage hormone-positive breast cancer, there were conflicting results that AR expression was not associated with prognosis and was not a suitable biomarker for determining adjuvant endocrine therapy [16]. Other studies reported that AR expression was a favorable prognostic factor in women with ER-positive breast cancer than with ER-negative breast cancer [17, 18].
The National Comprehensive Cancer Network guidelines recommend neoadjuvant chemotherapy for locally advanced breast cancer, as it shows a significantly good survival rate in cases with pCR after neoadjuvant chemotherapy [1]. According to a neoadjuvant setting study based on AR mRNA expression, high AR expression was associated with lower pCR rates (OR, 0.66; 95% CI, 0.55 to 0.78; p < 0.001) [19]. Our study showed that AR positivity was associated with a low pCR rate in a univariate analysis. Among breast cancer subtypes, the HR+/HER2-subtype had the highest AR-positivity, but the pCR rate after neoadjuvant chemotherapy was lower in the HR+/HER2− subtype than the HR+/HER+ subtype, HR−/HER2+ subtype, or HR−/HER− subtype.
A previous study found that AR expression was associated with better therapeutic responsiveness in HER2 type. The AR-positive group was a significantly high pCR rate than the AR-negative group (72.1% vs. 23.1%, p < 0.001). In a multivariate analysis, AR-positive was associated with pCR in HER2 type (OR, 9.4; 95% CI, 3.31 to 26.71; p < 0.001) [15]. Our study showed that there was no significant association between pCR and AR expression in the HR+/HER2+ subtype and HR−/HER2− subtype. The results between the previous study and our study may be due to differences in the sample size distribution of the subtypes.
AR expression may help determine neoadjuvant chemotherapy according to breast cancer subtype. Another study demonstrated that chemotherapy-sensitive patients with the TNBC subtype had negative AR status and higher Ki-67 values [20].
Patients with TNBC accomplishing non-pCR have an unfavorable prognosis and require additional treatment after surgery [21]. A retrospective study showed that compared with the AR-negative TNBC subtype, AR-positive breast cancers were significantly associated with better disease-free survival and overall survival despite a significantly lower rate of pCR to neoadjuvant chemotherapy (AR-positive 12.8% vs. AR-negative 25.4%) [22]. Based on gene ontologies and differential gene expression in TNBC, the luminal androgen receptor (LAR) subtype had a lower pCR rate compared to other subtypes, but a favorable prognosis [23]. The LAR type showed a lower pCR rate compared to other subtypes, but a better prognosis.
The strengths of this study are as follows: First, this study is comprised of a large sample size of Asians for the analysis of AR expression following neoadjuvant chemotherapy. In a previous study in Germany with a similar sample size to our study, the patterns of AR expression were observed as similar results to our study [22]. It was possible to confirm whether there was a difference between Asians and other ethnicities. Second, we analyzed AR positivity in various subtypes of breast cancer including HR-positive and HER2-positive subtypes. In clinical settings, HR-positive tumor including clinical stages I and II is treated with surgery rather than neoadjuvant chemotherapy. Additional chemotherapy is considered after performing an Oncotype test or Mammaprint test based on final pathology results. Previous studies regarding AR expression and clinical significance have mainly focused on the TNBC subtype. Our study confirmed pCR patterns in different subtypes of breast cancer.
However, our study also has several limitations. The main limitation regards the lack of information to identify the relationship between pCR and overall survival. We could not confirm the prognostic effect of AR positivity on overall survival. Further follow-up research is necessary to investigate AR expression and prognostic value for overall survival. Another limitation regards the comparison of treatment effectiveness. As the patients were treated with different chemotherapy regimens according to breast cancer subtype, it was difficult to compare the effectiveness of treatment.
Nevertheless, this is the first large-scale study to analyze AR expression in all subtypes of breast cancer patients who received neoadjuvant chemotherapy in Asia.
In conclusion, we discovered the correlation between AR expression and neoadjuvant chemotherapy outcomes. AR expression is predominant in the HR+/HER2− subtype. AR negativity is more effective in a neoadjuvant treatment setting of the HR+/HER2− subtype. Therefore, we need to regard AR expression as a pCR predictive marker for the HR+/HER2− subtype considering neoadjuvant chemotherapy.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Ethical Committee of the National Cancer Center (NCC 2020-0224) and the written informed consent was not required due that the design of this study was retrospective.
Author Contributions
Conceived and designed the analysis: Lee EG, Jung SY.
Collected the data: Lee DG, Jung SY.
Contributed data or analysis tools: Lee DE, Jung SY.
Performed the analysis: Lee DE.
Wrote the paper: Lee EG, Jung SY.
Writing - review and editing: Lee EG, Lee DE, Kim HE, Han JH, Lee S, Kang HS, Lee ES, Chae H, Sim SH, Lee KS, Kwon Y, Jung SY.
Funding acquisition: Jung SY. Supervision: Jung SY.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This work was supported by the National Cancer Center Grant (NCC2210541-1).