PIK3CA Mutation Is Associated with Poor Response to HER2-Targeted Therapy in Breast Cancer Patients
Article information
Abstract
Purpose
Mutations in the PIK3CA gene occur frequently in breast cancer patients. Activating PIK3CA mutations confer resistance to human epidermal growth factor receptor 2 (HER2)–targeted treatments. In this study, we investigated whether PIK3CA mutations were correlated with treatment response or duration in patients with HER2-positive (HER2+) breast cancer.
Materials and Methods
We retrospectively reviewed the clinical information of patients with HER2+ breast cancer who received HER2-targeted therapy for early-stage or metastatic cancers. The pathologic complete response (pCR), progression-free survival (PFS), and overall survival were compared between patients with wild-type PIK3CA (PIK3CAw) and those with mutated PIK3CA (PIK3CAm). Next-generation sequencing was combined with examination of PFS associated with anti-HER2 monoclonal antibody (mAb) treatment.
Results
Data from 90 patients with HER2+ breast cancer were analyzed. Overall, 34 patients (37.8%) had pathogenic PIK3CA mutations. The pCR rate of the PIK3CAm group was lower than that of the PIK3CAw group among patients who received neoadjuvant chemotherapy for early-stage cancer. In the metastatic setting, the PIK3CAm group showed a significantly shorter mean PFS (mPFS) with first-line anti-HER2 mAb. The mPFS of second-line T-DM1 was lower in the PIK3CAm group than that in the PIK3CAw group. Sequencing revealed differences in the mutational landscape between PIK3CAm and PIK3CAw tumors.
Conclusion
Patients with HER2+ breast cancer with activating PIK3CA mutations had lower pCR rates and shorter PFS with palliative HER2-targeted therapy than those with wild-type PIK3CA. Precise targeted therapy is needed to improve survival of patients with HER2+/PIK3CAm breast cancer.
Introduction
From the anti–human epidermal growth factor receptor 2 (HER2) monoclonal antibody (mAb) and antibody-drug conjugates (ADCs) to novel tyrosine kinase inhibitors, effective targeted therapy has revolutionized the treatment of HER2-positive (HER2+) breast cancer [1,2]. Although these therapies have markedly improved the prognosis of patients with HER2+ breast cancer, resistance to targeted therapy can develop, with some tumors showing de novo resistance [3]. Continued use of the anti-HER2 mAb trastuzumab beyond progression remains one of the standards of care even after the failure of dual HER2-blockades [4,5]; however, precise guidelines based on individual tumor biology are still lacking in clinical practice [6,7].
Results from preclinical and translational studies have highlighted mutations in the phosphatidyl inositol-4,5 bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) gene as a major driver of resistance to HER2-targeted agents [8–10]. The phosphoinositide-3-kinase (PI3K)-alpha catalytic subunit (p110α) acts as a key component in controlling the PI3K/ AKT/mammalian target of rapamycin (mTOR) signaling pathway [11], which regulates various cellular processes including cell growth, survival, and apoptosis [12]. Tumors with PIK3CA gain-of-function mutations respond poorly to anti-HER2 therapy and are associated with reduced pathological complete response (pCR) rates [13]. However, the relationship between palliative treatment response and PIK3CA mutations has not yet been fully characterized. Accordingly, we conducted a retrospective study to determine whether PIK3CA aberrations correlate with the duration of anti-HER2 therapy in patients with HER2+ breast cancer.
Materials and Methods
1. Patients
Among patients who had received systemic chemotherapy for early or metastatic HER2+ breast cancer at our hospital, those with available next-generation sequencing (NGS) data and clinical response to treatment were chosen for this study. Clinical characteristics such as age, sex, date of diagnosis, molecular subtype, treatment history, and survival data of each patient were collected from their medical records.
2. Tumor NGS analysis
Mutational analysis of tumor tissues was performed using targeted NGS. DNA was extracted, purified, and quantified from formalin-fixed paraffin-embedded breast tumor specimens, according to the K-MASTER protocol [14]. Using the K-MASTER panel, which allows the detection of variants in 409 representative genes using the HiSeq sequencing platform, we investigated the mutation profiles of the collected tissues. After passing the quality control process, the pipeline returned single-nucleotide variants (SNVs), copy number variants, and genomic fusion data from each sample. Detailed laboratory and bioinformatic protocols are available in the Supplementary Methods. In this study, the average depth of tumor-targeted sequencing coverage, duplication rate, on-target rate, pass rate score, and uniformity were 718.07 (range, 344.17 to 1,128.29), 28.49 (range, 86.65 to 99.14), 93.32 (range, 89.65 to 99.14), 99.27 (range, 69.88 to 100), and 79.62% (range, 69% to 91%), respectively.
3. Cell-free DNA analysis by liquid biopsy
A liquid biopsy was performed in patients with no available NGS data. Cell-free DNA (cfDNA) was extracted from plasma samples using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. After cfDNA quantification and purification, targeted sequencing was performed using Axen Cancer Panel 1 (ACP1), which included the exons of 88 cancer-related genes and intronic regions of three genes. Detailed laboratory and bioinformatic protocols for cfDNA analysis are available in the Supplementary Methods.
4. Response assessment
All the patients included in this study received at least one additional HER2-targeted therapy during their disease course. pCR was defined as the complete disappearance of invasive breast cancer in both the breast and lymph nodes and was assessed in patients who had received anti-HER2 mAb as neoadjuvant therapy (ypT0/is and ypN0). Response evaluation in the metastatic setting was performed according to the Response Evaluation Criteria in Solid Tumors 1.1 [15]. Invasive disease-free survival (iDFS) in patients who received (neo)adjuvant therapy was defined as the period from the date of curative surgery to the date of the local/distant recurrence, second primary invasive breast cancer, or death attributable to any cause. Progression-free survival (PFS) in metastatic or recurrent disease was assessed as the time from the start of specific treatment to disease progression or death from any cause.
5. Statistical analysis
Continuous variables were analyzed using Student’s t test, while categorical variables were analyzed using the chi-squared test. The median PFS and overall survival (OS) were calculated using the Kaplan-Meier method. Hazard ratios and p-values were assessed using a multivariate Cox regression analysis. IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY) was used for statistical analyses, and the results were visualized using SPSS ver. 4.1.0 (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
After screening 366 patients with HER2+ breast cancer who were treated at our hospital from August 2008 to September 2020, 90 patients were selected for this study. Their clinical characteristics are summarized in Table 1. Pathogenic mutations in PIK3CA (PIK3CAm) were found in 34 patients (37.8%), and 57.6% of these mutations were found in the kinase domain (H1047X). The median age of the patients was 50.2 years, and all patients except one were women. Estrogen receptor (ER) positivity was observed in 68.9% of the patients. The ER and PIK3CAm statuses of the analyzed HER2+ breast cancer samples are shown in S1 Fig. Among the analyzed population, 62 patients (68.9%) were ER positive, and the proportion of patients whose ER status was positive or negative was similar between patients with PIK3CAm and those with wild-type PIK3CA (PIK3CAw) (67.6% vs. 69.9%). For palliative chemotherapy, patients received multiple lines of anti-HER2 mAbs (trastuzumab and pertuzumab), ADCs (T-DM1), or tyrosine kinase inhibitors (lapatinib) as the disease progressed.
2. Treatment outcome in early-stage patients
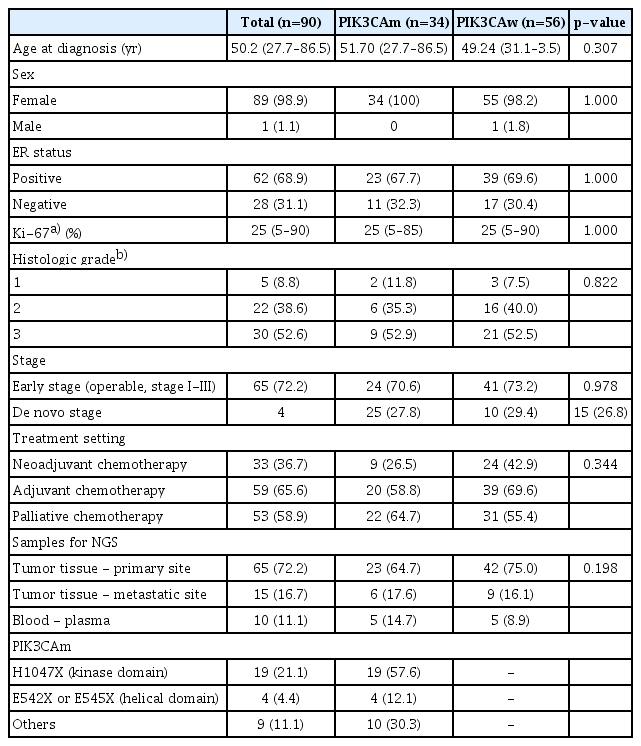
The overall treatment response and treatment duration for patients with PIK3CAw or PIK3CAm are shown in Fig. 1 and summarized in S2 Table. Among patients with early-stage cancers, 33 received standard neoadjuvant therapy, including single or dual anti-HER2 mAb with trastuzumab or trastuzumab plus pertuzumab. Twenty-six of them had pathologic report of curative breast surgery. Among the patients who received a single anti-HER2 mAb, 57.1% of patients with PIK3CAw achieved pCR (4 of 7), while none of the patients with PIK3CAm (0/3, 0%) achieved pCR. Treatment with the dual anti-HER2 mAbs trastuzumab and pertuzumab improved pCR rates in patients with PIK3CAw (72.7%) and PIK3CAm (40%). Altogether, 25% of patients with PIK3CAm and 66.7% with PIK3CAw were reported to have achieved pCR, and the difference between these two groups was marginally significant (p=0.090, chi-square test). At a median follow-up of 43.4 months (range, 14.2 to 222.2), the iDFS showed no significant difference between the two groups (31.0 months vs. 30.5 months).
Comparison of treatment duration and clinical course of human epidermal growth factor receptor 2–positive (HER2+) breast cancer with or without PIK3CA mutation. Treatment response and duration of HER2-targeted therapy according to PIK3CA status. The pathologic complete response (pCR) rate after neoadjuvant anti-HER2 monoclonal antibody (mAb) was significantly lower in the mutated PIK3CA (PIK3CAm) group, while disease-free survival (DFS) after curative treatment was similar between the two groups. In palliative treatment for advanced-stage cancer, patients with PIK3CAm presented shorter mean progression-free survival (mPFS) with first-line anti-HER2 mAb and second-line T-DM1.
3. Progression-free survival
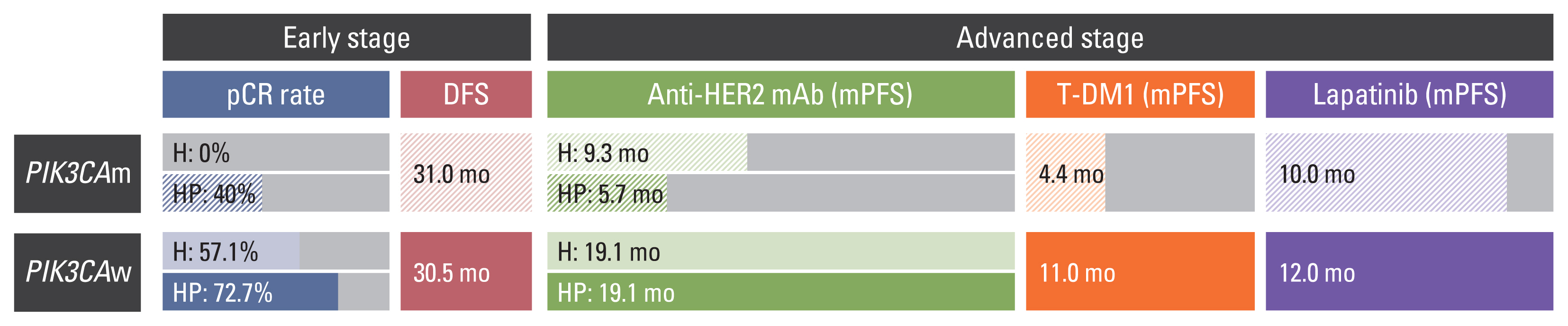
The PFS of palliative HER2-directed therapy was assessed using Kaplan-Meier analysis (Fig. 2). After a median 36.4 months of the total follow-up period (range, 3.5 to 206.7), the median PFS of the patients who were treated with palliative chemotherapy with anti-HER2 mAb as the first-line treatment was 32.8 months (95% confidence interval [CI], 4.5 to 23.9) in the PIK3CAw group and 8.8 months (95% CI, 5.8 to 11.9) in the PIK3CAm group (p < 0.001, Mantel-Cox log rank test), with a hazard ratio (HR) of 4.602 (95% CI, 2.057 to 10.514; p < 0.001, Cox regression analysis) (Fig. 2A). When we compared the HR based on the mutational status of PIK3CA among the patients who received anti-HER2 mAbs, the HR of patients with PIK3CAm was the highest (HR, 8.181; 95% CI, 2.780 to 24.075; p=0.001) (Fig. 2B). However, the median PFS after first-line treatment with anti-HER2 mAb was not influenced by ER positivity (HR, 0.761; p=0.577) (S3A Fig.). The risk of progression was higher in patients with PIK3CAm, regardless of ER status (S3B Fig.).
Progression-free survival (PFS) in patients who received human epidermal growth factor receptor 2 (HER2)–targeted therapy according to PIK3CA status. (A) PFS of palliative first-line anti-HER2 monoclonal antibody (mAb). (B) PFS according to single/dual anti-HER2 mAb and PIK3CA status. (C) PFS of trastuzumab emtansine (T-DM1). (D) PFS of lapatinib and capecitabine; CI, confidence interval; HR, hazard ratio; PIK3CAm, mutated PIK3CA; PIK3CAw, wild-type PIK3CA; Tx, therapy.
The PFS for T-DM1, which is usually prescribed as the second-line treatment after anti-HER2 mAb treatment, also significantly differed between patients with PIK3CAw and those with PIK3CAm (3.5 months vs. 11.8 months; HR, 3.018; 95% CI, 1.054 to 8.644; p=0.040) (Fig. 2C). The median PFS of lapatinib, usually prescribed as a later line of treatment combined with capecitabine, showed no differences between the two groups (7.8 months vs. 6.2 months; HR, 1.189; 95% CI, 0.247 to 5.732; p=0.767) (Fig. 2D). There were four patients who received lapatinib earlier than T-DM1 (3 patients with PIK3CAm and one with PIK3CAw). The average PFS of lapatinib and T-DM of PIK3CAm patients were 19.5 months and 2.1 months, respectively.
4. Overall survival
Although the data were often censored, the median OS of patients without the PIK3CA mutation was 135.9 months and the median OS of patients with the PIK3CA mutation was 137.1 months, and this difference was not statistically significant (HR, 1.755; 95% CI, 0.553 to 5.571; p=0.340) (S4A Fig.). When we compared the OS of patients with PIK3CA mutations in different regions, including the helical domain (E542X or E545X), kinase domain (H1047X), and other minor sites, as well as of patients with the wild-type PIK3CA, the median OS in patients with mutations in the helical domain was the shortest (HR, 84.368; 95% CI, 6.031 to 1,191.384; p=0.006) (S4B Fig.).
5. Mutational landscape of HER2+ breast cancer according to the PIK3CA genotype
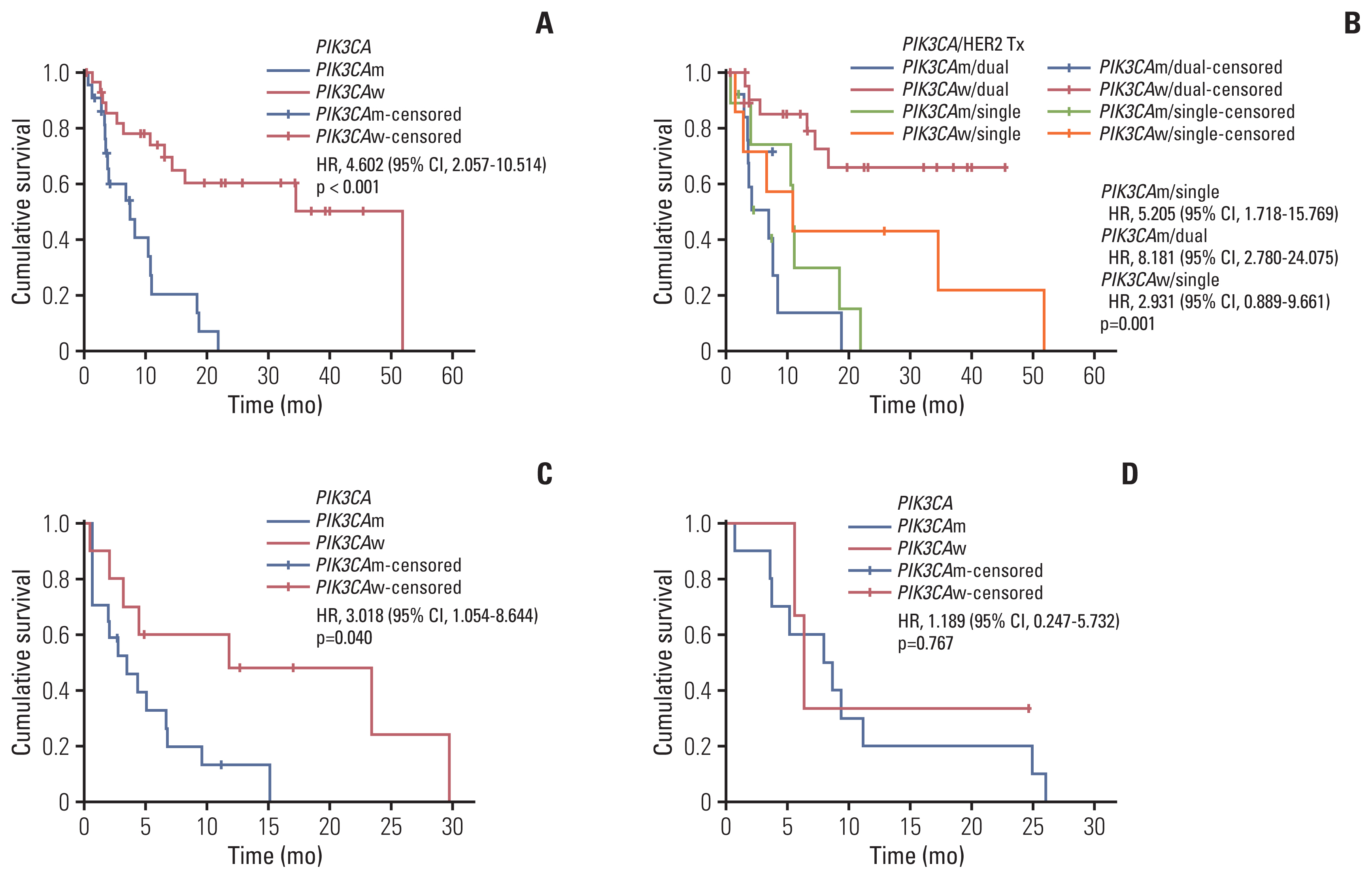
We analyzed the mutation profiles of available tumor tissues by targeted sequencing using a cancer panel (Fig. 3). Among the 34 patients with PIK3CAm, TP53 was the most frequently co-occurring pathogenic mutation (74%), follow-ed by ERBB2 (35%), and MSH3 (29%) (Fig. 3A). ALK and BRCA2 mutations co-occurred with PIK3CAm in 24% and 21% of patients, respectively, some of which are known to be clinically pathogenic. BRCA1 was not among the top 20 mutated genes in the PIK3CAm group. In the 56 patients with PIK3CAw, alterations in TP53, NOTCH1, and ERBB2 were frequently observed (58%, 28%, and 26%, respectively) (Fig. 3B). BRCA2 and BRCA1 mutations were identified in 19% and 17% of the patients with PIK3CAw, respectively.
Tumor mutational landscape of breast cancer according to PIK3CA status. Heatmap visualization of gene alterations analyzed using next-generation sequencing. The 20 most frequently mutated genes are shown in each figure. (A) Tumor mutational landscape of breast cancer with the PIK3CA mutation. (B) Tumor mutational landscape of breast cancer with wild-type PIK3CA. ER, estrogen receptor; SNV, single-nucleotide variant.
We compared the mutational landscapes in tumor tissues of patients with different tumor statuses. A total of 67 patients whose primary tumor tissue was sequenced and 23 patients who underwent NGS at their recurrence time points were included in the analysis (Fig. 4). Fig. 4A shows the mutational landscape of the primary HER2+ cancer cells. TP53 was the most frequently mutated gene in primary tumor tissue (58%), followed by PIK3CA (33%) and MSH3 (30%). Upon sequencing the patients’ recurrent tumor tissue or blood cfDNA, TP53, ERBB2, and NOTCH1 were revealed to be the three most frequently altered genes (78%, 52%, and 48%, respectively), whereas mutations in PIK3CA were determined to be the fourth most common set of genetic mutations (Fig. 4B).
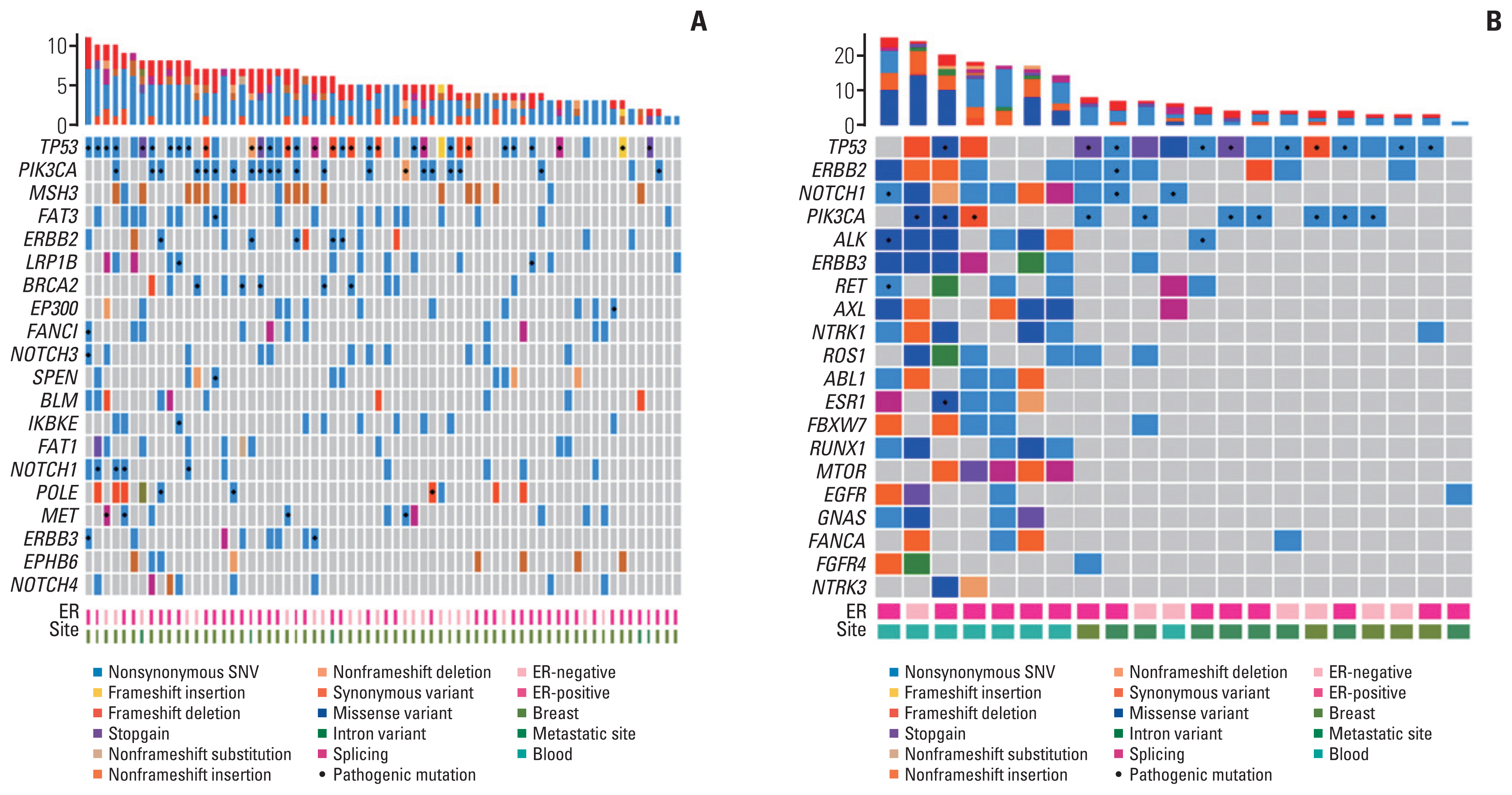
Tumor mutational landscape of breast cancer according to primary or recurrent status. Heatmap of somatic alterations analyzed using next-generation sequencing. The 20 most frequently mutated genes are shown in each figure. (A) Tumor mutational landscape of primary breast cancer. (B) Tumor mutational landscape of recurrent breast cancer. ER, estrogen receptor; SNV, single-nucleotide variant.
6. Association between the number of variants and anti-HER2 mAb treatment duration
We examined the sequencing results in the context of the anti-HER2 treatment duration (Fig. 5). A total of 45 patients who underwent palliative first-line anti-HER2 mAb treatment and whose tumor tissues were available for NGS were included in the analysis. The median number of reported SNVs was 16 (range, 5 to 27) and the median number of pathologic SNVs was 3 (range, 0 to 6). The PFS of palliative first-line anti-HER2 mAb therapy was compared between subgroups with the median number of SNVs or pathological SNVs. The median PFS was 11.2 months (standard deviation [SD], 15.4; range, 0.5 to 51.8) in patients with fewer numbers of SNVs and 10.8 months (SD, 12.0; range, 0.7 to 39.9) in patients with more numbers of SNVs (p=0.720). Among the patients with pathologic SNVs, patients with more pathologic variants showed shorter PFS (mean PFS [mPFS], 8.7 months; SD, 11.0; range, 2.7 to 39.9) than patients with fewer pathologic variants (mPFS, 14.4 months; SD, 15.4; range, 0.5 to 51.8), but the difference was not statistically significant (p=0.097).
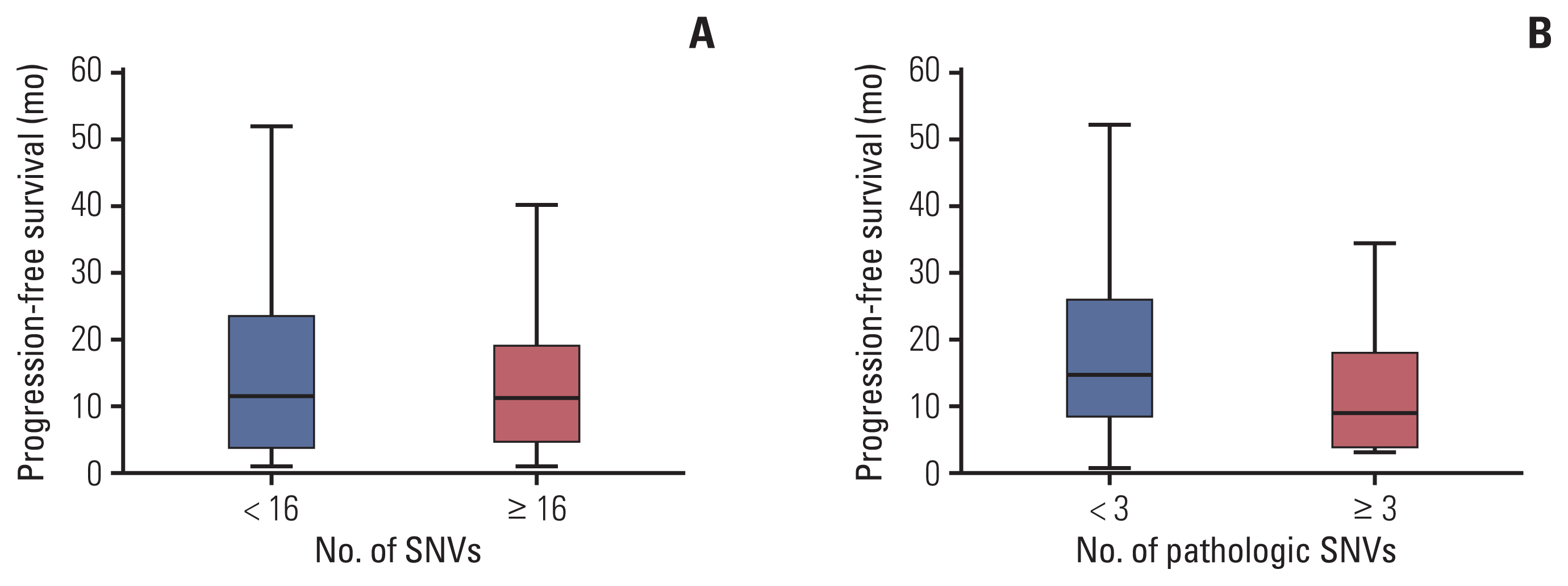
Progression-free survival (PFS) with anti–human epidermal growth factor receptor 2 (HER2) monoclonal antibody (mAb) therapy according to the number of pathologic variants. PFS distribution with palliative first-line anti-HER2 mAb by subgroup according to the number of pathologic single-nucleotide variants (SNVs). Patients with fewer pathologic variants tended to show longer PFS than those with more pathologic SNVs. (A) PFS according to the number of SNVs. (B) PFS according to the number of pathologic SNVs.
Discussion
In this study, we retrospectively analyzed treatment duration and clinical response to HER2-directed treatment based on PIK3CA mutations in patients with HER2+ breast cancer. Patients with pathogenic PIK3CA mutations had lower pCR rates after neoadjuvant therapy and significantly shorter PFS with anti-HER2 monoclonal antibody therapy or ADC than those with PIK3CAw in a palliative setting. The PFS with lapatinib was similar in both groups. In addition, clinically targeted sequencing revealed that TP53 was the most frequent co-occurring mutation in HER2+/PIK3CAm breast cancer tissues. Patients whose tumor tissues had a higher number of pathological SNVs tended to show shorter PFS with palliative anti-HER2 mAb treatment.
Anti-HER2 mAbs such as trastuzumab and pertuzuma have several antitumor effects, including the inhibition of signal transduction through HER, induction of antibody-dependent cellular cytotoxicity in tumor cells with HER2 amplification [16], and inhibition of HER2 extracellular domain cleavage [17]. Most importantly, the downregulation of intracellular signaling through the PI3K/AKT/mTOR pathway [18] is the main cellular event after the binding of trastuzumab to domain IV or of pertuzumab to domain II of HER2. However, constitutive activation of the PI3K/AKT/mTOR pathway is known to drive resistance to HER2-directed therapy, and pathologic mutations in PIK3CA are found in approximately 23%–30% of patients with HER2+ breast cancer [19].
The significance of PIK3CAm in primary early-stage breast cancer has been addressed in several prospective clinical trials. In a pooled analysis of 967 patients who were undergoing chemotherapy and received neoadjuvant treatment with either trastuzumab or lapatinib or both [13], the pCR rate was significantly lower in the PIK3CAm group than in the wild-type group (16.2% vs. 29.6%; p < 0.001). In the NeoALTTO trial, which compared the efficacy of dual therapy with trastuzumab and lapatinib to lapatinib alone, patients with PIK3CAm had poorer outcomes in all treatment groups (pCR rate: 28.6% vs. 53.1%, p=0.012) [20]. Similar results were reported in the TBCRC006 trial, which evaluated neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy, and the pCR rate was significantly lower in patients with PIK3CA mutations or low PTEN levels (4% vs. 39%, p=0.006) [21]. In the era of dual blockade using trastuzumab and pertuzumab, a retrospective analysis of the NeoSphere study showed an inferior pCR rate in patients with HER2+/PIK3CAm tumors [22]. Our data from Korean patients support these previous findings, suggesting that patients with PIK3CAm have significant unmet needs despite receiving dual HER2-directed neoadjuvant treatment as the standard of care.
In a retrospective analysis of adjuvant trastuzumab treatment in combination with chemotherapy, the presence of PIK3CAm did not change the prognosis of patients who participated in the ShortHER or FinHER trials [23,24], with a 5-year disease-free survival (DFS) rate of 90.6% for PIK3CAm and 86.2% for PIK3CAw (p=0.417) [23]. However, a previous study reported that patients who retained the initial PIK3CAm after neoadjuvant therapy had worse DFS after surgical resection, implying a possible role of PIK3CAm in predicting worse clinical outcomes in early-stage breast cancer [25]. Our study presented lower pCR rate in PIK3CAm comparing with PIK3CAw, but the iDFS of two groups showed no remarkable difference. This might be because we classified patients only based on PIK3CA status, not considering other clinical factors such as menopausal status, molecular subtype, histological grade of tumor, or adjuvant therapy. As a retrospective data with small sample size, our iDFS result should be interpreted with caution.
In contrast to the marked differences of PFS with HER2-targeted mAb or T-DM1 between groups, there were no significant differences in PFS with lapatinib in our study. This phenomenon has been demonstrated with multiple translational studies before. BioPATH study reported that PTEN deletion/downregulation or PIK3CA mutation were not predictive of clinical outcomes on lapatinib-based treatment in advanced breast cancer patients [26]. In addition, retrospective analysis from EGF20009 and EGF100151 studies found out equally beneficial effect of lapatinib in breast cancer with p95HER2, which was one of the key mechanisms of trastuzumab resistance [27]. Previous studies and our data suggest that lapatinib could be a clinically viable option for the patients with trastuzumab-resistance, PIK3CA-mutated cancer as the tyrosine kinase inhibitor effectively blocks not only HER2 but also signal through p95HER2 and epidermal growth factor receptor.
Substantial efforts have been made to identify biomarkers of resistance to anti-HER2 treatment in metastatic HER2+ breast cancer. In preclinical studies, breast cancer cell lines with BRAF, KRAS, and PIK3CA mutations were less sensitive to trastuzumab than wild-type cells [28], and drug susceptibility also differed depending on the mutation site in PIK3CA [29]. Genome-wide loss-of-function genetic screens have also shown that reduced ARID1A expression confers resistance to HER2-targeted therapies [19]. Nevertheless, treatment of patients with HER2+ breast cancer still employs a “one-size-fits-all” approach based on positive results from key trials. In a retrospective analysis of the CLEOPATRA study, PIK3CA mutation status had the greatest prognostic impact on PFS in palliative first-line therapy (docetaxel, trastuzumab, and pertuzumab) [30]. Biomarker analysis performed in clinical trials assessing T-DM1 and lapatinib in later lines of treatment led to similar findings [31,32].
Our study has several limitations, including its retrospective nature. First, the use of HER2 antibodies was switched from a single to a dual initial trastuzumab therapy combined with pertuzumab in both the early and metastatic settings. Thus, the number of patients in each treatment group was divided according to the genotype, resulting in a small sample size. Second, because of the retrospective nature of our study, we could not control for other potential prognostic factors, such as levels of the HER2 protein, HER2 and HER3 mRNA, soluble HER2, or PIK3CA genotype. In addition, access to tissue biopsies is limited in some patients with metastatic cancers. Considering that 8%–10% of patients are known to acquire PIK3CA mutations in a metastatic setting [33], our data should be interpreted with caution. Relatively short follow-up duration and lack of genetic interaction analysis should also be considered in interpretation. To build affirmative clinical evidence, a prospective study with large-scale and serial genomic data would be needed in the future.
In our study, patients with PIK3CA mutations showed weakened responses to single or dual HER2 antibodies combined with taxanes and subsequent T-DM1. Different genomic landscapes were identified according to PIK3CA status. Thus, there is a significant unmet need for these patients that may be addressed by targeting the aberrantly activated PI3K/AKT/mTOR pathway. Given the promise of the Food and Drug Administration-approved α-specific PI3K inhibitor alpelisib for the treatment of HR+/HER2− advanced-stage cancers, studies examining the clinical utility of targeting PIK3CA as well as HER2 are warranted.
In this retrospective analysis, pathogenic aberrations of PIK3CA were associated with a lower rate of pCR after neoadjuvant therapy and shorter PFS after palliative HER2-targeted therapy. Patients with activating PIK3CA mutations in HER2+ breast cancer have substantially unmet clinical needs and require more individualized therapeutic approaches.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB No. 2017AN0401), and the need for individual patient consent was waived.
Author Contributions
Conceived and designed the analysis: Kim JW, Park KH.
Collected the data: Kim JW, You JY, Lee JH, Song SE, Lee NK, Jung SP, Cho KR, Kim CY.
Contributed data or analysis tools: Kim JW, Lee JH, Song SE, Lee NK, Jung SP, Cho KR, Kim CY.
Performed the analysis: Kim JW, Lim AR, Park KH.
Wrote the paper: Kim JW, Park KH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
We thank the patients and their families, nurses, and trial coordinators, who helped with this trial.
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health &Welfare, Republic of Korea (Grant number: HI17C2206).