Real-World Study of Osimertinib in Korean Patients with Epidermal Growth Factor Receptor T790M Mutation–Positive Non–Small Cell Lung Cancer
Article information
Abstract
Purpose
Although osimertinib is the standard-of-care treatment of epidermal growth factor receptor (EGFR) T790M mutation–positive non–small cell lung cancer, real-world evidence on the efficacy of osimertinib is not enough to reflect the complexity of the entire course of treatment. Herein, we report on the use of osimertinib in patients with EGFR T790M mutation–positive non–small cell lung cancer who had previously received EGFR tyrosine kinase inhibitor (TKI) treatment in Korea.
Materials and Methods
Patients with confirmed EGFR T790M after disease progression of prior EGFR-TKI were enrolled and administered osimertinib 80 mg daily. The primary effectiveness outcome was progression-free survival, with time-to-treatment discontinuation, treatment and adverse effects leading to treatment discontinuation, and overall survival being the secondary endpoints.
Results
A total of 558 individuals were enrolled, and 55.2% had investigator-assessed responses. The median progression-free survival was 14.2 months (95% confidence interval [CI], 13.0 to 16.4), and the median time-to-treatment discontinuation was 15.0 months (95% CI, 14.1 to 15.9). The median overall survival was 36.7 months (95% CI, 30.9 to not reached). The benefit with osimertinib was consistent regardless of the age, sex, smoking history, and primary EGFR mutation subtype. However, hepatic metastases at the time of diagnosis, the presence of plasma EGFR T790M, and the shorter duration of prior EGFR-TKI treatment were poor predictors of osimertinib treatment. Ten patients (1.8%), including three with pneumonitis, had to discontinue osimertinib due to severe adverse effects.
Conclusion
Osimertinib demonstrated its clinical effectiveness and survival benefit for EGFR T790M mutation–positive in Korean patients with no new safety signals.
Introduction
Lung cancer is a leading cause of cancer-associated death worldwide [1]. Non–small cell lung cancer (NSCLC) is the most common subtype of lung cancer based on histological diagnosis, and approximately 85% of new lung cancer patients are diagnosed as NSCLC [2]. Since more than half of patients with NSCLC are diagnosed at the advanced stage, various systemic therapies have been considered, including chemotherapy, targeted therapy, and immunotherapy [1,3]. Targeted therapy based on the molecular diagnosis for specific genetic alterations has recently emerged as an effective treatment option in advanced NSCLC. Among the several molecular targets, the epidermal growth factor receptor (EGFR) was the most common treatment target [4]. Similar to other Asian populations, EGFR mutations in Korea are often accompanied by lung adenocarcinoma (overall 29% to 50%) [5].
First- and second-generation EGFR tyrosine kinase inhibitor (TKI) agents demonstrated improved progression-free survival (PFS) with tolerable side effects in NSCLC patients with EGFR mutations [6–8]. In Korea, first- and second-generation EGFR-TKI agents have been approved by the Ministry of Food and Drug Safety as first-line therapy for patients with EGFR mutation–positive adenocarcinoma [5]. However, patients receiving first- or second-generation EGFR-TKI tend to experience a disease progression after a median of 10–14 months on EGFR-TKI treatment [2,9]. Thr790Met point mutation (T790M) in exon 20 was identified in more than half of patients administrated with first- and second-generation EGFR-TKI whose disease had progressed [10].
Osimertinib was developed as a third-generation oral, irreversible EGFR-TKI agent [11]. In the AURA3 trial, osimertinib presented favorable efficacy and safety profile compared with platinum-based chemotherapy plus pemetrexed in EGFR T790M–positive patients whose disease had progressed after first-line EGFR-TKI therapy [12]. Thereafter, the ASTRIS study demonstrated real-world data pertaining to the efficacy and safety of osimertinib in EGFR T790M–positive patients with advanced NSCLC, who had previously used first- or second-generation EGFR-TKI [13]. This study revealed that the efficacy and safety profile of osimertinib were in line with the results of the AURA3 trial. Osimertinib has been approved as a second or more line setting in EGFR T790M-positive NSCLC patients since December 2017 [14]. However, there have only been a limited number of real-world studies investigating the efficacy and safety of osimertinib in the Asian population. In addition, there is no sufficient evidence regarding the clinical features associated with favorable outcomes of osimertinib.
In this study, we aimed to assess the efficacy and safety of osimertinib in EGFR T790M–positive NSCLC patients in South Korea by investigating its PFS, other treatment efficacy outcomes, and safety profile. In addition, we performed subgroup analysis to evaluate clinical characteristics related to osimertinib treatment outcomes.
Materials and Methods
1. Study design and participants
This study was a retrospective cohort study performed at 19 medical centers in South Korea. Eligible patients were NSCLC patients treated with osimertinib between January 1, 2018, and June 30, 2020. The inclusion criteria were as follows: (1) patients at least 20 years old, (2) EGFR mutation–positive locally advanced or metastatic NSCLC patients who experienced disease progression after a prior EGFR-TKI treatment, and (3) patients with confirmed presence of EGFR T790M mutation in tissue biopsy, plasma, or body fluid cytology samples. The exclusion criteria were as follows: (1) enrollment in other clinical trials, (2) inadequate medical records for review, (3) combined malignancy other than lung cancer, (4) use of third-generation EGFR-TKI agents before enrollment, (5) osimertinib used as first-line chemotherapy, and (6) no previous use of first- and second-generation EGFR-TKI agents before enrollment.
Patient information from their electronic medical records was assessed by researchers in each hospital. Data were then collected to the Asan Medical Center for data cleaning and statistical analysis. The study was approved by Asan Medical Center’s Institutional Review Board (IRB No. 2021-0802) and by each participating institution. Per their local regulations, all institutions provided approval to use the required data for the aim of this study. The requirement for informed consent was waived due to the retrospective nature of the analysis. The study protocol was registered at Clinical Research Information Service, established by the Korea Centers for Disease Control and Prevention (KCT0006341).
2. Measurements
Patients’ demographic characteristics included age, sex, smoking history, and the Eastern Cooperative Oncology Group performance score (ECOG PS). In addition, we evaluated cancer-related information, including histologic type of lung cancer, clinical stage, specimen and results of EGFR mutation test, other combined EGFR mutations, metastatic organ, and previous anticancer treatment history.
To assess the efficacy and safety of osimertinib, we analyzed the following primary and secondary outcomes and adverse events of osimertinib. The primary outcome of this study was PFS. We evaluated PFS from the first day of osimertinib treatment to disease progression or death. Researchers evaluated the status of disease progression based on the Response Evaluation Criteria in Solid Tumors, ver. 1.1. In addition, we compared the PFS between subgroups delineated by various baseline characteristics. We investigated and defined the secondary outcomes as follows: overall survival (OS), the time from initiation of osimertinib treatment to death from any cause; time-to-treat discontinuation (TTD), the time from initiation of osimertinib treatment to discontinuation due to any cause; best overall response, the percentage of patients with a best reported response during study periods; overall response rate (ORR), the percentage of patients who had at least one complete response (CR) or partial response (PR) prior to any evidence of progression; and duration of response (DoR), the time from the first documentation of objective tumor response (CR or PR) to the first documentation of objective tumor progression or death due to any cause, whichever came first. Furthermore, we evaluated potential adverse effects that led to dose reduction or discontinuation of osimertinib.
3. Statistical analysis
We presented data as median and 95% confidence interval (CI) for the continuous variables and as number and percentages for the categorical variables. We used Kaplan-Meier methods with log-rank test for survival analysis and to estimate the median time to event, including 95% CI. All tests of significance were two sided. We considered p-values of < 0.05 to be indicative of statistical significance. The data cutoff date was November 7, 2021. Statistical analyses were performed using SAS software ver. 9.4 (SAS Institute Inc., Cary, NC).
Results
1. Demographic characteristics of the study population
Eligibility screening identified 617 with EGFR T790M-positive NSCLC patients treated with osimertinib between January 1, 2018, and June 30, 2020. Of them, 59 patients were not included in this study in accordance with our exclusion criteria (S1 Fig.). Median follow-up duration of enrolled patients was 623.5 days (95% CI, 627.9 to 684.2). The demographic and clinical characteristics of the 558 eligible patients are shown in Table 1. The median age was 65 years old, and 336 patients (60.2%) were female. More than 70% of patients had no history of smoking. Of the 496 patients available for ECOG performance status, ECOG PS 0, 1, and ≥ 2 were 21.9%, 59.1%, and 7.9%, respectively.
Furthermore, 26.3% of patients relapsed after surgery, and patients who had central nervous system (CNS) metastases at the time of diagnosis were 26.7%. Primary EGFR mutations were exon 19 deletion (56.2%), L858R (33.3%), and other rare EGFR mutations, including L861Q, S768I, G719X, and exon 20 insertion. All study populations had prior EGFR-TKI treatment before study enrollment. The commonly prescribed EGFR-TKI agents were in order of gefitinib (57.9%), afatinib (31.0%), and erlotinib (17.9%). Presence of EGFR T790M mutation was confirmed in all patients by tissue biopsy (63.4%), plasma (26.7%), or cytology and other samples (15.4%). Finally, 38.5% of the study population was alive at the data cutoff date, although 119 patients were lost to follow-up.
2. Treatment efficacy of osimertinib
By the data cutoff date, 415 individuals (74.4%) had already experienced disease progression or death after receiving osimertinib. The median time for PFS, the primary outcome in this study, was 14.2 months (95% CI, 13.0 to 16.4) (Fig. 1A). The PFS of osimertinib did not differ according to age, sex, smoking history, and primary EGFR mutation subtype. Fig. 2 demonstrates differences in PFS according to clinical characteristics. The presence of plasma EGFR T790M (11.0 months vs. 16.6 months, p < 0.001), hepatic metastasis (7.7 months vs. 14.7 months, p < 0.001), and shorter use (less than 8 months) of prior EGFR-TKI (9.1 months vs. 16.4 months, p < 0.001) were associated with worse PFS with osimertinib.
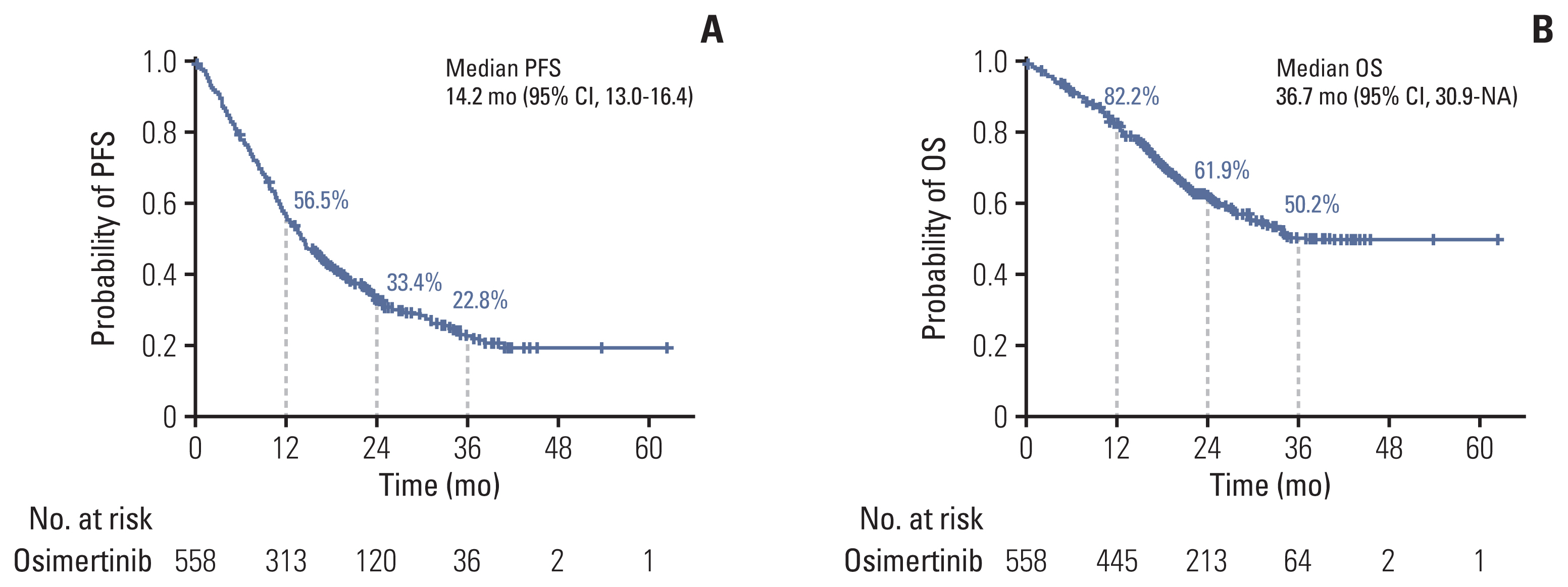
Progression-free survival (PFS) (A) and overall survival (OS) (B) in patients treated with osimertinib. We illustrated the median PFS and OS with 95% confidence intervals (CI) in patients treated with osimertinib. NA, not available.
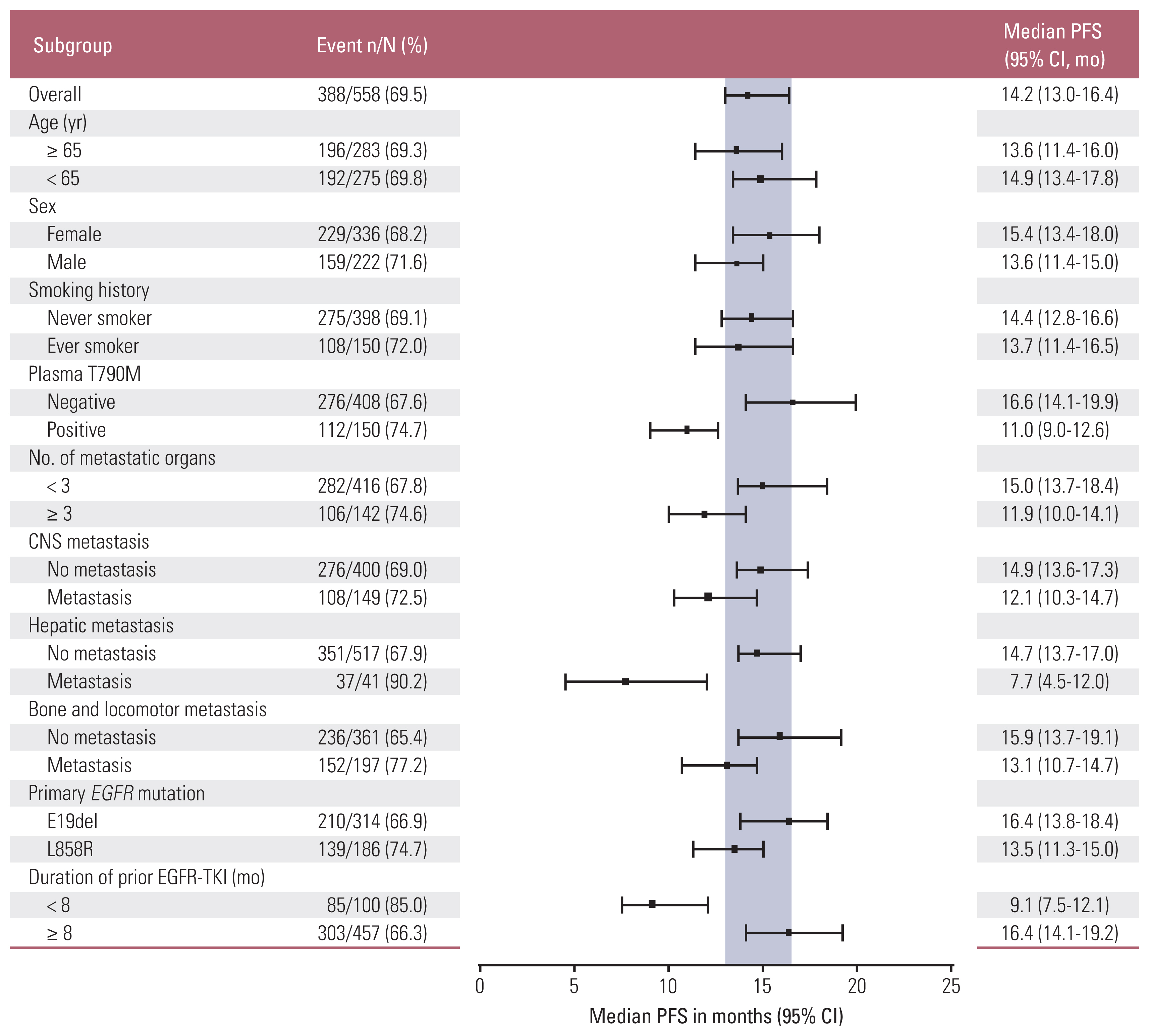
Forest plots of progression-free survival (PFS) according to subgroups. We presented the median PFS with 95% confidence interval (CI) in patients treated with osimertinib according to subgroups. Positive blood EGFR (epidermal growth factor receptor) T790M mutation test results, concurrent hepatic metastasis at diagnosis, and use of prior epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) agents for a period of less than 8 months were related to shorter PFS. CNS, central nervous system.
The median OS was 36.7 months (95% CI, 30.9 to not reached) (Fig. 1B). The estimated survival rates at 12, 24, and 36 months were 82.2%, 61.9%, and 50.2%, respectively. In subgroup analysis, the OS showed no differences according to age, sex, smoking history, and primary EGFR mutation subtype (S2 Fig.). In contrast, patients with multiple organ metastases (≥ 3 organs), CNS metastases, hepatic metastases, and bone metastases at diagnosis showed worse survival outcomes compared to patients without such metastases (Fig. 3). Presence of plasma EGFR T790M and shorter use (less than 8 months) of prior use of EGFR-TKI agents were also found to be poor prognostic factors (Fig. 3).

Overall survival (OS) in enrolled patients treated with osimertinib according to subgroups. We presented the median OS with 95% confidence interval (CI) in enrolled patients treated with osimertinib. We compared OS according to subgroups classified by the number of metastatic organs at diagnosis (A), concurrent central nervous system (CNS) (B), hepatic (C), bone and locomotor metastasis (D) at diagnosis, presence of plasma EGFR (epidermal growth factor receptor tyrosine) T790M mutation (E), and duration of prior epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) agents (F). All factors were related to considerable differences in OS. NA, not available.
The ORR was 55.2% among the 539 investigated patients. The median DoR was 12.1 months (95% CI, 10.9 to 13.2). At the time of data cutoff, 415 patients (74.4%) had discontinued treatment, with a median TTD of 15.0 months (95% CI, 14.1 to 15.9). Patients who did not have CNS metastases benefited from longer TTD with osimertinib compared to those with CNS metastases at diagnosis (15.9 months [95% CI, 14.8 to 17.0] vs. 12.5 months [95% CI, 11.0 to 14.0]) (Table 2).
3. Safety profile of osimertinib
Adverse events leading to dose reduction and treatment discontinuation during osimertinib treatment are shown in Table 3. A total of 24 patients (4.3%) required dose reduction or discontinuation of osimertinib during the study periods. Diarrhea (0.9%) was the most common adverse effect, followed by general weakness (0.7%) and hematologic toxicity (0.7%). Ten patients (1.8%) had to discontinue osimertinib due to severe adverse effects. Among them, drug-induced pneumonitis was the most common cause of discontinuation, which led to discontinuation in three patients, followed by general weakness and edema.
4. Treatment pattern of osimertinib
Table 4 shows the treatment pattern of osimertinib. A total of 37 patients (6.6%) needed dose adjustment during the treatment, and more than half of these patients finally halted the use of osimertinib. Thirteen patients (2.3%) maintained a dose of 40 mg once daily and five increased to a standard dose after adaptation. At the data cutoff date, 340 patients (60.8%) had experienced disease progression and 143 (25.6%) were still on osimertinib.
Discussion
The introduction of EGFR-TKIs has redefined treatment of EGFR mutation–positive NSCLC patients throughout the past decade. Osimertinib is the current standard-of-care treatment for EGFR T790M–positive advanced NSCLC pati-ents following progression on a prior EGFR-TKI. This real-world experience identified a group of patients that may benefit from osimertinib treatment, as well as survival data that reflected the entire course of treatment in EGFR mutation–positive NSCLC patients.
In this study, a PFS of 14.2 months was noted, which is longer than the PFS in the ASTRIS study (11.1 months) [13] or the ASTRIS study Korean subgroup analysis (12.4 months) [3]. This long PFS induced an OS benefit; thus, an OS of 36.7 months in this study was significantly longer compared to the OS reported in the AURA3 study (26.8 months) [12]. This longer PFS and OS might result from the difference in study populations between this study and AURA3. This study had more Asians (100% vs. 65%), less presence of plasma EGFR T790M (26.7% vs. 46.4%), and fewer brain metastases (26.7% vs. 34.4%) than the AURA3 study; notably, all of these factors are known to be associated with osimertinib efficacy [3,15,16]. In addition, this study showed longer median TTD of osimertinib than the AURA3 study (15.0 months vs. 8.1 months). TTD has been associated with PFS and OS in patients receiving molecular-targeted therapies [17]. In clinical practice, EGFR-TKIs, which are well tolerated and induce dramatic responses, can be occasionally maintained beyond Response Evaluation Criteria in Solid Tumor–defined progression. Performing EGFR-TKI maintenance, in particular, along with local consolidative therapy showed clinical benefits in some populations such as oligoprogression [18]. Therefore, Pan-Asian clinical practice guidelines suggest continuing molecular-targeted therapy with a radical local treatment in patients with driver mutations with oligoprogression [19].
The PFS of osimertinib was consistent regardless of age, sex, smoking history, and primary EGFR mutation subtype. The CIs overlapped within these subgroups. In contrast, we observed that patients with extrathoracic metastases at the time of diagnosis showed shorter PFS than those without metastases. In particular, the PFS of patients with hepatic metastases at diagnosis was only 7.7 months, which resulted in the highest hazard ratio (2.36) in OS among the subgroups. Hepatic metastasis has been known to be an independent poor prognostic factor in EGFR-mutant NSCLC patients receiving EGFR-TKI [20].
Plasma EGFR T790M–positive patients also had shorter PFS (11.0 [9.0–12.6] months vs. 16.6 [14.1–19.9] months) than plasma EGFR T790M–negative patients. The shorter PFS observed in plasma EGFR T790M–positive patients was generally consistent with that observed in the ASTRIS (9.7 months; 95% CI, 8.6 to 10.3) and AURA3 (8.2 months; 95% CI, 6.8 to 9.7) studies [12]. Plasma EGFR T790M positivity may reflect the increase in tumor burden and metastasis [21]. Indeed, this study showed that plasma EGFR T790M–positive patients often had three or more multi-organ metastases at the time of diagnosis (37.3% vs. 21.1%, p < 0.001) and had more CNS metastases compared to negative patients (35.4% vs. 24.1%, p=0.009). In addition, shorter treatment duration of prior EGFR-TKI is related to shorter PFS of osimertinib. When compared with the period of prior EGFR-TKI treatment, patients who were treated for less than 8 months with first- or second-generation EGFR-TKI had a significantly shorter PFS (9.1 months; 95% CI, 7.5 to 12.1) than patients treated for a period longer than 8 months (16.4 months; 95% CI, 14.1 to 19.2). This difference was the largest numerical between-group difference in the PFS period. Earlier development of resistance to first- and second-generation EGFR-TKI suggests the possibility of harboring other concurrent alterations in addition to T790M, which could lead to the early development of resistance to osimertinib as well. Among several studies, Yu et al. [22] reported that co-occurring mutations other than EGFR were frequently observed in patients with short PFS, suggesting that this could be a major factor that affects resistance to EGFR-TKI therapy [23]. Preliminary data from the FLAURA study revealed that first-line osimertinib resistance mechanisms triggered by less frequent alterations are similar to those observed in patients who have resistance to other first- and second-generation EGFR-TKIs [24].
Despite the longer PFS of osimertinib compared to platinum therapy plus pemetrexed after first-line EGFR-TKI in the AURA3 trial, the PFS benefit with osimertinib did not result in a concurrent OS benefit [12]. In this study, some patients treated with multiple lines of therapies (35.5% of patients received osimertinib as the third-line therapy or over) were included. Therefore, caution is needed when interpreting the OS advantage of osimertinib. However, the ultimate goal of EGFR-mutant NSCLC treatment is to achieve long survival using multiple available treatment modalities. Although osimertinib is currently available, its use in clinical application as a first-line treatment in South Korea is limited because it is not reimbursed. Consequently, in cases where chemotherapy is usually considered the next treatment option after osimertinib, sequential use of EGFR-TKI might be an effective strategy for maximizing chemo-free treatment in some populations to obtain long OS benefits [25]. Thus, clinicians need to investigate treatment strategies that can show the best clinical results based on the characteristics of each patient.
With respect to the adverse events noted in this study, 4.3% of patients had adverse events of any grade, and only 1.8% of patients had severe adverse events that led to discontinuation. Pneumonitis was observed in only three patients from a total of 558 (0.5%). None of the patients showed cardiac toxicity leading to dose reduction or discontinuation, one of the most fatal adverse effects observed in other studies [13,26,27]. Regarding the frequency of adverse events, our study did not find any new safety signals that had not been previously reported. However, caution is necessary when interpreting this finding, especially considering the limitations of a real-world study. In addition to the fact that adverse events reporting was not based on protocol-specified adverse events, a limitation of this study was that, similar to other real-world studies, the treatment responses were assessed according to investigators.
In terms of the aim of the study, only T790M-positive patients after a prior EGFR-TKI treatment were included in this study. Approximately half of the patients showed a resistance mechanism other than EGFR T790M, and some patients did not survive before receiving a subsequent treatment following first- or second-generation EGFR-TKI; thus, these populations do not benefit from the subsequent osimertinib treatment. Consequently, it is essential to identify the characteristics of these patients and determine an appropriate population for upfront or sequential treatment of osimertinib to improve efficacy of the entire treatment.
To the best of our knowledge, this study is the largest real-world experience of osimertinib in EGFR T790M–positive advanced NSCLC patients reporting OS benefits in Korean patients. In the future, in addition to EGFR T790M, identification of co-occurring alterations and determination of appropriate therapies based on the respective molecular-resistance profiles should be considered to maximize the entire duration of treatment in EGFR mutation–positive lung cancer.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study was approved by the Asan Medical Central Institutional Review Board (IRB No. 2021-0802) and also approved by each participating institution. Per their local regulations, all institutions provided approval to use the required data for the aim of this study. The requirement for informed consent was waived due to the retrospective nature of the analysis.
Author Contributions
Conceived and designed the analysis: Lee JH, Kim EY, Park CK (Cheol-Kyu Park), Lee SY (Shin Yup Lee), Lee MK, Yoon SH, Lee JE, Lee SH (Sang Hoon Lee), Kim SJ, Lee SY (Sung Yong Lee), Lim JH, Jang TW, Jang SH, Lee KY, Lee SH (Seung Hyeun Lee), Yang SH, Park DW, Park CK (Chan Kwon Park), Kang HS, Yeo CD, Choi CM, Lee JC.
Collected the data: Lee JH, Kim EY, Park CK (Cheol-Kyu Park), Lee SY (Shin Yup Lee), Lee MK, Yoon SH, Lee JE, Lee SH (Sang Hoon Lee), Kim SJ, Lee SY (Sung Yong Lee), Lim JH, Jang TW, Jang SH, Lee KY, Lee SH (Seung Hyeun Lee), Yang SH, Park DW, Park CK (Chan Kwon Park), Kang HS, Yeo CD, Choi CM, Lee JC.
Contributed data or analysis tools: Lee JH, Kim EY, Choi CM, Lee JC.
Performed the analysis: Lee JH, Kim EY, Choi CM, Lee JC.
Wrote the paper: Lee JH, Kim EY, Choi CM, Lee JC.
Reviewed and edited the paper: Lee JH, Kim EY, Park CK (Cheol-Kyu Park), Lee SY (Shin Yup Lee), Lee MK, Yoon SH, Lee JE, Lee SH (Sang Hoon Lee), Kim SJ, Lee SY (Sung Yong Lee), Lim JH, Jang TW, Jang SH, Lee KY, Lee SH (Seung Hyeun Lee), Yang SH, Park DW, Park CK (Chan Kwon Park), Kang HS, Yeo CD, Choi CM, Lee JC.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This research was conducted with support from an Investigator Sponsored Study Program of AstraZeneca (ESR-20-20804) and in part by Korea Medical Device Development Fund (KMDF) (Grant No. 202011B13). There was no additional conflict of interest to declare for any authors.




