Role of Local Treatment for Oligometastasis: A Comparability-Based Meta-Analysis
Article information
Abstract
Purpose
We intend to investigate the oncological efficacy and feasibility of local consolidative therapy (LCT) through a meta-analysis method.
Materials and Methods
Four databases including PubMed, MEDLINE, Embase, and Cochrane library were searched. Target studies are controlled trials comparing outcomes of LCT versus a control group. Primary endpoints are overall survival (OS) and progression-free survival (PFS).
Results
A total of 54 studies involving 7,242 patients were included. Pooled analyses showed that the LCT arm could achieve improved OS with pooled odds ratio of 2.896 (95% confidence interval [CI], 2.377 to 3.528; p < 0.001). Regarding PFS, pooled analyses showed pooled odds ratio of 3.045 (95% CI, 2.356 to 3.937; p < 0.001) in favor of the LCT arm. In the subgroup analyses including the studies with reliable comparability (e.g. randomized studies or intentionally matched studies without significant favorable prognosticator in LCT arms), pooled odds ratio was 2.548 (95% CI, 1.808 to 3.591; p < 0.001) favoring the LCT arm regarding OS. Regarding PFS, pooled OR was 2.656 (95% CI, 1.713 to 4.120; p < 0.001) which also favored the LCT arm. Subgroup analyses limited to the randomized controlled trials (RCT) were also performed and pooled odds ratios on OS and PFS were 1.535 (95% CI, 1.082 to 2.177; p=0.016) and 1.668 (95% CI, 1.187 to 2.344; p=0.003). The rates of grade ≥ 3 complications related to LCT was mostly low (< 10%) and not significantly higher compared to the control arm.
Conclusion
Pooled analyses results of all included studies, selected studies with reliable comparability, and RCT’s demonstrated the survival benefit of LCT. These consistent results suggest that LCT was beneficial to the patients with oligometastasis.
Introduction
With the classic general oncologic concept, the role of aggressive local treatment in the patients with systemic metastatic lesions used to be limited in a few clinical situations. Nevertheless, the benefit of locally ablative therapy for metastatic lesion(s) and/or primary disease has recently been proposed in the patients with “oligometastasis”, which has been defined as the disease status with only a few, but not disseminated, metastatic foci [1]. Resection of limited metastatic lesions involving the lung or liver, for example, enabled favorable long-term survival outcome in significant portion of the colorectal cancer patients [2,3]. Given the advances in radiation therapy (RT) techniques capable of high-dose delivery with precise targeting, the utilization of local consolidative therapy (LCT) in oligometastatic setting has become dramatically and increasingly popular over the recent past years [4,5].
Many recently published studies have shown that improved long-term survival outcomes were achieved, by applying LCT to oligometastasis, when compared to the historic controls. Majority of these studies, however, were single arm studies or small phase 2 comparative series [6–10]. Furthermore, there was no preclinical evidence on the role of local treatment in the process of tumors undergoing the events of metastatic cascade. Therefore, it is unclear whether the improved outcomes following LCT were by virtue of the provided local therapy per se, or the bias of selecting out more favorable patients’ subgroup having better clinical conditions and indolent disease nature. In clinical practice, there is still insufficient consensus in regards to the role of additional LCT to systemic therapy or supportive care in oligometastatic setting.
In this meta-analysis, the oncologic benefit of LCT in oligometastic disease was investigated by analyzing the literatures that explored the role of LCT in terms of survival outcomes as the endpoints with their comparative groups. In particular, the clinical balancing between the LCT and control arms was taken into account in further detail.
Materials and Methods
1. Study design and eligibility criteria
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [11] were strictly observed. The population, intervention, comparison, and outcome (PICO) question of the hypothesis was as follows: “Did LCT confer an oncologic benefit (regarding overall survival [OS] and progression-free survival [PFS]) in managing the patients with oligometastasis?” The following inclusion criteria were used to include the eligible studies: (1) controlled trial involving the patients with oligometastasis that compared the outcomes of those who underwent LCT versus a control group; (2) 10 or more patients in each arm; (3) at least one primary endpoint provided; and (4) oligometastasis defined as five or fewer metastases or as the metastatic lesions that could definitely be encompassed and treated by the provided LCT.
2. Protocol registration
This study is registered in PROSPERO (protocol No. CRD42022316613).
3. Information sources and search strategy
Four databases including PubMed, MEDLINE, Embase, and Cochrane library were systematically searched, as recommended by Cochrane handbook [12], and the last date of the search was the 14th of March, 2022. Detailed searching strategy including the search terms are as shown in the Supplement Data 1. The conference abstracts and in-press studies were also searched and included if they met the inclusion criteria. No language limitation was applied. For the studies possibly having the overlapping patients’ cohort, those with the larger number of patients or those published more recently, if the number of patients are similar between competing studies, were chosen. Searching process was performed independently by two investigators (CH Rim, WK Cho) and any disagreements were resolved by discussion or re-evaluation of the databases in question.
4. Data items and collection process
The primary endpoints were OS and PFS. The incidences and types of grade 3 or higher adverse events were collected and subjectively reviewed. A pre-designed data sheet included the followings.
General information including the author, affiliation, year of publication, patient recruitment, type of study, target disease, and definition of oligometastasis.
Clinical data including the number of patients in each arm (LCT arm vs. control arm), target sites for LCT (e.g., metastatic or primary site), number of oligometastasis, treatment modality employed, OS, PFS, and adverse events of grade 3 or higher.
The survival data were acquired from the descriptive graphs if the numerical data were not provided in the articles. Data collection processes were also performed by two independent investigators (CH Rim, WK Cho) and any disagreements were resolved by re-evaluation of the literature.
5. Risk of bias and subgroup analyses
Although the current study intended to investigate on the studies that had the control arms (LCT vs. control), only few were in randomized study design, whereas majority were in retrospective ones. Possible confounders were carefully analyzed following the guidelines provided by the Cochrane group [13]. Reliable comparability was defined as either randomized controlled trials (RCT) or the studies with clinical balancing effort (e.g., propensity score matching) without any major prognosticators skewed in favor of any arm. Major prognosticators include the number of metastases, patients’ age, performance status, and TNM stage, respectively, which were common and important clinical factors across various cancer primaries. The studies were regarded as having non-reliable comparability, if any of the above prognosticators or disease-specific factors were regarded important at the authors’ discretion (e.g., prostate specific antigen in prostate cancer and or α-fetoprotein in hepatocellular carcinoma, respectively) and had favorable slant toward the LCT arm (e.g., statistically significant or > 20% difference). After the pooled analyses of all included studies, subgroup analyses were serially performed for the studies with reliable comparability and RCT’s, and RCT’s only, respectively.
Since the included studies dealt with heterogenous primary sites, subgroup analyses per primary were also subsequently performed. Subgroup analyses were performed according to hierarchical comparability and study designs, as suggested by Shin and Rim [14].
6. Quality assessment
Considering that most eligible studies were non-randomized, Newcastle-Ottawa scales of the included studies were used for the quantitative quality analyses [15]. The studies having quality scale of high (8 or 9 points) and moderate (6 or 7 points) were included in the pooled analysis, but not those with low score (5 or lower points) [13].
7. Statistics
The effect measures of primary endpoints (OS and PFS) were assessed as the odds ratio (OR) in comparison to percentile OS or PFS rates at 2-years between the LCT and control arms. 1- or 5-year rates were evaluated considering the natural courses of different primaries and histology (e.g., OS or PFS nearly nil at 2 years in small cell lung cancer [SCLC] studies; minimal OS or PFS changes within 2 years in prostate cancer studies). For pooled analyses of OR’s, the random effects model was used based on the possible heterogeneity in clinical setting and study designs, referencing the Cochrane handbook [13]. In subgroup analyses that included RCT’s only, fixed-effect model was applied if heterogeneity among the studies were regarded insignificant (p < 0.1 and I2 ≤ 50%). In addition, pooled analyses of temporal OS percentile were performed according to the primary sites, using the random effects model.
In pooled analyses, heterogeneity was assessed using the Cochrane Q test [16] and I2 statistics [17]. Studies with an I2 statistic of 25%, 50%, and 75% were regarded to have low, moderate, and high heterogeneity, respectively. Publication bias was assessed in pooled analyses including 10 or more studies, using visual funnel plot evaluation and quantitative Egger’s test [18]. If 2-tailed p-value was < 0.1 in Egger’s test and asymmetry was noted in funnel plot, Duval and Tweedie’s trim and fill methods were performed for the sensitivity analyses [19]. All the statistical analyses were performed using the Comprehensive Meta-Analysis ver. 3 (Biostat Inc., Englewood, NJ).
Results
1. Study selection and characteristics
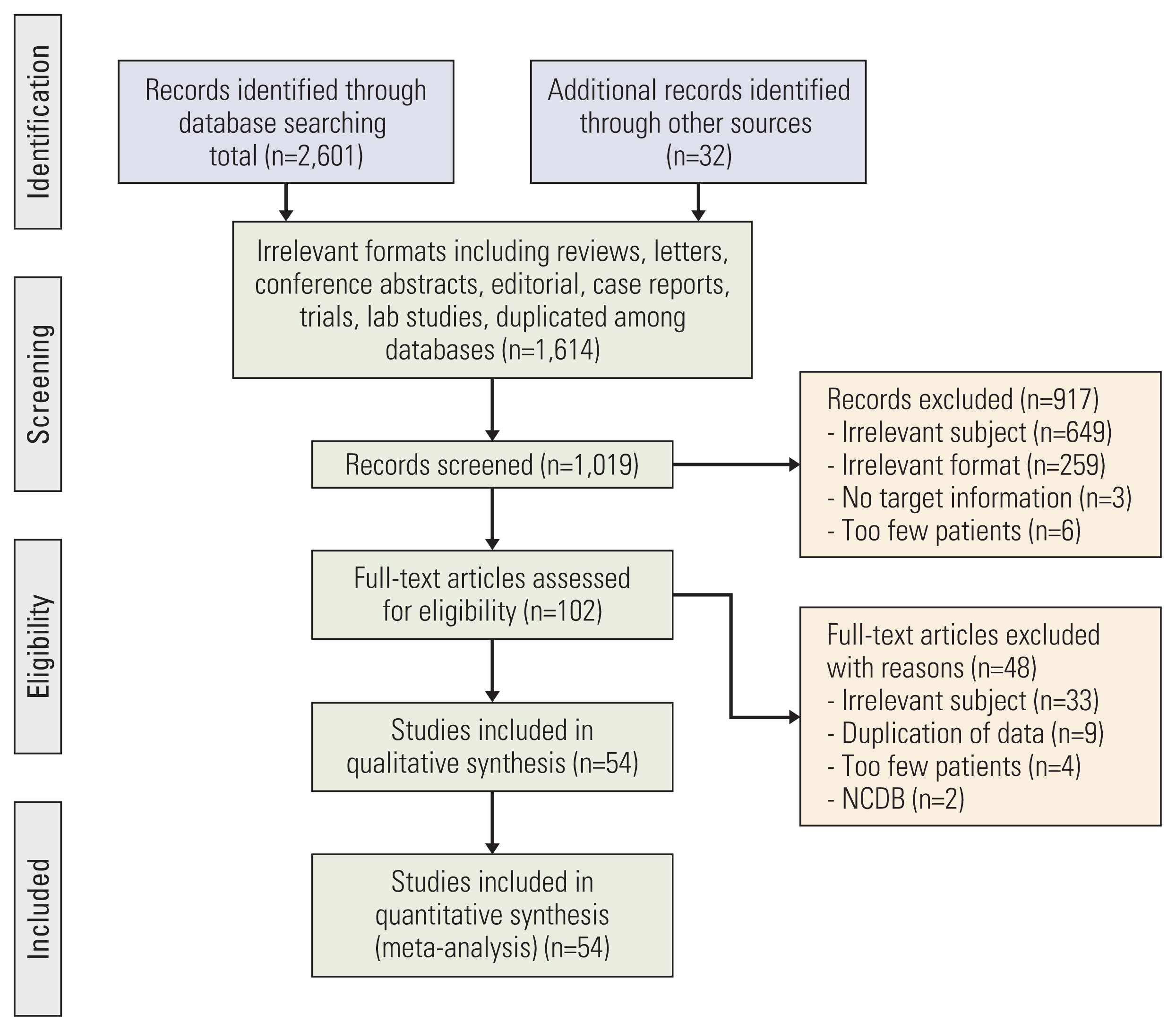
The selection process is illustrated in Fig. 1. At the initial search across the databases, a total of 2,601 studies were identified. Thirty-two studies were added from the reference lists of the searched studies. After filtering of 1,614 studies with irrelevant format or duplicates among databases, abs-tracts of 1,019 studies were screened. Full-text evaluation was performed for 102 studies, and 54 studies finally fulfilled the inclusion criteria, which comprised as the final cohort of the current study [6,7,20–71].
Regarding the study design, eight studies were prospective RCTs, whereas the remainders were retrospective series. Fourteen studies did clinical balancing effort (e.g., propensity score matching) between the LCT and control arms. Among all 54 selected studies, 26 studies (48.1%) defined oligometastases as having metastatic foci of 5 or less, four studies (7.4%) as having 4 or less, and 14 studies (25.9%) as having 3 or less, respectively. Remaining studies used various individualized clinical definitions such as “resectable” “controllable with surgery”, “within RT portal”, or “confined to a single organ”, respectively (Fig. 2A). Regarding the LCT modality, RT was performed in 42 studies (77.8%), surgery in 25 studies (46.3%), and radiofrequency ablation in 10 studies (18.5%), respectively (Fig. 2B). Twenty-two studies investigated oligometastasis of lung primary (40.7%, 20 on non-small cell lung cancer (NSCLC) plus 2 on SCLC, 10 of prostate primary (18.5%), four of colorectal primary, four of esophagus primary, three of liver primary, three of pancreatobiliary primary, and three of head and neck primary, respectively. There were three studies that focused only on single disease site: soft tissue sarcoma; renal cell carcinoma; and breast cancer, respectively. Two studies included various primaries (Fig. 2C). Further information on the included studies are summarized in S1 and S2 Tables.
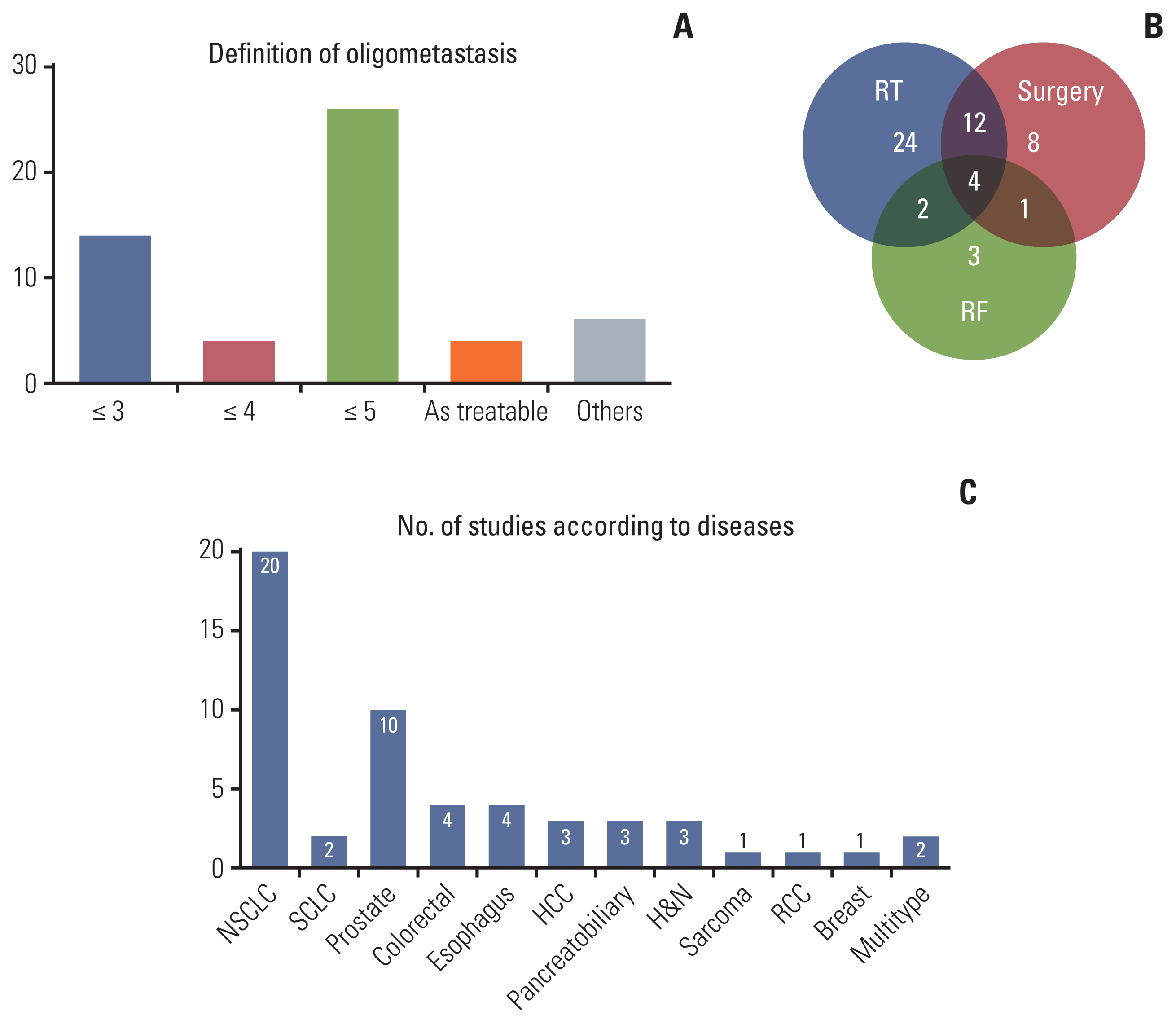
Descriptive summary of definition of oligometastases among included studies (A), modality of local consolidative therapy used in included studies (B), and number of studies included according to site of origin (C). HCC, hepatocellular carcinoma; H&N, head and neck; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; SCLC, small cell lung cancer.
2. Quality assessment
As for the selection category of Newcastle-Ottawa scale, all included studies acquired 4 points. All included studies had high representativeness as investigating a specific disease condition (oligometastases of cancers), adequate selection of non-exposed cohort (drawn from the same community), ascertainment of exposure (all studies acquired data from the secure medical records), and demonstrated the outcomes of interest (e.g., death or recurrence) not present at the initiation of study, respectively. Regarding the outcome category, majority of studies acquired 3 points as they acquired data based on the medical records and few or negligible proportion of follow-up loss. Several studies, however, were regarded as having 2 points if the duration of follow-up was less than one year. Since RCTs and studies with matched control compared at least two known clinical prognosticators, they acquired 2 points in comparability category, whereas others acquired 0 points. The resulting quality points of the selected studies were at least 6 (S3 Table), which met the pre-defined cut-off value, and all were included in the pooled analyses.
3. Synthesized results
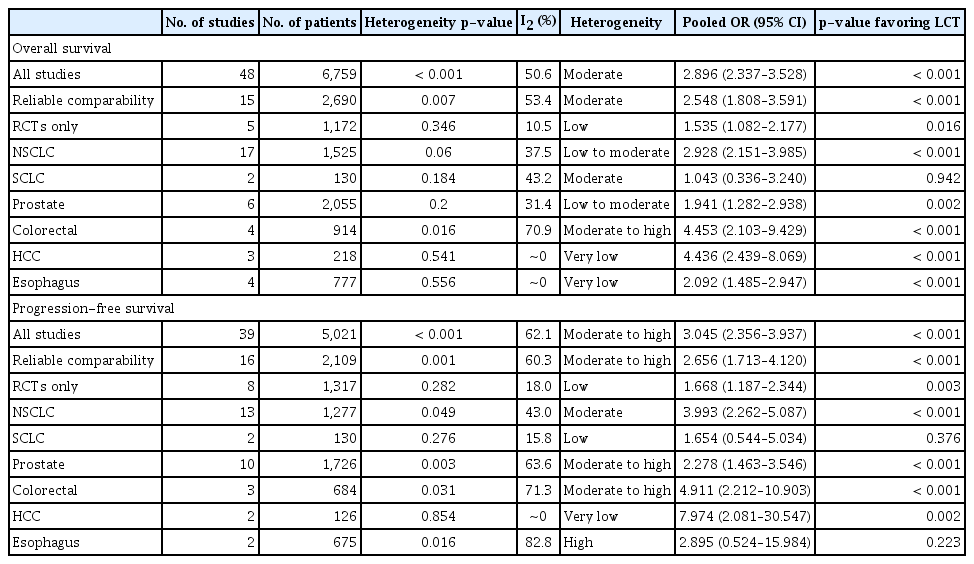
Pooled analyses of all included studies showed that the patients in the LCT arm could achieve improved OS with pooled OR of 2.896 (95% confidence interval [CI], 2.377 to 3.528; p < 0.001), with moderate heterogeneity (p < 0.001, I2=50.6%) (Table 1, Fig. 3A). Regarding PFS, pooled analyses showed pooled OR of 3.045 (95% CI, 2.356 to 3.937; p < 0.001), with moderate heterogeneity (p < 0.001, I2=62.1%) in favor of the LCT arm (Table 1, Fig. 4A).
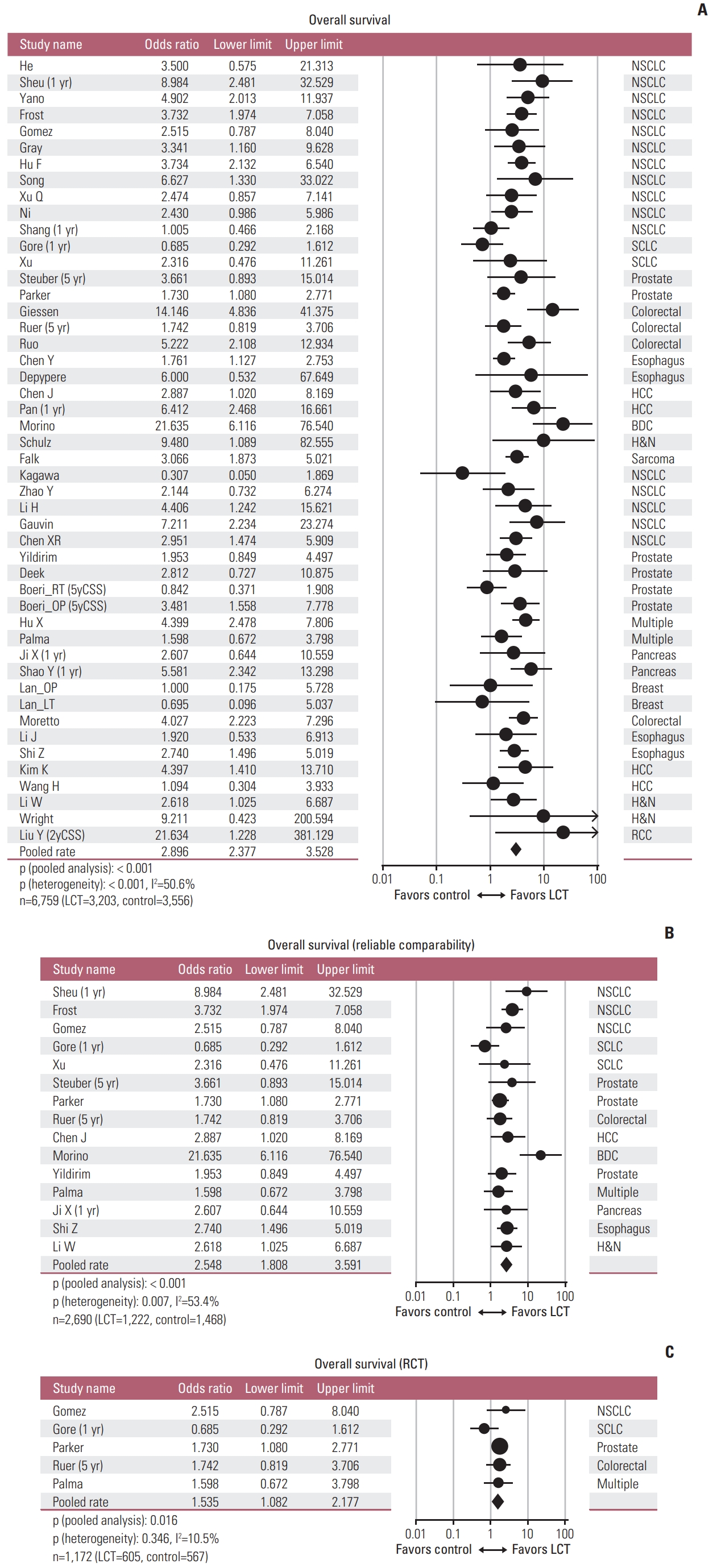
Forest plots of pooled analyses regarding overall survival, including all studies (A), studies with reliable comparability (B), and randomized controlled trials (C) [7,8,21–31,34,35,37–45,47–53,55–66,68–71,73]. BDC, biliary duct cancer; HCC, hepatocellular carcinoma; H&N, head and neck; LCT, local consolidative therapy; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; SCLC, small cell lung cancer; 2yCSS, 2-year cancer-specific survival; 5yCSS, 5-year cancer-specific survival.
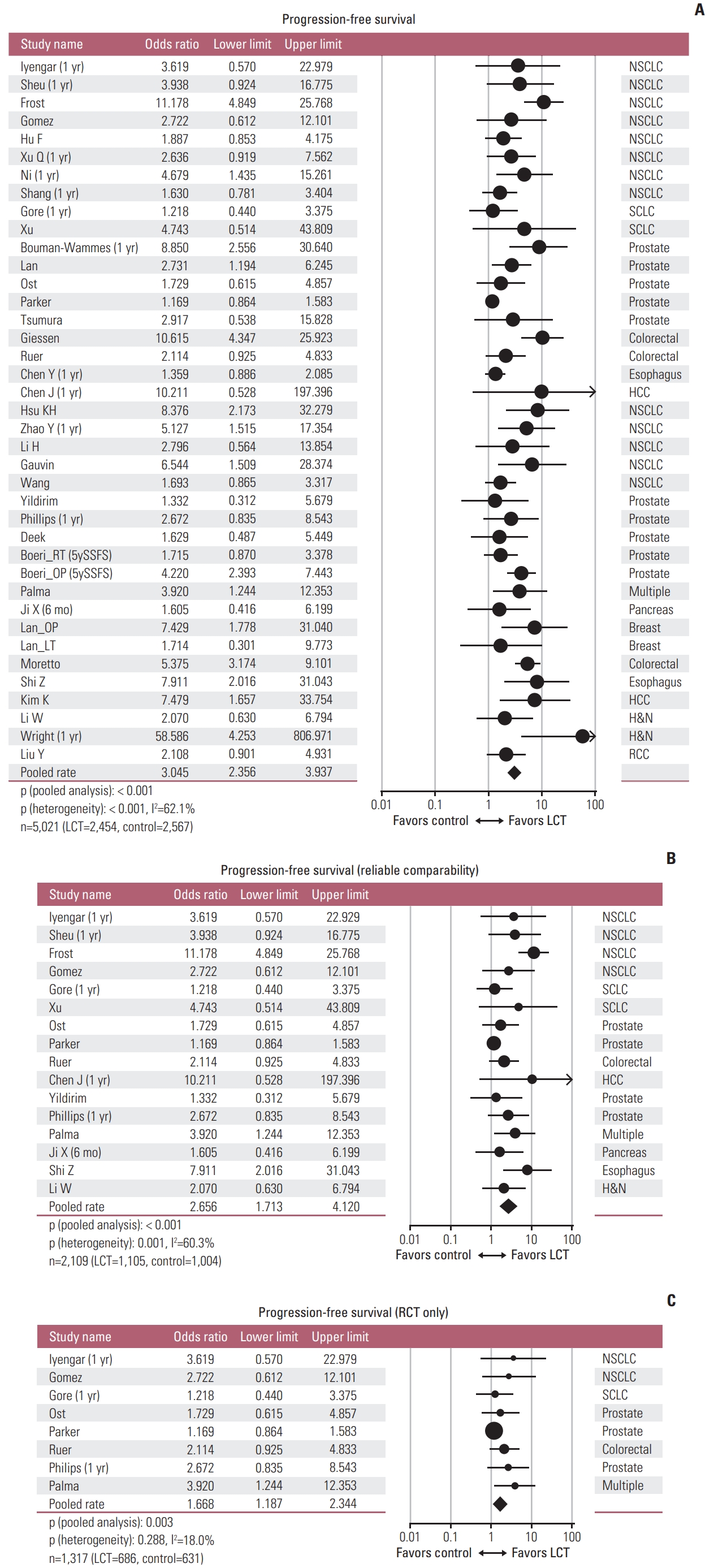
Forest plots of pooled analyses regarding progression-free survival, including all studies (A), studies with reliable comparability (B), and randomized controlled trials (C) [6–8,20–22,25–28,31–33,35,36,38,39,42,43,46–48,50,52–54,56,58–60,62–64,66,67,69–71,73]. HCC, hepatocellular carcinoma; H&N, head and neck; LCT, local consolidative therapy; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; SCLC, small cell lung cancer; 5ySSFS, 5-year second-line systemic therapy free survival.
In the subgroup analyses including the studies with reliable comparability (RCTs and intentional matched studies without known favorable prognosticator in the LCT arms), pooled OR was 2.548 (95% CI, 1.808 to 3.591; p < 0.001) favoring the LCT arm regarding OS, with moderate heterogeneity (p=0.007, I2=53.4%) (Table 1, Fig. 3B). Regarding PFS, pooled OR was 2.656 (95% CI, 1.713 to 4.120; p < 0.001) which also favored the LCT arm, with moderate to high heterogeneity among studies (p=0.001, I2=60.3%) (Table 1, Fig. 4B). Subgroup analyses limited to the RCT’s only were also performed and all favored the LCT arm: pooled ORs on OS and PFS were 1.535 (95% CI, 1.082 to 2.177; p=0.016) with low heterogeneity (p=0.346, I2=10.5%) (Fig. 3C) and 1.668 (95% CI, 1.187 to 2.344; p=0.003) with low heterogeneity (p=0.282, I2=18.0%) (Table 1, Fig. 4C), respectively.
4. Pooled survival according to the primary site
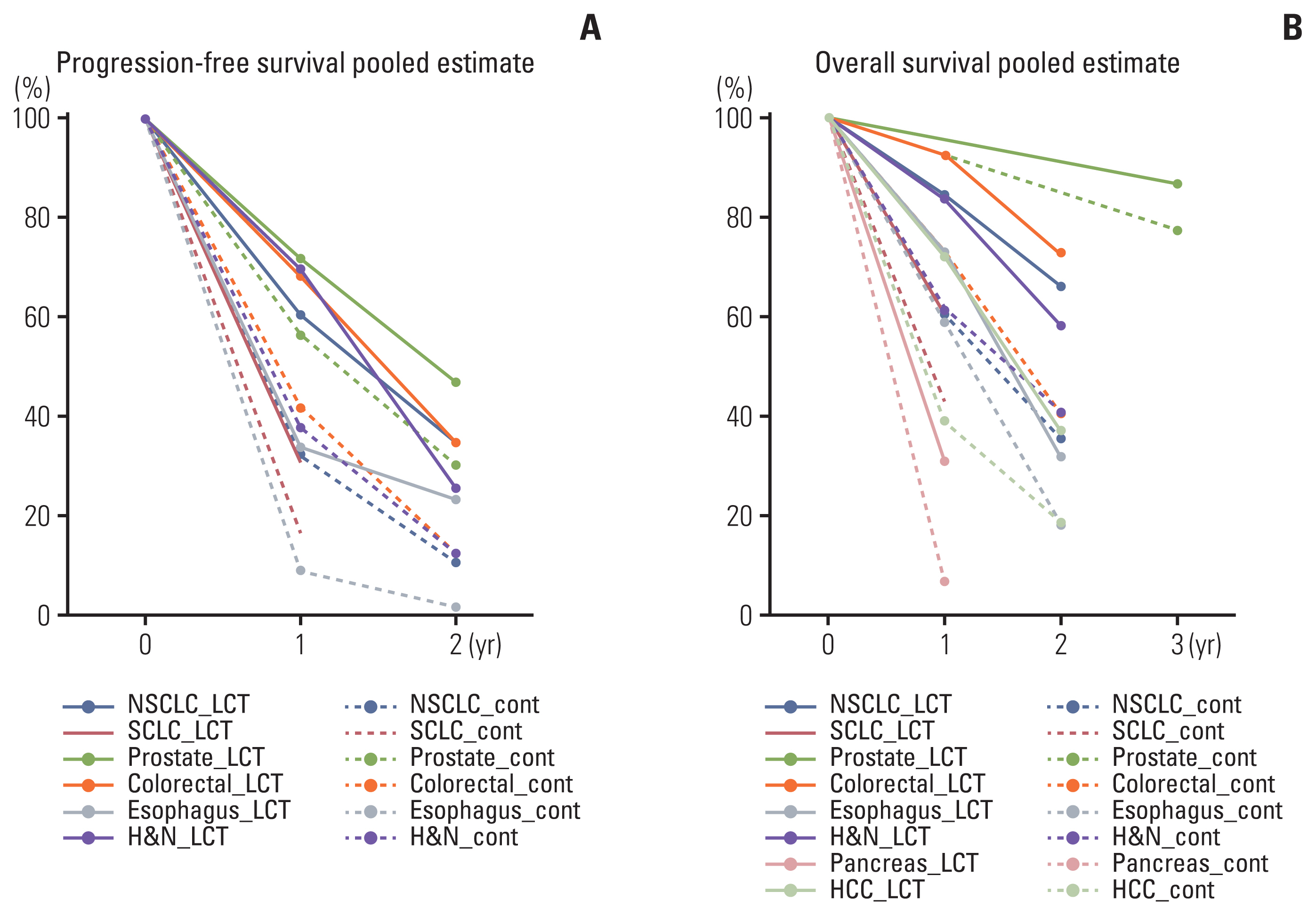
Subgroup pooled analyses were performed according to the primary sites (Table 1). Pooled OR’s for OS in NSCLC, SCLC, prostate cancer, colorectal cancer, liver cancer, and esophageal cancer were 2.928 (95% CI, 2.151 to 3.985; p < 0.001), 1.043 (95% CI, 0.336 to 3.240; p=0.942), 1.941 (95% CI, 1.282 to 2.938; p=0.002), 4.453 (95% CI, 2.103 to 9.429; p < 0.001), 4.436 (95% CI, 2.439 to 8.069; p < 0.001), and 2.092 (95% CI, 1.485 to 2.947; p < 0.001), respectively. Improved OS was achievable in the LCT arm in all disease sites except in SCLC. For PFS, pooled OR’s for NSCLC, SCLC, prostate cancer, colorectal cancer, liver cancer, and esophageal cancer were 3.993 (95% CI, 2.262 to 5.087; p < 0.001), 1.654 (95% CI, 0.554 to 5.034; p=0.376), 2.278 (95% CI, 1.463 to 3.546; p < 0.001), 4.911 (95% CI, 2.212 to 10.903), 7.974 (95% CI, 2.081 to 30.547; p=0.002), and 2.895 (95% CI, 0.524 to 15.984; p=0.223), respectively. Again, improved PFS was achievable in the LCT arm in all disease sites except SCLC. The percentile rates of OS and PFS by pooled analyses according to the primary sites are illustrated and summarized in Table 2 and Fig. 5.
5. Publication bias
Regarding OS, no significant publication bias was noted (Egger’s p=0.234). However, publication bias was highly suggested in the pooled analysis regarding PFS (Egger’s p < 0.001). The trimmed OR using Duval and Tweedie’s method was 2.278 (95% CI, 1.753 to 2.961). Funnel plots and results of quantitative Egger’s test are shown in S4 Fig.
6. Adverse events
Twenty studies (seven on lung cancer; five on prostate cancer; two on pancreas cancer; two on esophageal cancer; one on colorectal cancer; one on liver cancer; and two on various cancers, respectively) involving 2,963 patients (1,487 in the LCT arm, 1,476 in the control arm) provided comparative information on the incidences and grade of adverse events. Regarding lung cancer studies, LCT-related adverse events were relatively more frequent when compared to other primaries, with grade 3 or higher rates ranging from 8% to 28.6%. Three studies reported the possibility of excessive grade 3 or higher adverse events related to LCT [28,35,67]. Grade 5 adverse event, potentially related to the LCT, occurred in three cases among seven lung cancer studies (3 of 281, 1.07%). Regarding prostate cancer studies, grade 3 or higher adverse events related to the LCT was quite rare, and no studies reported significantly excessive adverse events related to the LCT, and grade 5 case, however, was also not reported. Palma et al. [7] reported three grade 5 adverse events among 66 patients (4.5%) following stereotactic body radiotherapy (SBRT) (radiation pneumonitis, pulmonary abscess, gastric ulcer). Ruo et al. [40] reported two out of 127 patients (1.6%) with postoperative death, and significant postoperative morbidity incidence of 20.5%. The types and rates of adverse events varied in other studies. The rates of grade 3 or higher adverse events related to LCT was mostly low (< 10%) and not significantly excessive when compared to the control arm (Table 3).
Discussion
There is little disagreement that the patients with a lower metastatic burden have a far better prognosis, when compared to those with higher metastatic burden. There exist controversies, however, whether aggressive local treatment directed to oligometastasis may derive oncological benefits either by delaying disease progression or hindering metastatic cascade [5,72,73]. In addition to the several previous prospective studies which reported their conclusive results, the current analysis could provide a support on the role of LCT in managing the patients with oligometastatic disease.
In the present study, application of LCT resulted in significant benefit in terms of OS or PFS through the analyses for all included studies. In a subsequent subgroup analysis confined to the studies with reliable comparability, the effect size and the degree of hetererogeneity were similar to those from all studies. Finally, in an analysis limited to randomized controlled studies, the benefit of LCT remained significant in favor of the LCT, and the heterogeneity between studies was small. In another subgroup analyses on the various primary sites, the effect sizes related to the benefit of LCT varied and the degree of heterogeneity generally tended to decrease, when compared to those on all studies. As the benefit of LCT was consistently significant across all the analyses, we would speculate that the current study results strongly support the role of LCT in oligometastatic disease. The benefit of LCT in subgroup of RCTs with low heterogeneity suggested significant clinical advantages of LCT in oligometastatic patients. In addition, the effect size was different for primary sites, which suggests the needs for disease-specific approach.
The key question that remains is whether LCT can alter the biologic course of the oligometastatic patients. Several researchers recently suggested their hypotheses based on their own observations. Gomez et al. [8,27] investigated the role of LCT in the oligoprogression following the first-line chemotherapy for NSCLC, and reported that PFS benefit from LCT might have led to long-term OS benefit. Moreover, they also suggested that LCT could have removed the treatment-resistant cancer clones, or at least, could have slowed down the progression of metastatic spread by reduction of residual disease burden [27]. In another recent study, Phillips et al. [6] reported that total ablation of disease detectable by prostate-specific membrane antigen positron emission tomography–computed tomography using SBRT could reduce the development of new metastases. Based on these results, they suggested that the application of SBRT to metastatic lesions would not only delay the time for reemergence of detectable metastases, but also could prevent the progression of remaining micrometastases. Although the initial report by Palma et al. [7], with follow-up of approximately 2 years, failed to demonstrate the survival benefit following LCT, they later proved significant OS benefit in favor of LCT arm in the latest update with 51-months’ follow-up (median OS difference of 22 months). As such, majority of clinical studies suggested the oncologic benefit by LCT and relevant hypotheses. However, preclinical studies are insufficient to provide biologic rationale and underlying mechanism. Phillips et al. [6] found that the circulating tumor DNA (ctDNA) was lower in the oligometastatic patients, when compared to that in polymetastaic patients, but failed to elucidate the relationship between ctDNA and oncologic outcomes [6]. Ongoing studies by Palma et al. [7], namely SABR-COMET 3 and SABR-COMET 10, intend to elucidate the biologic characteristics of oligometastasis by collecting ctDNA and circulating tumor cells, whose results are highly awaited.
Current study is not free from a few limitations. Relative significance of meta-analysis including the observational studies could be debated, because the uncontrolled confounders and possible heterogeneity could have affected pooled analyses [13]. However, the clinical decisions in oncology field could not be based solely on the level-1 evidence drawn from multiple well-designed prospective randomized clinical trials [74]. Furthermore, with the increasing evidence in favor of the role of LCT, it might be quite difficult to initiate a large-scale prospective trials in this clinical setting. On the other hand, there are suggestions that well-designed observational studies may provide high level of evidence similar to those from the prospective randomized trials [75]. In addition, our study comprehensively analyzed various cancer types, which is a rather unfamiliar method in oncology studies. Such approach can yield the heterogeneity of the pooled analysis. However, because the existence of oligometastic status having potential benefit of local treatment is still controversial, an integrated study is needed for overall clinical decisions [76]. To overcome the heterogeneity of pooled analysis, we performed hierarchical analysis, disease-specific subgroup analyses, and quantitative heterogeneity analyses. However, through the comprehensive analysis, the authors’ could demonstrate, more or less, consistent and reliable results in favor of LCT based on various primaries, which may be shared in the clinical setting of the intermediate metastatic cascade called oligometsatasis. Another weakness of the current study included the fact that the innate mechanism of LCT could not be verified, as the speculations of the current study are based on external integration of the published clinical series. Based on these perspectives, we would strongly believe that the current analyses would be fruitful in our routine clinical practice, through our comparability-based formal meta-analyses, to support the necessity of applying LCT to the oligometastatic patients, and also in promoting the relevant basic biologic researches on oncologic mechanism of LCT, respectively.
The result of the current analyses suggested that LCT application be beneficial to the oligometastatic patients, based on the consistent findings by pooled analyses among (1) all included studies, (2) selected studies with reliable comparability, and (3) RCT’s, respectively. LCTs might have different magnitude of oncologic benefits according to the primary sites, since the pooled survival percentiles varied among different primaries. Additional adverse events related to LCT, however, need to be considered in the treatment decision process, especially for optimizing the potency of LCT when treating the lesions adjacent to the critical organs at risk.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author Contributions
Conceived and designed the analysis: Ahn YC, Chie EK, Lee JH, Kim YS, Suh YG, Kim KH, Rim CH, Cho WK.
Collected the data: Rim CH, Cho WK.
Contributed data or analysis tools: Ahn YC, Chie EK, Lee JH, Kim YS, Suh YG, Kim KH, Rim CH, Cho WK.
Performed the analysis: Rim CH
Wrote the paper: Rim CH, Cho WK, Ahn YC, Chie EK.
Conflicts of Interest
Yong Chan Ahn, the editor-in-chief of the Cancer Research and Treatment, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Acknowledgments
This work has been approved and assisted by the board of directors of Korean Cancer Association.