Clinical Evidence of Chemotherapy or Endocrine Therapy Maintenance in Patients with Metastatic Breast Cancer: Meta-Analysis of Randomized Clinical Trials and Propensity Score Matching of Multicenter Cohort Study
Article information
Abstract
Purpose
This study aims to comprehensively evaluate the clinical efficacy of chemotherapy or endocrine therapy maintenance in metastatic breast cancer (MBC) patients.
Materials and Methods
The meta-analysis of randomized clinical trials (RCTs) and propensity score matching of multicenter cohort study evaluated MBC patients who underwent first-line chemotherapy or endocrine therapy maintenance. This study is registered with PROSPERO: CRD42017071858 and ClinicalTrials.gov: NCT04258163.
Results
A total of 2,867 patients from 15 RCTs and 760 patients from multicenter cohort were included. The results from meta-analysis showed that chemotherapy maintenance improved progression-free survival (PFS) (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.54 to 0.73; p < 0.001; moderate-quality evidence) and overall survival (OS) (HR, 0.87; 95% CI 0.78 to 0.97; p=0.016; high-quality evidence) than observation. In the cohort study, for hormone receptor–positive MBC patients, chemotherapy maintenance improved PFS (HR, 0.67; 95% CI, 0.52 to 0.85; p < 0.001) and OS (HR, 0.55; 95% CI, 0.42 to 0.73; p < 0.001) compared with observation, and endocrine therapy maintenance also improved PFS (HR, 0.65; 95% CI, 0.53 to 0.80; p < 0.001) and OS (HR, 0.55; 95% CI, 0.44 to 0.69; p < 0.001). There were no differences between chemotherapy and endocrine therapy maintenance in PFS and OS (all p > 0.05). Regardless of the continuum or switch maintenance therapy, showed prolonged survival in MBC patients who were response to first-line treatment.
Conclusion
This study provided evidences for survival benefits of chemotherapy and endocrine therapy maintenance in MBC patients, and there was no difference efficacy between chemotherapy and endocrine therapy maintenance for hormone receptor–positive patients.
Introduction
The primary goals of treatment for metastatic breast cancer (MBC) are to reduce symptoms, maintain quality of life, slow tumor progression, and extend survival. After first-line chemotherapy, further treatment always determined by the patient’s response, individual tolerance, and physician preferences. However, there are several options for MBC patients who are responding to chemotherapy, to continue treatment with a fix number of cycles or until disease progression, stop chemotherapy, take a watch and wait strategy, and the optimal maintenance treatment has not been determined [1–4].
For hormone receptor–positive MBC patients, switch endocrine therapy maintenance is also a common option following first-line chemotherapy. A GINECO group study, phase III trial of taxane plus bevacizumab compared with exemestane plus bevacizumab duration in estrogen receptor–positive, human epidermal growth factor receptor 2 (HER2)–negative MBC patients after first-line taxane and bevacizumab indicated that maintenance therapy with exemestane plus bevacizumab did not achieve longer progression-free survival (PFS) [5]. The current clinical practice has established neither a clear clinical benefit of chemotherapy over endocrine therapy maintenance for hormone receptor–positive MBC patients.
Overall, high-quality studies are warranted to further clarify the association between chemotherapy or endocrine therapy maintenance and clinical benefit in patients with MBC after first-line chemotherapy, specifically hormone receptor–positive MBC patients. This study aimed to perform a comprehensive meta-analysis of randomized controlled trials (RCTs) and machine learning propensity score matched analysis of multicenter cohort data to evaluate the efficacy of chemotherapy or endocrine therapy maintenance in MBC patients after first-line chemotherapy.
Materials and Methods
1. Meta-analysis of randomized clinical trials
The meta-analysis was conducted according to the Cochrane Collaboration recommendations and PRISMA statement [6]. We searched PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov for RCTs up to December 30, 2019 using the following terms: “chemotherapy” or “endocrine therapy”, “breast cancer” and “randomized clinical trials.” The proceedings of American Society of Clinical Oncology, European Society for Medical Oncology and American Society for Therapeutic Radiology and Oncology, and the references in the included RCTs and relevant meta-analysis were also reviewed manually.
Trials with any of the following study designs were included: trials comparing a fixed number of cycles of with a longer cycle, regardless of whether the longer cycle is a few more cycles or until the disease progresses, it also doesn’t matter whether maintenance therapy is the original regimen or alternative, chemotherapy or endocrine therapy. We have excluded studies whose abstracts or full texts were no English, and studies that do not have available data. Three investigators (Y.Y., Q.G. and D.L.) screened the eligibility of the studies. The risk of bias was assessed based on the recommendation of the Cochrane Collaboration Handbook [7].
2. Propensity score matched analysis of multicenter cohort study
The multicenter cohort study was reported according to the CONSORT and STROBE guideline. hormone receptor–positive MBC patients who underwent chemotherapy, endocrine therapy maintenance, or observation were retrospectively collected from three hospitals in China between January 2003 and September 2017. A total of 760 patients were recruited from at the Sun Yat-sen Memorial Hospital of Sun Yat-sen University (Guangzhou, China), the Sun Yat-sen University Cancer Center (Guangzhou, China), and the Foshan Afflicted Hospital of Sun Yat-sen University (Foshan, China).
Patient selection was performed according to the following inclusion criteria: (1) primary diagnosed as hormone receptor–positive breast cancer, which was defined as immunohistochemical staining showed that at least 1% of the nuclei were positive for either estrogen receptor or progesterone receptor. (2) Patients with measurable disease, who have response to first-line chemotherapy, including the patients were evaluated as complete response, partial response, or stable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, ver. 1.1 [8]. (3) After the last cycle of first-line chemotherapy, it was still in a state of no progress for at least 4 weeks, otherwise it was considered to be a failure of first-line chemotherapy. The key exclusion criteria were as follows: (1) the presence of immeasurable disease, and (2) the endocrine therapy was administered before first-line chemotherapy. The data were censored on April 30, 2018. The follow-up was performed according to the recommendation of the National Comprehensive Cancer Network guidelines.
3. Endpoint definition
The primary endpoints were PFS and overall survival (OS). The PFS was defined as the time from therapy to the first assessed progression, or death. The OS was defined as the time from the date of the histologically documented diagnosis to the date of death or final follow-up.
4. Statistical analysis
For the meta-analysis, we pooled the data from different studies using a DerSimonian-Laird random effects model weighted by the sample size in each trial [9]. Then, to incorporate the indirect comparison with the direct comparison, we conducted a random effects Bayesian network meta-analysis. The treatment effect on the time-to-event outcome was estimated by the hazard ratio (HR) with 95% confidence interval (CI). Weighted averages of treatment effects were calculated by pooling log HRs for PFS and OS across the studies, by inverse variance weighting. The I2 statistic was used to assess the heterogeneity across the trials. I2 ≥ 50% was considered substantial heterogeneity [10]. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used to assess the quality of evidence as high, moderate, low, or very low [11].
For the individual patient-level analysis, the exact chi-square test was used to compare the patient characteristics. Propensity score matching was used to reduce baseline bias based on neural network machine learning [12]. The PFS and OS were calculated using the Kaplan-Meier method and the log-rank test. Univariate and multivariable Cox regression models were applied to determine the independent prediction factors. Furthermore, we developed a model to predict the OS and evaluate the suitability of chemotherapy or endocrine therapy maintenance. The optimal cutoff values were used to separate patients into low-risk and high-risk groups were generated using the “survminer” package in R (R Foundation for Statistical Computing, Vienna, Austria).
Additionally, to evaluate the potential correlation between the different outcomes, a matrix correlation analysis was first conducted, and a weighted linear regression model was further applied to quantify any existing correlations. An F-statistical significance test of the regression coefficient (β) was performed to confirm the validity of this model. Pearson correlation coefficients (ρ) and the coefficient of determination (R2) with its 95% CI were used to estimate the strength of the correlation. All statistical tests were two-sided, and p-values less than 0.05 were considered statistically significant. The statistical analyses were performed using R ver. 3.4.3.
This study combined a meta-analysis of RCTs that was registered on PROSPERO (Identifier: CRD42017071858), and a retrospectively, machine learning propensity score matched analysis of multicenter cohort study that has been registered at ClinicalTrials.gov (Identifier: NCT04258163).
Results
1. Trials and patients’ characteristics
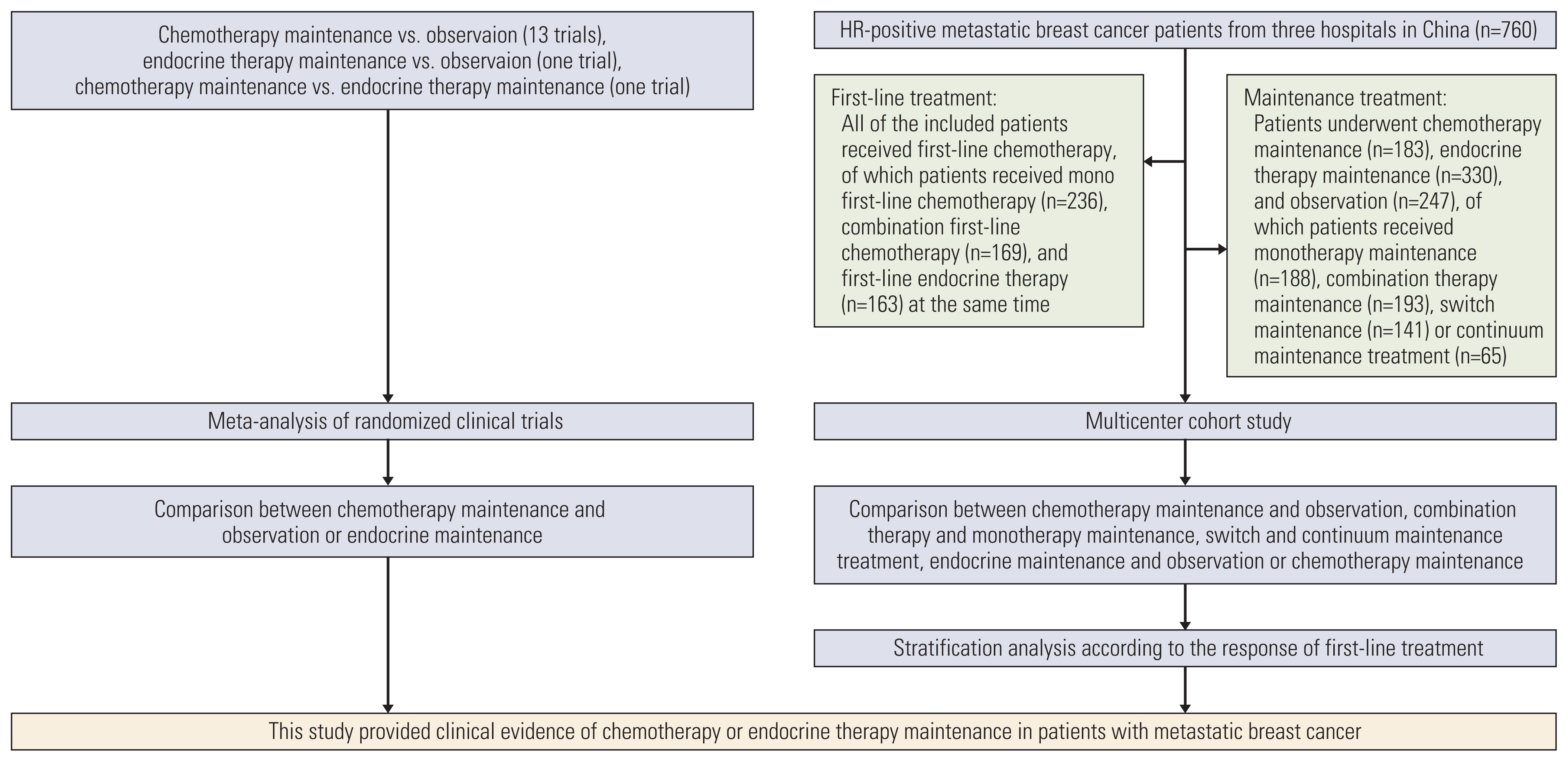
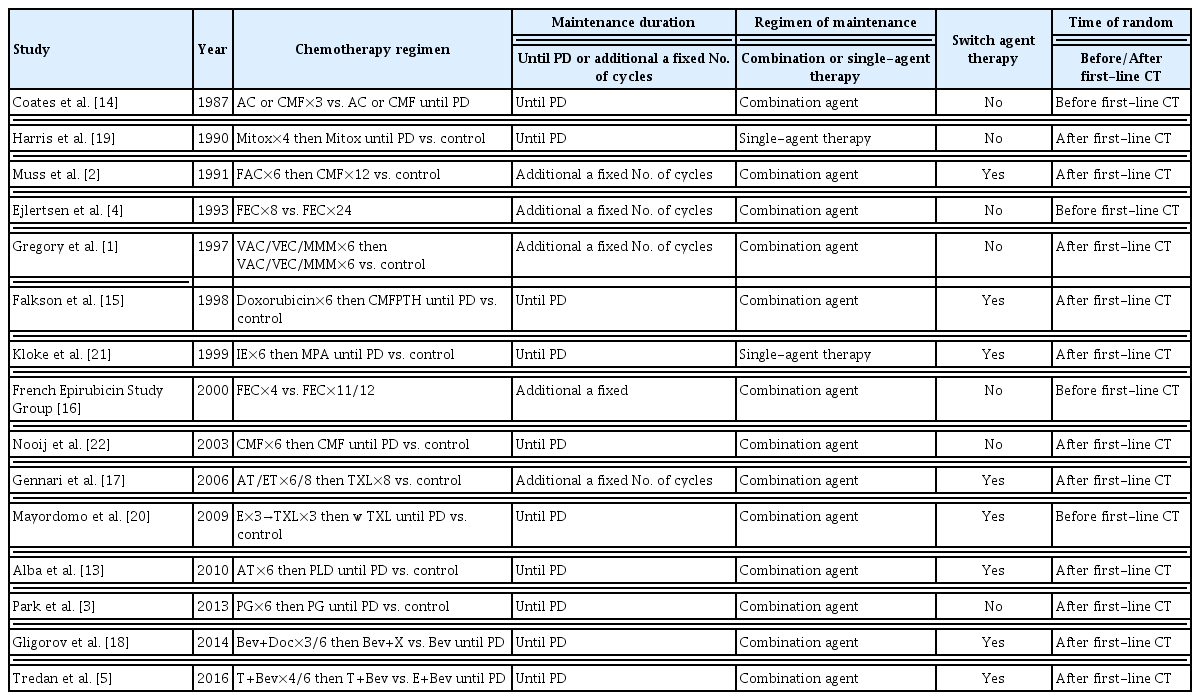
The study design and patient recruitment shows in Fig. 1. The characteristics of RCTs are summarized in Table 1. This included 15 trials including 2,867 patients, 13 trials [1–4,13–22] compared chemotherapy maintenance and observation, one trial [5] compared endocrine therapy maintenance and observation, and one trial [21] compared chemotherapy and endocrine therapy maintenance. Most trials had a low risk of bias (S1 Fig.).
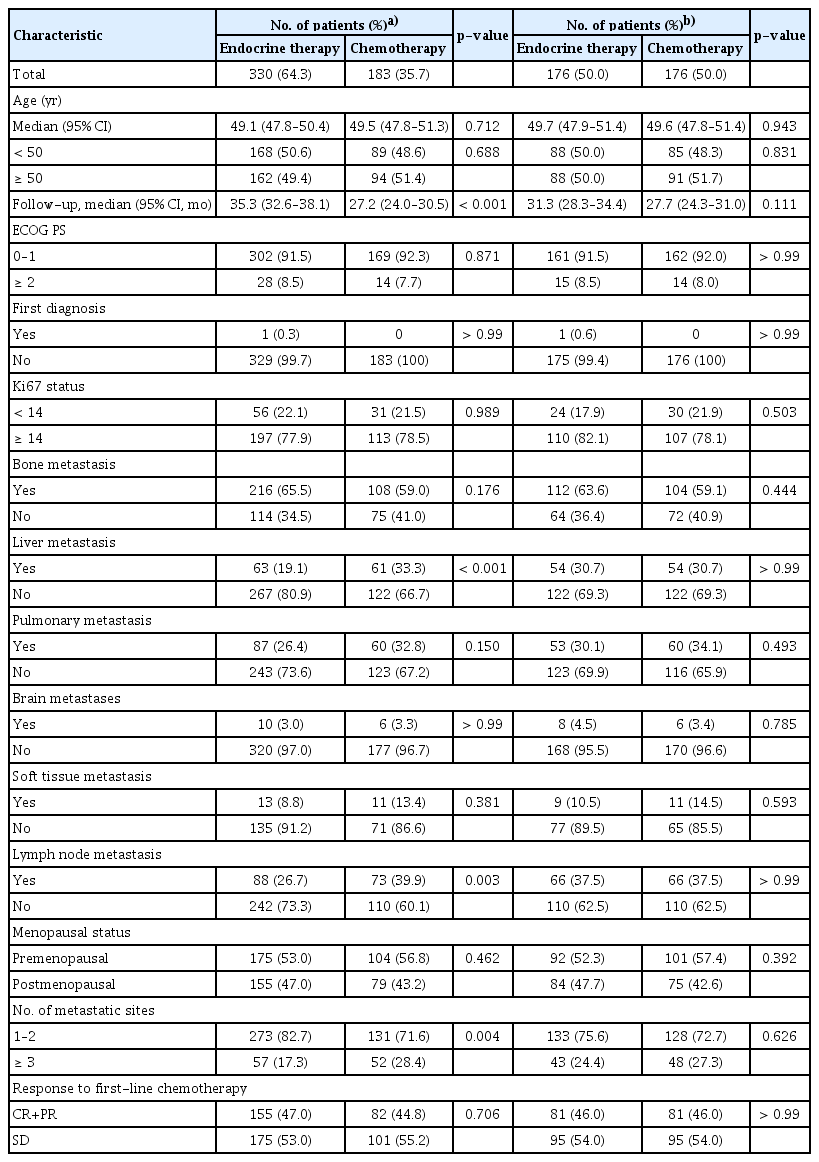
The multicenter cohort recruited 760 patients, including 183 patients (24.1%) who underwent chemotherapy maintenance, 330 patients (43.4%) received endocrine therapy maintenance, and 247 patients (32.5%) were observation after first-line chemotherapy. All of the included patients received first-line chemotherapy, of which 236 patients (31.1%) received mono first-line chemotherapy, 169 patients (22.2%) received combination first-line chemotherapy, and 163 patients (21.4%) received first-line endocrine therapy at the same time. As for the maintenance treatment, 188 patients (24.7%) were treated with monotherapy of cytotoxic chemotherapy (n=28) or endocrine therapy (n=160), 193 (25.4%) patients received combination therapy of cytotoxic chemotherapy (n=74) or endocrine therapy (n=119), 141 (18.6%) patients underwent switch chemotherapy maintenance (n=42) or endocrine therapy (n=99), and 65 (8.6%) patients received continuum maintenance treatment of cytotoxic chemotherapy (n=20) or endocrine therapy (n=45). Via machine learning-based propensity score matching, there were 176 patients in each group for the comparison between chemotherapy maintenance and observation, 221 patients in each group for the comparison between endocrine therapy maintenance and observation, and 176 patients in each group for the comparison between chemotherapy and endocrine therapy maintenance. The demographic features are detailed in Tables 2, 3, and 4, the baseline bias was reduced after matching.
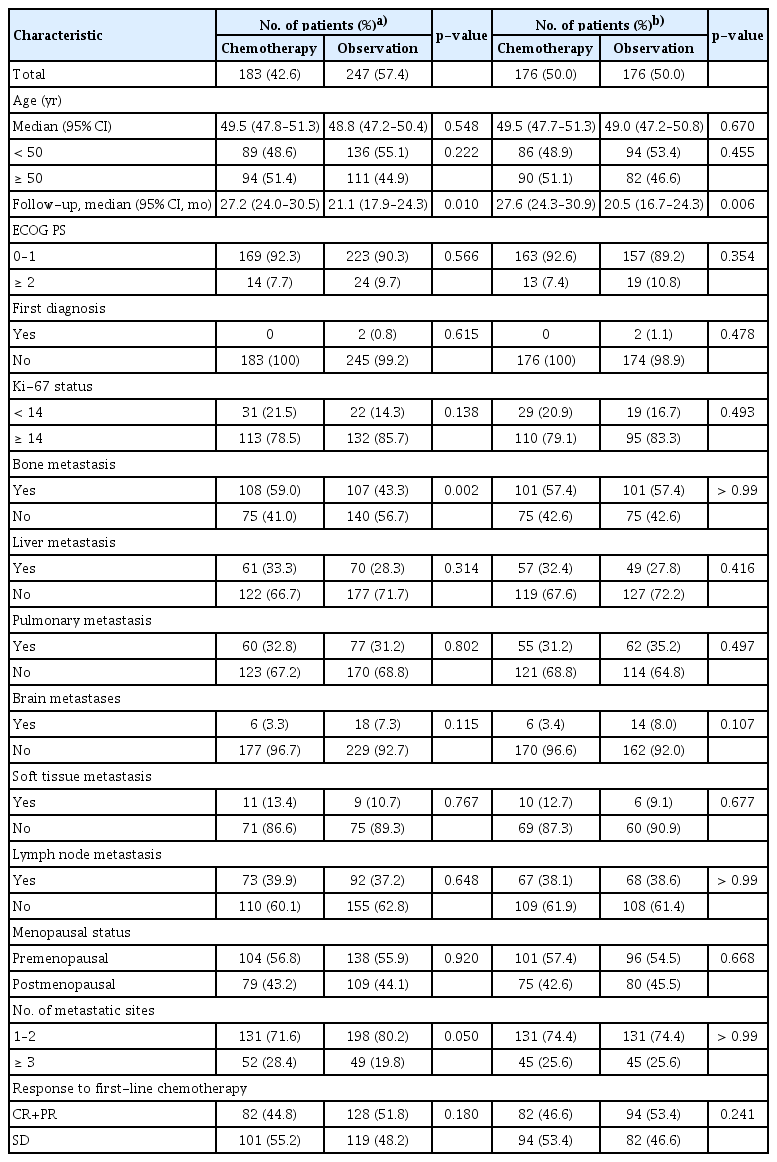
Clinical characteristics among patients with chemotherapy maintenance versus observation before and after propensity score matching in multicenter cohort

Clinical characteristics among patients with endocrine therapy maintenance versus observation before and after propensity score matching in multicenter cohort
2. Chemotherapy maintenance with better clinical benefit than observation in RCTs
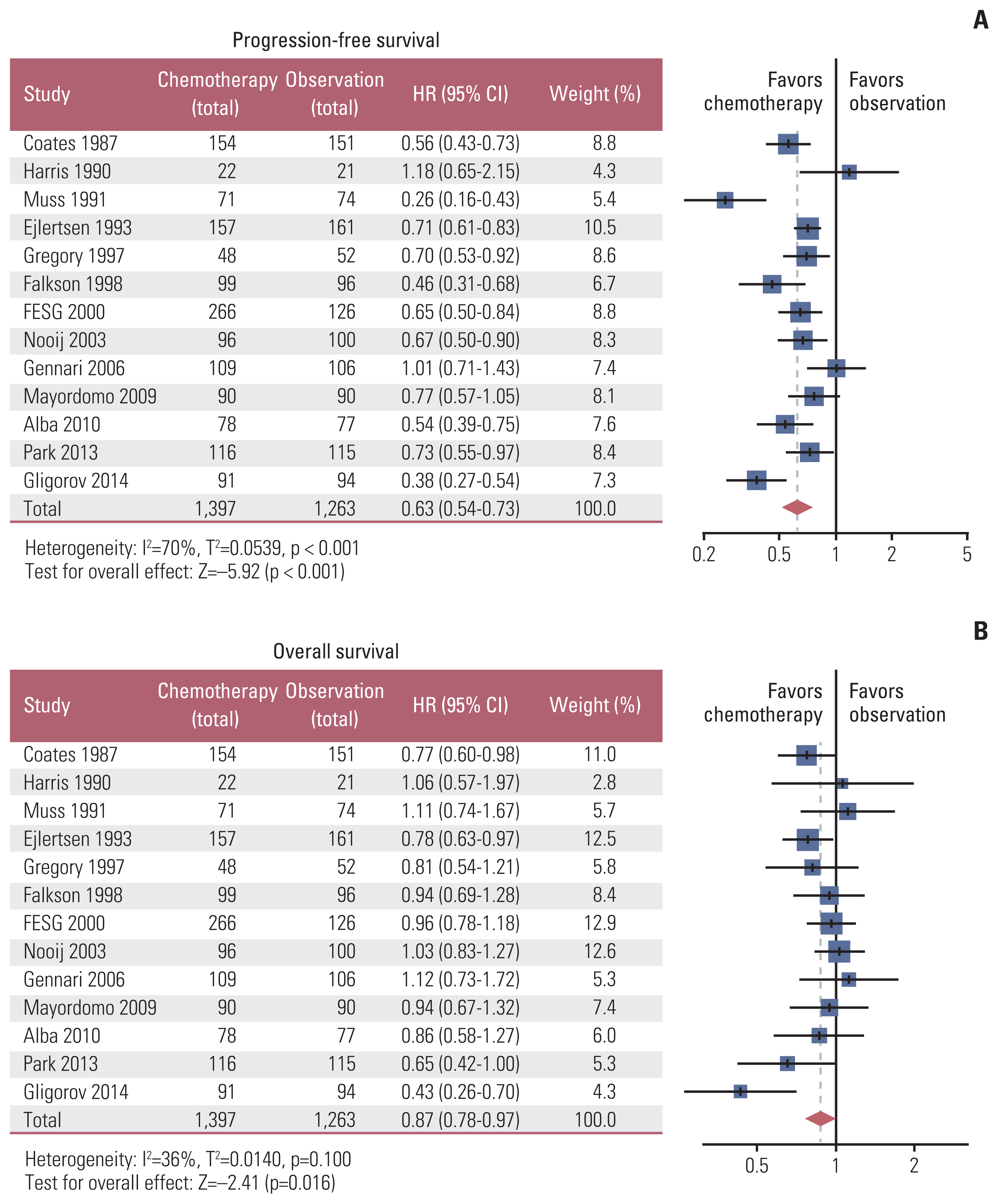
In the meta-analysis of RCTs, comparing with observation, chemotherapy maintenance significantly improved PFS (HR, 0.63; 95% CI, 0.54 to 0.73; p < 0.001; moderate-quality evidence) (Fig. 2A) and OS (HR, 0.87; 95% CI, 0.78 to 0.97; p=0.016; high-quality evidence) (Fig. 2B). Similar survival benefits were also recorded in subgroups defined by timing of random assignment, duration of maintenance chemotherapy in the study arm, combined agent or single-agent chemotherapy maintenance, and switch agent therapy or not (S2 and S3 Tables). The GRADE evidence ranged from moderate to high quality.
3. No difference between chemotherapy and endocrine therapy maintenance in RCTs
In the meta-analysis of RCTs, patients who received endocrine therapy showed similar PFS than chemotherapy maintenance (HR, 1.00; 95% CI, 0.70 to 1.50; p=0.998) (S4A Fig.). Only one trial comparing endocrine therapy maintenance and observation, which found that endocrine therapy maintenance could extend the time to progression (p=0.020), but no improved survival (p=0.390) [21]. The overall network meta-analysis comparison between chemotherapy and endocrine therapy maintenance showed similar PFS (HR, 1.00; 95% CI, 0.73 to 1.37; p > 0.99) (S4B Fig.). Patients who received treatment with chemotherapy or endocrine therapy maintenance had similar OS (HR, 1.15; 95% CI, 0.59 to 2.22; p=0.679) (S4C Fig.). The HR of OS for the overall network meta-analysis comparison between chemotherapy and endocrine therapy maintenance was 1.02 (95% CI, 0.68 to 1.53; p=0.920) (S4D Fig.).
4. Chemotherapy maintenance with better clinical benefit than observation in cohort study
In the multicenter cohort study, before matching, chemotherapy maintenance was associated with a significant improvement in PFS (HR, 0.66; 95% CI, 0.53 to 0.83; p < 0.001) (Fig. 3A) and OS (HR, 0.56; 95% CI, 0.43 to 0.73; p < 0.001) (Fig. 3B) compared with observation. After matched, chemotherapy maintenance also significantly improved PFS (HR, 0.67; 95% CI, 0.52 to 0.85; p < 0.001) (Fig. 3C) and OS (HR, 0.55; 95% CI, 0.42 to 0.73; p < 0.001) (Fig. 3D) compared with observation. The majority of subgroups showed the OS advantage of chemotherapy maintenance compared with the observation (S5 Fig.). Furthermore, comparing with combination therapy of cytotoxic chemotherapy, monotherapy of cytotoxic chemotherapy after first-line treatment in MBC showed similar PFS (HR, 0.94; 95% CI, 0.56 to 1.59; p=0.832) and OS (HR, 0.71; 95% CI, 0.40 to 1.27; p=0.241). The comparison between the switch and continuum chemotherapy maintenance treatment was also performed. Results showed that there were no differences between switch and continuum chemotherapy maintenance in PFS (HR, 0.67; 95% CI, 0.32 to 1.42; p=0.285) and OS (HR, 0.54; 95% CI, 0.19 to 1.58; p=0.254) (S6 Fig.).
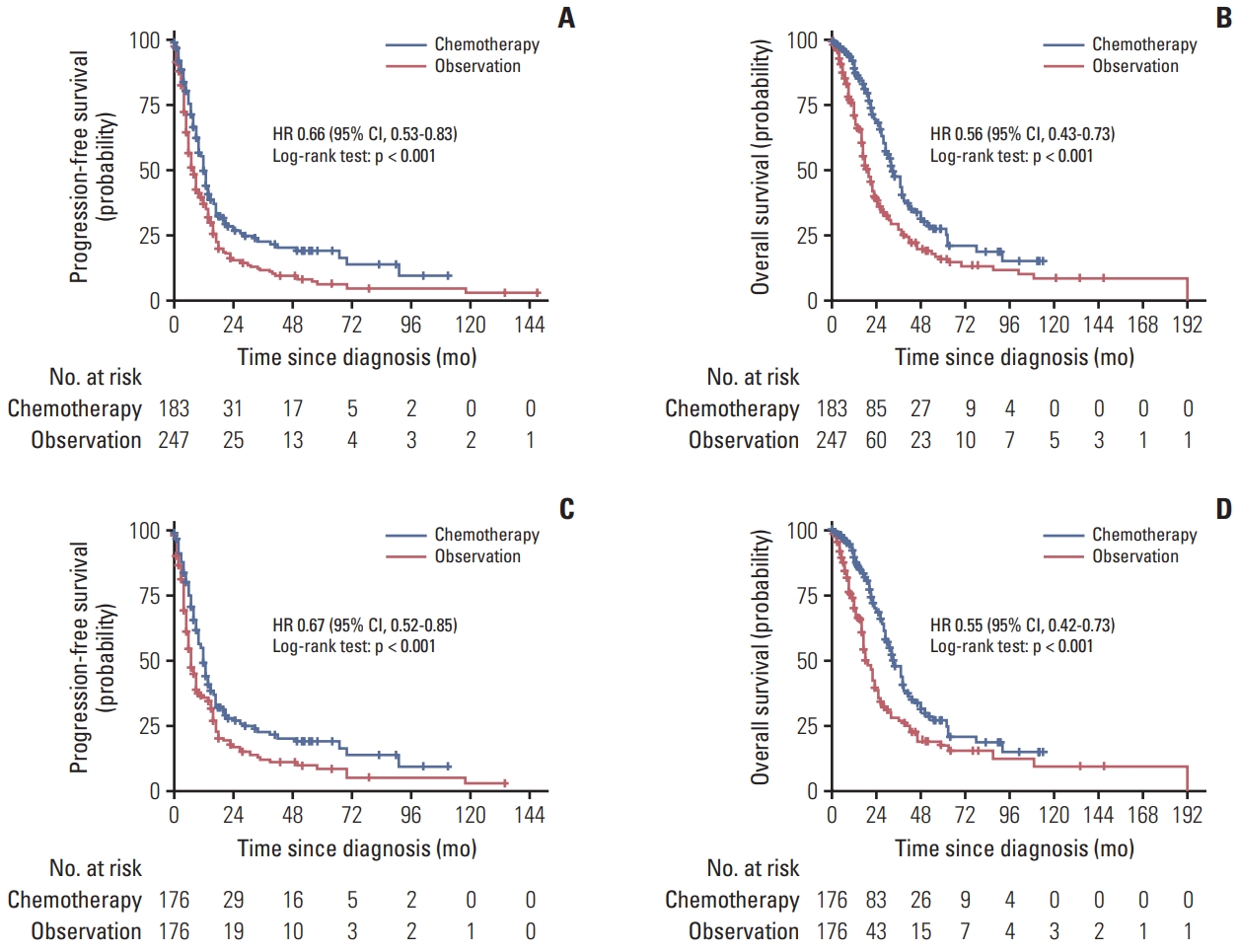
Progression-free survival and overall survival among patients with chemotherapy maintenance versus observation before and after matching in multicenter cohort: progression-free survival before matching (A), overall survival before matching (B), progression-free survival after matching (C), and overall survival after matching (D). CI, confidence interval; HR, hazard ratio.
5. Endocrine therapy maintenance with better clinical benefit than observation in cohort study
In the cohort study, for hormone receptor–positive MBC patients, before matching, compared with the observation group, the endocrine therapy maintenance group significantly prolonged the PFS (HR, 0.63; 95% CI, 0.53 to 0.76; p < 0.001) (Fig. 4A) and OS (HR, 0.56; 95% CI, 0.45 to 0.69; p < 0.001) (Fig. 4B). After matching, endocrine therapy maintenance also improved PFS (HR, 0.65; 95% CI, 0.53 to 0.80; p < 0.001) (Fig. 4C) and OS (HR, 0.55; 95% CI, 0.44 to 0.69; p < 0.001) (Fig. 4D). Compared with observation group, the endocrine therapy maintenance group showed OS superiority in most subgroups, more results are shown in S7 Fig. It was observed that there were no differences between combination therapy and monotherapy of endocrine therapy maintenance in PFS (HR, 1.12; 95% CI, 0.87 to 1.44; p=0.397) and OS (HR, 1.11; 95% CI, 0.82 to 1.49; p=0.497). Results of the comparison between switch and continuum endocrine therapy maintenance indicated that continuum endocrine therapy maintenance significantly improved PFS (HR, 0.63; 95% CI, 0.43 to 0.92; p=0.017), whereas no differences were presented in OS (HR, 0.99; 95% CI, 0.64 to 1.52; p=0.948) (S8 Fig.).
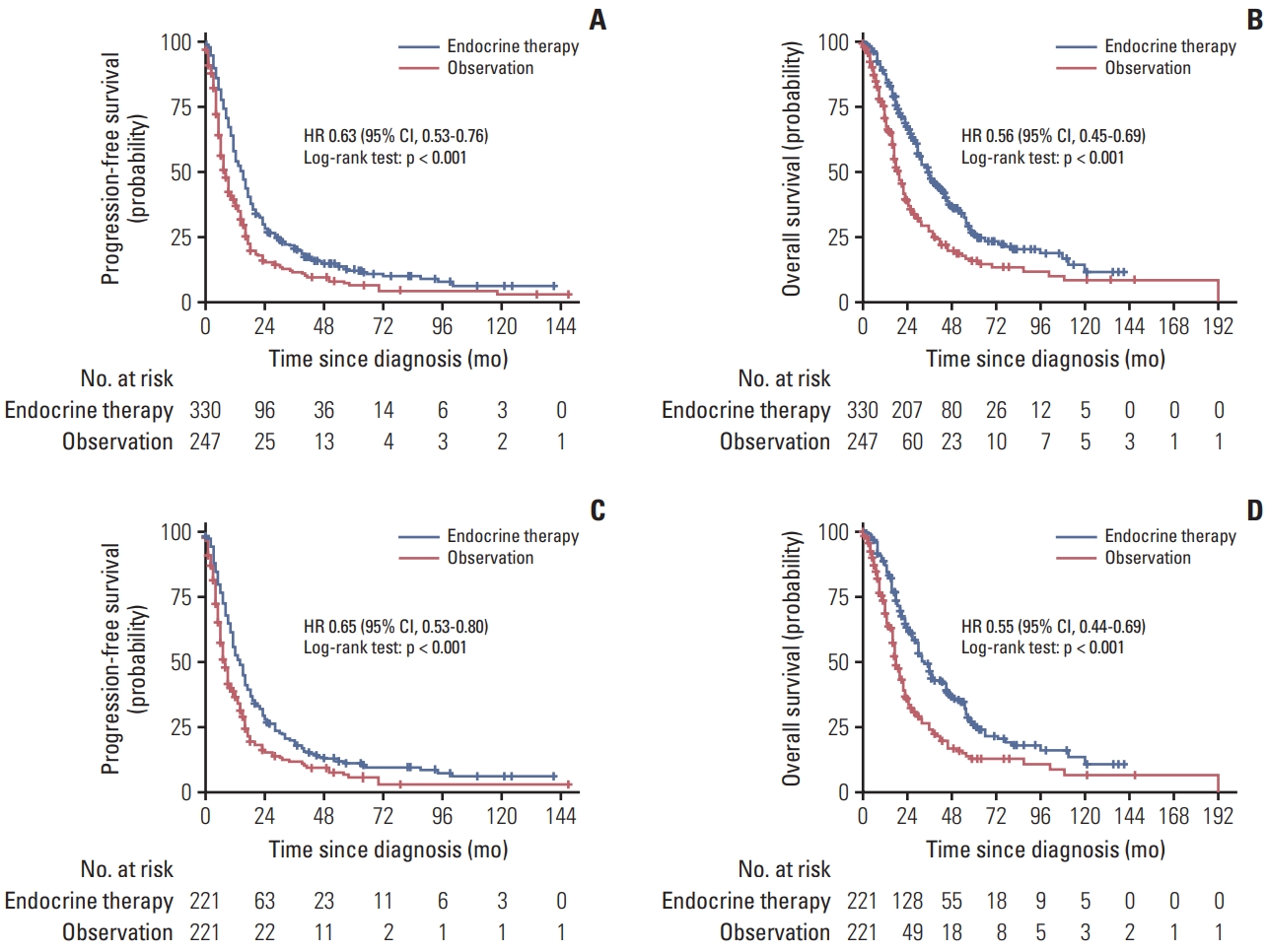
Progression-free survival and overall survival among patients with endocrine therapy maintenance versus observation before and after matching in multicenter cohort: progression-free survival before matching (A), overall survival before matching (B), progression-free survival after matching (C), and overall survival after matching (D). CI, confidence interval; HR, hazard ratio.
6. No difference between chemotherapy and endocrine therapy maintenance in cohort study
In the cohort study, for hormone receptor–positive MBC patients, before matching, there were no differences between chemotherapy and endocrine therapy maintenance in PFS (HR, 1.03; 95% CI, 0.84 to 1.27; p=0.760) (Fig. 5A) and OS (HR, 1.06; 95% CI, 0.83 to 1.35; p=0.660) (Fig. 5B). After matching, the chemotherapy was also similar to the endocrine therapy maintenance in PFS (HR, 0.96; 95% CI, 0.76 to 1.21; p=0.726) (Fig. 5C) and OS (HR, 0.85; 95% CI, 0.65 to 1.11; p=0.219) (Fig. 5D). Chemotherapy and endocrine therapy maintenance have comparable OS in most subgroups, more results are shown in S9 Fig.
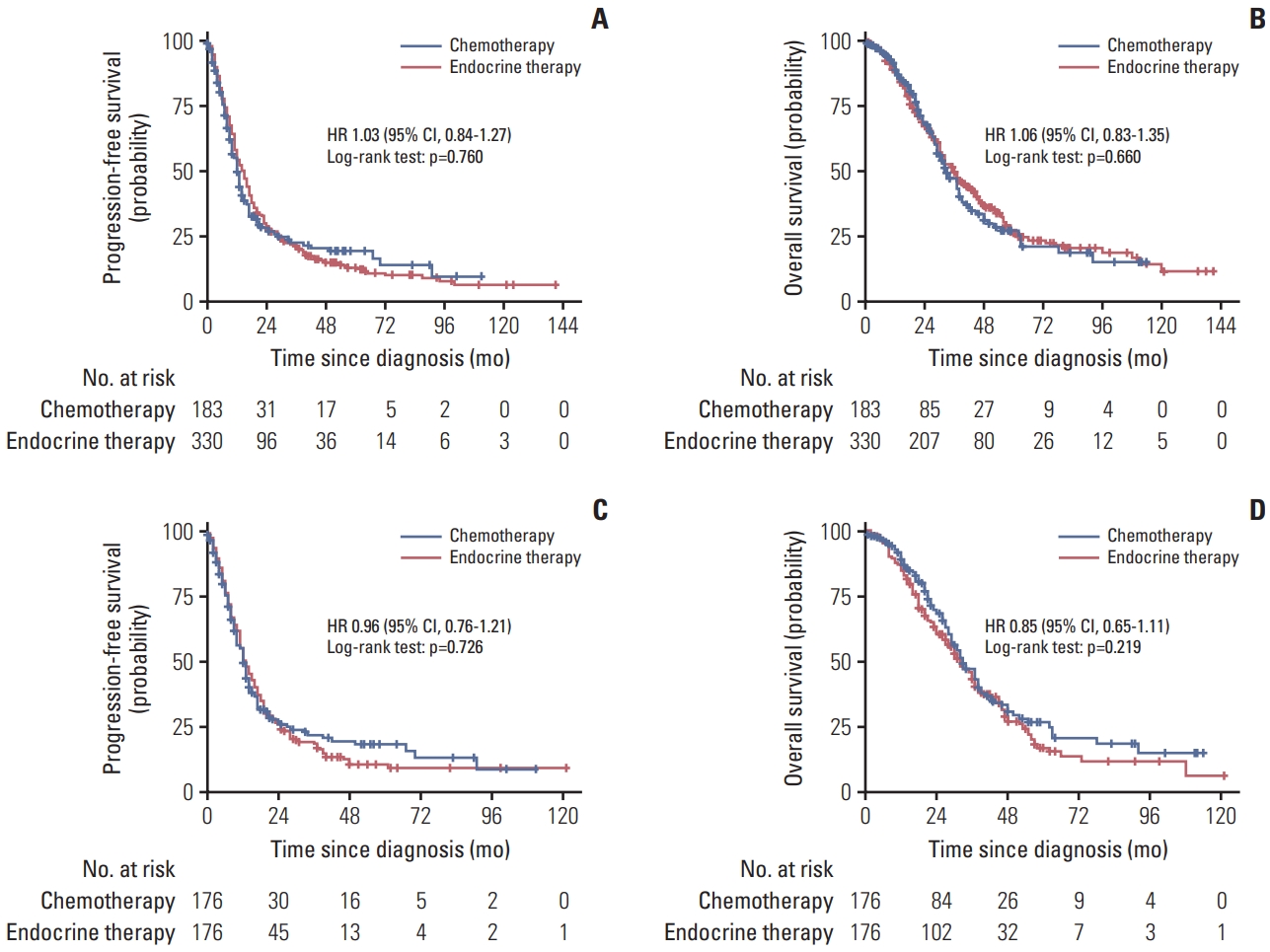
Progression-free survival and overall survival among patients with chemotherapy versus endocrine therapy maintenance before and after matching in multicenter cohort: progression-free survival before matching (A), overall survival before matching (B), progression-free survival after matching (C), and overall survival after matching (D). CI, confidence interval; HR, hazard ratio.
Furthermore, for all 513 patients who received chemotherapy or endocrine therapy maintenance, we built a prediction model incorporating factors with the response to first-line chemotherapy (HR, 1.83; 95% CI, 1.45 to 2.30), liver metastasis or not (HR, 2.05; 95% CI, 1.61 to 2.62), pulmonary metastasis or not (HR, 1.61; 95% CI, 1.27 to 2.03), soft tissue metastasis or not (HR, 1.20; 95% CI, 1.06 to 1.35), lymph node metastasis or not (HR, 1.90; 95% CI, 1.50 to 2.40), and Ki-67 ≥ 14% or < 14% (HR, 1.35; 95% CI, 1.17 to 1.55), and then a risk score of death was calculated for each patient using a formula derived from the levels of these predictive variables weighted by their corresponding regression coefficients as follows: Risk score= (0.49359×level of best response to chemotherapy)+(0.64785× level of liver metastasis)+(0.33138×level of pulmonary metastasis)+(0.28485×level of soft tissue metastasis)+(0.62951× level of lymph node metastasis)+(0.36584×level of ki67 expression) to categorize patients into high-risk or low-risk group according to OS. After obtaining the risk scores of deaths from the prediction model, the patients were separated into low-risk and high-risk groups (HR for PFS, 0.44; 95% CI, 0.35 to 0.55; p < 0.001; HR for OS, 0.30; 95% CI, 0.23 to 0.40; p < 0.001) (S10 and S11 Figs.). There was no significant difference in PFS or OS between chemotherapy or endocrine therapy maintenance in either high-risk (HR for PFS, 0.88; 95% CI, 0.70 to 1.12; p=0.296; HR for OS, 0.81; 95% CI, 0.62 to 1.06; p=0.122) (S12A and S12B Fig.) or low-risk groups (HR for PFS, 1.01; 95% CI, 0.63 to 1.61; p=0.971; HR for OS, 1.31; 95% CI, 0.74 to 2.35; p=0.358) (S12C and S12D Fig.).
7. Stratification analysis according to the response of first-line treatment in cohort study
In the cohort study, for the complete response patients after first-line treatment, patients received endocrine therapy showed similar PFS (HR, 0.39; 95% CI, 0.14 to 1.11; p=0.064) (S13A Fig.) and OS (HR, 1.17; 95% CI, 0.14 to 9.49; p=0.867) (S13B Fig.) than chemotherapy maintenance. Similar PFS (HR, 0.80; 95% CI, 0.31 to 2.07; p=0.622) (S13C Fig.) and OS (HR, 0.97; 95% CI, 0.24 to 3.93; p=0.969) (S13D Fig.) benefits were observed in patients received monotherapy and combination therapy. Patients who received switch or continuum maintenance treatment also had similar PFS (HR, 0.82; 95% CI, 0.22 to 2.98; p=0.718) (S13E Fig.) and OS (p=0.073) (S13F Fig.). For the patients who were partial response to the first-line treatment, there were no differences between chemotherapy and endocrine maintenance therapy in PFS (HR, 0.83; 95% CI, 0.56 to 1.23; p=0.343) (S14A Fig.) and OS (HR, 0.83; 95% CI, 0.52 to 1.34; p=0.444) (S14B Fig.). Comparing with combination therapy, monotherapy maintenance significantly improved PFS (HR, 1.48; 95% CI, 1.00 to 2.20; p=0.047) (S14C Fig.), whereas no differences were observed in OS (HR, 1.37; 95% CI, 0.86 to 2.18; p=0.177) (S14D Fig.). Moreover, the switch maintenance was similar to the continuum maintenance treatment in PFS (HR, 0.56; 95% CI, 0.31 to 1.02; p=0.058) (S14E Fig.) and OS (HR, 0.89; 95% CI, 0.46 to 1.70; p=0.701) (S14F Fig.). As for patients with stable disease after the first-line treatment, the chemotherapy was similar to the endocrine maintenance therapy in PFS (HR, 0.86; 95% CI, 0.60 to 1.23; p=0.409) (S15A Fig.) and OS (HR, 0.75; 95% CI, 0.50 to 1.14; p=0.176) (S15B Fig.). Monotherapy maintenance showed similar PFS (HR, 1.26; 95% CI, 0.88 to 1.80; p=0.197) (S15C Fig.) and OS (HR, 1.44; 95% CI, 0.94 to 2.21; p=0.087) (S15D Fig.) compared with combination therapy. Patients who received switch or continuum maintenance treatment had similar PFS (HR, 0.66; 95% CI, 0.39 to 1.14; p=0.138) (S15E Fig.) and OS (HR, 0.94; 95% CI, 0.49 to 1.79; p=0.854) (S15F Fig.).
8. PFS as surrogate for OS
In order to further explore the potential surrogate value of PFS for OS in maintenance treatment in MBC patients. Differences were greater with PFS than OS for trials of chemotherapy maintenance compared with observation (HR, 0.72; 95% CI, 0.59 to 0.80; p < 0.001) (S16 Fig.), and the correlation coefficient R2 between treatment effects on PFS and on OS was 12% (95% CI, 8% to 16%) when all trials were considered to 40% (95% CI, 30% to 54%) after exclusion of one highly influential trial3 by sensitivity analysis (S17 Fig.). Additionally, in the cohort study, among patients (n=513) who were treated with chemotherapy or endocrine therapy maintenance, the association between PFS and OS was R2=0.609 (p < 0.001) (S18 Fig.).
Discussion
This study based on 15 RCTs including 2,867 patients and a multicenter cohort recruited 760 patients quantitatively evaluated the clinical benefits of chemotherapy or endocrine therapy maintenance after first-line chemotherapy for MBC, which indicated that maintenance treatment has clinically benefits for both PFS and OS than observation in MBC patients, and there were no difference efficacy between chemotherapy and endocrine therapy maintenance for hormone receptor–positive MBC patients. Additionally, treatment effect sizes were greater for OS than for PFS, and a moderate correlation between PFS and OS was identified for determining the effectiveness of maintenance treatment.
Results of this study were consistent with a meta-analysis of the duration of chemotherapy for MBC [23], which showed prolonged chemotherapy had a statistically significant survival advantage, thus support policies to extend treatment until the disease progresses without unacceptable toxicity. We included several new trials [3,5,18,21] that were not included in the previous study [23], in particular studies that included new antitumor drugs including gemcitabine and capecitabine. A meta-analysis [24] included four RCTs with 1,044 participants and found that the combination of doublet chemotherapy with trastuzumab compared with single-agent chemotherapy as first-line therapy for HER2-positive MBC is associated with longer PFS and OS, and recommended that doublet chemotherapy appears to be an appropriate regimen for good performance status patients. This meta-analysis with multicenter cohort study findings support PFS and OS benefit of chemotherapy than observation after first-line chemotherapy in MBC. Furthermore, it was observed that comparing with combination therapy, monotherapy maintenance significantly improved PFS for the patients who were partial response to the first-line treatment. Overall, we recommend the use of sequential monotherapy chemotherapy maintenance for MBC patients, and combination of doublet chemotherapy recommend for patients who with rapid disease progression, or life-threatening visceral metastases occurs, or the need for rapid symptom or disease control is present.
Previous meta-analysis study included eight RCTs with 4,580 participants indicated that cyclin-dependent kinases 4 and 6 inhibitors combined with endocrine therapy can significantly prolong PFS, OS and improve the objective response rate, clinical benefit response in patients with hormone receptor–positive, HER2-negative advanced breast cancer [25]. But even in the first-line treatment of hormone receptor–positive MBC patients, more than half of the patients receive chemotherapy as the first-line treatment, for the reasons that patients with high tumor load and visceral crisis, clinicians who consider that some patients need a fast response, and the efficacy of chemotherapy is higher than that of endocrine therapy [26]. It is worth noting that there are few studies provide high evidence for endocrine therapy maintenance after disease control by previous chemotherapy in hormone receptor–positive MBC patients.
Moreover, in previous clinical practice, using endocrine therapy maintenance before disease progression might not be recommended after first-line chemotherapy, patients who received endocrine therapy before disease progression may lose the opportunity to receive other endocrine therapy after disease progression [27]. Due to the limited trials comparing endocrine therapy maintenance and observation in hormone receptor–positive MBC patients, our multicenter cohort data analysis provides evidence that the endocrine therapy maintenance plays an important role in hormone receptor–positive MBC patients.
This study further confirmed the benefit of maintenance endocrine therapy in hormone receptor–positive MBC patients, which could provide clinical evidence for further clinical trials. Although the results revealed that chemotherapy and endocrine therapy maintenance have similar effects on PFS and OS, further validation in prospective clinical trials was needed. Independent biomarkers, such as circulating tumor cell, long noncoding RNAs and tumor immune-microenvironment were adequately predicting therapeutic response and identifying patients who could derive the greatest therapeutic benefit in breast cancer [28–30]. But there were no clear evidences that tumor immune-microenvironment biomarkers can guide maintenance therapy. Therefore, in order to further validate the results of this study, overcome within-tumor microenvironments heterogeneity, and explore the mechanism of maintenance therapy efficacy, we conducted a phase 3 randomized trial comparing the efficacy of fulvestrant versus capecitabine as maintenance therapy after first-line combination chemotherapy in patients with hormone receptor+/HER2− MBC, and the trial recruitment is ongoing (NCT04263298).
There are some limitations in this study. The heterogeneity of molecular subtype of MBC, and the schedule of chemotherapy that some of the regimens used in the study are outdated from the current point of view. Although prolonged chemotherapy maintenance has significant clinical benefits, which can reduce symptoms and improve quality of life by delaying disease progression, however, only two studies included in the meta-analysis focused on the quality of life. Due to the retrospective nature of the multicenter cohort study and the meta-analytic approach taken in this study, not all of the included patients have available data for us to further analyze. Additionally, due to a lack of available tumor microenvironment-based variables, we were unable to further consider the potential mechanisms driving the interaction between clinical benefit and tumor microenvironment, which warrants further investigation to better guide chemotherapy or endocrine therapy maintenance precisely.
In conclusion, this study provided evidences for PFS and OS benefits of chemotherapy or endocrine therapy maintenance over observation after first-line chemotherapy in MBC, and there was no difference efficacy between chemotherapy and endocrine therapy maintenance for hormone receptor–positive MBC patients. Additionally, treatment effect sizes were greater for OS than for PFS, and a moderate correlation between PFS and OS was identified and suggested that both PFS and OS should be evaluated to determine the effectiveness of maintenance therapy in future clinical trials.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
The study protocol was approved by the ethics committee (SYSEC-KY-KS-2019-171-001) of the Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Author Contributions
Conceived and designed the analysis: Ren W, Yu Y, Hong H, Wang Y, Yao H.
Collected the data: Ren W, Yu Y, Hong H, Wang Y, Gao Q, Chen Y, Chen P, Zhao J, Ou Q, Lin D, Fu T, Tan Y, Li C, Xie X, Ye G, Tang J, Yao H.
Contributed data or analysis tools: Ren W, Yu Y, Hong H, Wang Y, Gao Q, Chen Y, Yao H.
Performed the analysis: Ren W, Yu Y, Hong H, Wang Y, Gao Q, Chen Y, Yao H.
Wrote the paper: Ren W, Yu Y, Hong H, Wang Y, Gao Q, Chen Y, Chen P, Zhao J, Ou Q, Lin D, Fu T, Tan Y, Li C, Xie X, Ye G, Tang J, Yao H.
Supervision: Yao H.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
We thank the patients who participated in this study and all investigators contributed to these trials. This study was supported by grants 81972471 and 82073408 from the National Natural Science Foundation of China, grants 202206010078 and 202201020574 from the Guangzhou Science and Technology Project, grant 2018007 from the Sun Yat-Sen University Clinical Research 5010 Program, grant SYS-C-201801 from the Sun Yat-Sen Clinical Research Cultivating Program, grant A2020558 from the Guangdong Medical Science and Technology Program, grant 7670020025 from Tencent Charity Foundation, grant YXQH202209 from the Scientific Research Launch Project of Sun Yat-Sen Memorial Hospital.