Lobular Carcinoma In Situ during Preoperative Biopsy and the Rate of Upgrade
Article information
Abstract
Purpose
There is a potential risk that lobular carcinoma in situ (LCIS) on preoperative biopsy might be diagnosed as ductal carcinoma in situ (DCIS) or invasive carcinoma in the final pathology. This study aimed to evaluate the rate of upgrade of LCIS on preoperative biopsy to DCIS or invasive carcinoma.
Materials and Methods
Data of 55 patients with LCIS on preoperative biopsy were analyzed. All patients underwent surgery between 1991 and 2016 at Severance Hospital in Seoul, Korea. We analyzed the rate of upgrade of preoperative LCIS to DCIS or invasive cancer in the final pathology. The clinicopathologic features related to the upgrade were evaluated.
Results
The rate of upgrade of LCIS to DCIS or invasive carcinoma was 16.4% (9/55). In multivariate analysis, microcalcification and progesterone receptor expression were significantly associated with the upgrade of LCIS (p=0.023 and p=0.044, respectively).
Conclusion
The current study showed a relatively high rate of upgrade of LCIS on preoperative biopsy to DCIS or invasive cancer. The presence of microcalcification and progesterone receptor expression may be potential predictors of upgradation of LCIS on preoperative biopsy. Surgical excision of the LCIS during preoperative biopsy could be a management option to identify the concealed malignancy.
Introduction
Traditionally, an excision is recommended for patients with lobular carcinoma in situ (LCIS) diagnosed on core needle biopsy [1]. However, the management of LCIS has been controversial, and some authors advocate observation rather than surgical excision [2,3].
LCIS was excluded from the malignant category in the 8th American Joint Committee on Cancer (AJCC) staging system for breast cancer [4]. The National Comprehensive Cancer Network (NCCN) guidelines recommend active surveillance, surgical excision, and/or other interventions such as counseling for lifestyle modification, medication, or surgery for reducing the risk of breast cancer in patients with LCIS diagnosed on core needle biopsy [1]. Surgical excision should be considered only in patients with pleomorphic LCIS or lesions that are non-concordant with imaging findings [1]. Patients with classic LCIS and those with lesions that are concordant with imaging findings can be followed up with close observation [1].
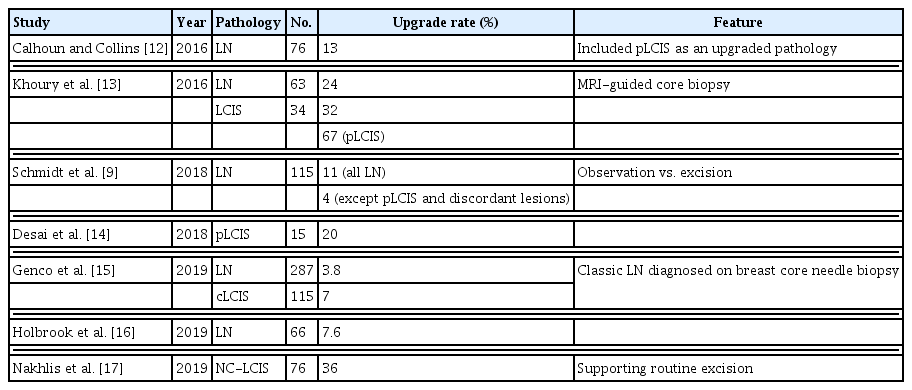
Across the literature, the upgrade rates of LCIS on core needle biopsy to invasive carcinoma or ductal carcinoma in situ (DCIS) at surgical excision have been reported from 0% to 50% [5]. Previous studies have shown that the upgrade rate varies, and there is still no consensus about surgical treatment or observation in cases of LCIS [5].
In this study, we analyzed the upgrade rate and risk factors associated with the upgrade of LCIS diagnosed at preoperative biopsy and performed surgical excision in a single institution.
Materials and Methods
We reviewed electronic medical records (EMR) and data from the Breast Cancer Registry database of Severance Hospital, Yonsei University Health System, and conducted a retrospective study. The computerized medical database included information about the clinical, radiological, and pathological characteristics of patients; treatment methods; preoperative and postoperative pathologic findings; preoperative findings on physical examination, mammography, and ultrasonography; recurrence and mortality; and follow-up data, as previously described [6].
We reviewed the data of 80 patients who underwent breast surgery for LCIS at Severance Hospital between January 1991 and December 2016 using EMRs and data from the database. We excluded patients diagnosed with invasive cancer at preoperative biopsy (n=6) and non-LCIS at preoperative biopsy (n=18); we also excluded one case involving unavailable data. Finally, 55 cases of LCIS diagnosed at preoperative biopsy were enrolled in the study (Fig. 1).
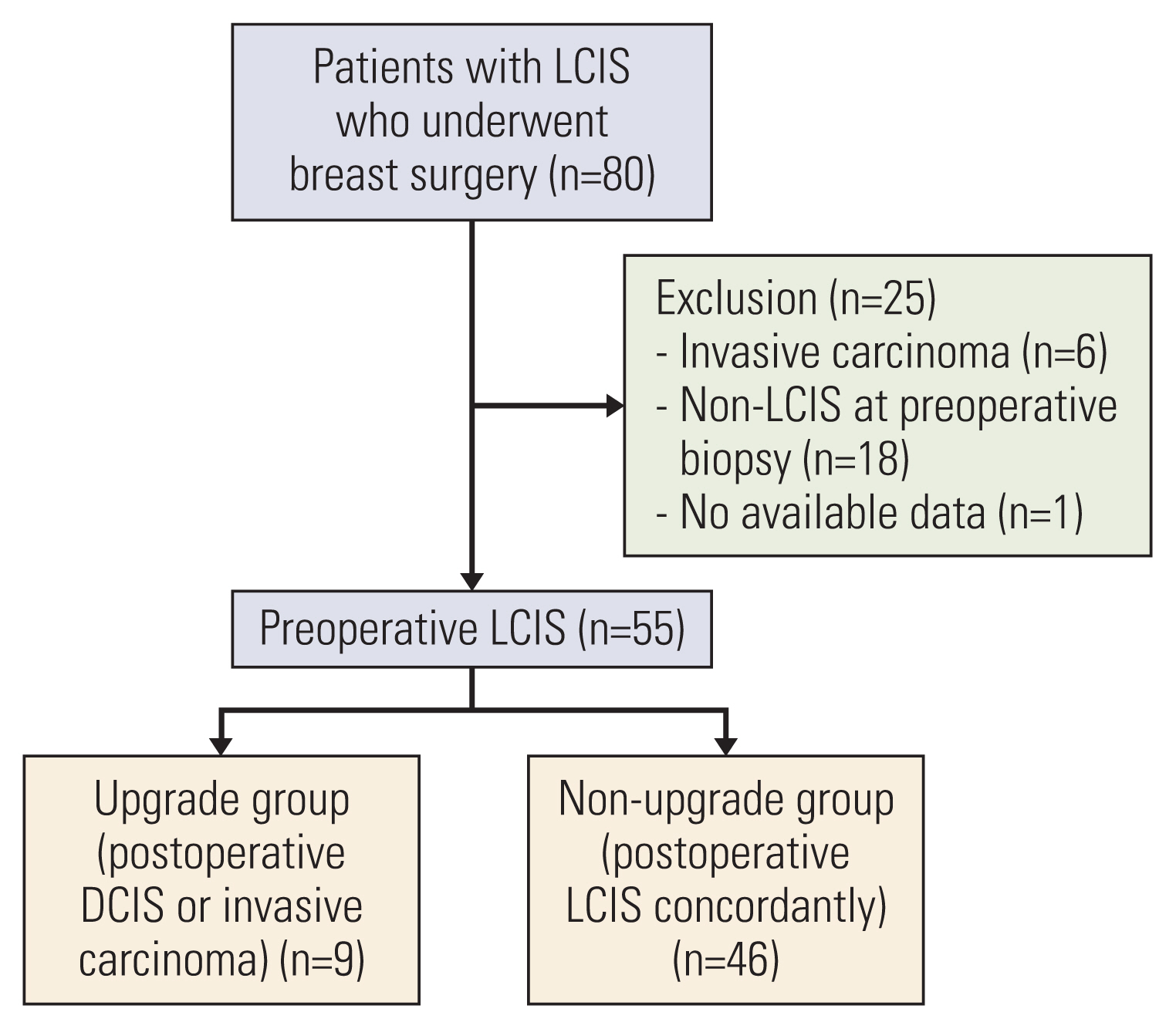
Schema of the study design to analyze the upgrade rate of preoperative LCIS. DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
The patients underwent breast-conserving surgery or mastectomy, according to the patients’ and surgeons’ preferences based on the tumor size, location, and multiplicity of tumors. After surgery, some patients who underwent breast-conserving surgery received adjuvant radiotherapy according to the multidisciplinary team approach.
Patient characteristics such as age, clinical findings, preoperative biopsy methods, pathological findings, and treatment methods were reviewed. A preoperative physical examination was performed by experienced surgeons, and a palpable mass was described in the medical database with or without information about the location or size of the lesion. Preoperative imaging evaluations including mammography, ultrasonography, and magnetic resonance imaging (MRI) were performed. The initial reports of preoperative imaging studies were reviewed for their correlation with the final pathology.
Final pathology records were reviewed to analyze histopathological variables including tumor size, hormone receptor status, E-cadherin expression, pleomorphism, and comedo necrosis. Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2)/neu expression were evaluated on formalin-fixed, paraffin-embedded whole sections of the surgically resected breast specimens using immunohistochemistry (IHC). The cutoff value for ER and PR positivity was > 1% staining on IHC. Pleomorphism and comedo necrosis were reviewed by an experienced breast pathologist (J.S.K.) and categorized as either absent or present.
Categorical variables were analyzed using the chi-square test or Fisher exact test. Continuous variables were analyzed using the Student’s t test or Mann-Whitney U test. Univariate and multivariate analyses for calculating the odds ratios of significant risk factors for the upgrade of preoperative LCIS were performed using binary logistic regression. Multivariate analysis was adjusted for age, microcalcification on mammography, and PR status as covariates. Risk factors were selected using backward stepwise regression based on the probability of the likelihood ratio. A p < 0.05 was considered significant; all tests were two-sided. Statistical analyses were conducted using a commercially available statistical software SPSS Statistics ver. 25 (IBM Corp., Armonk, NY).
Results
Overall, 55 cases of preoperative LCIS were identified. They were classified into two different groups based on the final pathology: nine cases of postoperative DCIS or invasive carcinoma in the upgrade group and 46 cases of postoperative LCIS in the non-upgrade group (Fig. 1). The upgrade rate of preoperative LCIS to DCIS or invasive carcinoma was 16.4% (9/55) (Fig. 2A).
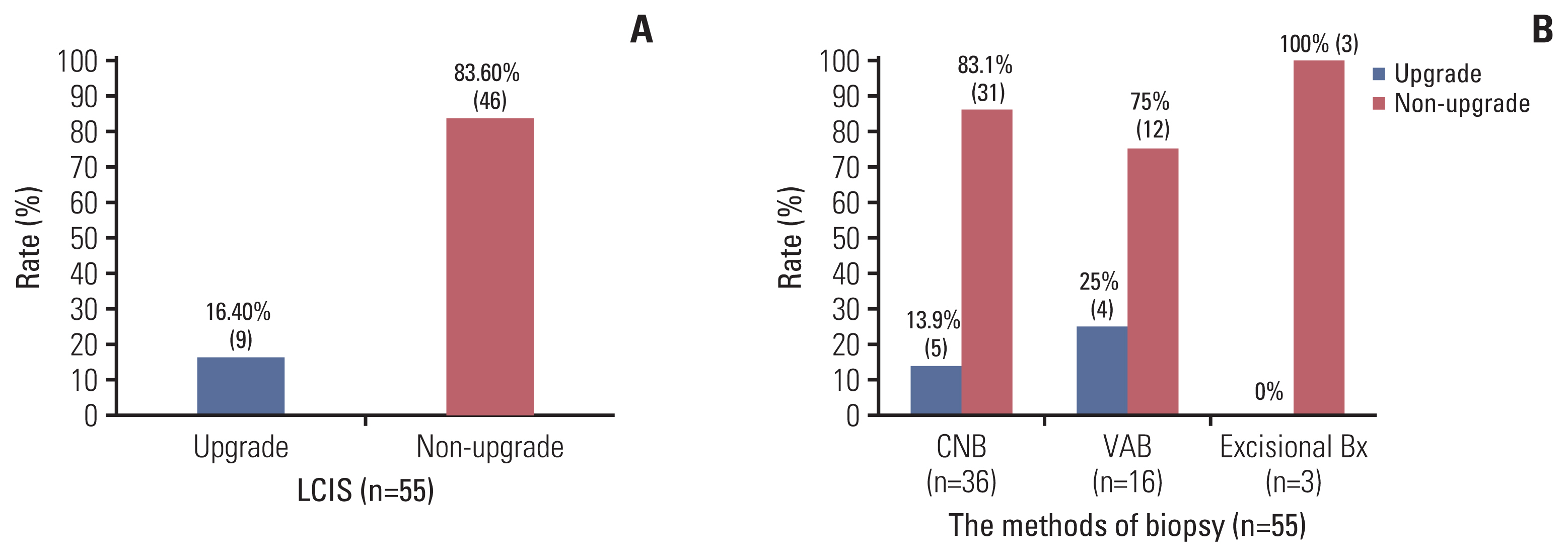
The rate of upgrade of preoperative LCIS. (n=55). (A) The rate of upgrade of preoperative LCIS. (B) The rate of upgrade of preoperative LCIS according to the methods of preoperative biopsy. Bx, biopsy; CNB, core needle biopsy; LCIS, lobular carcinoma in situ; VAB, vacuum assisted biopsy.
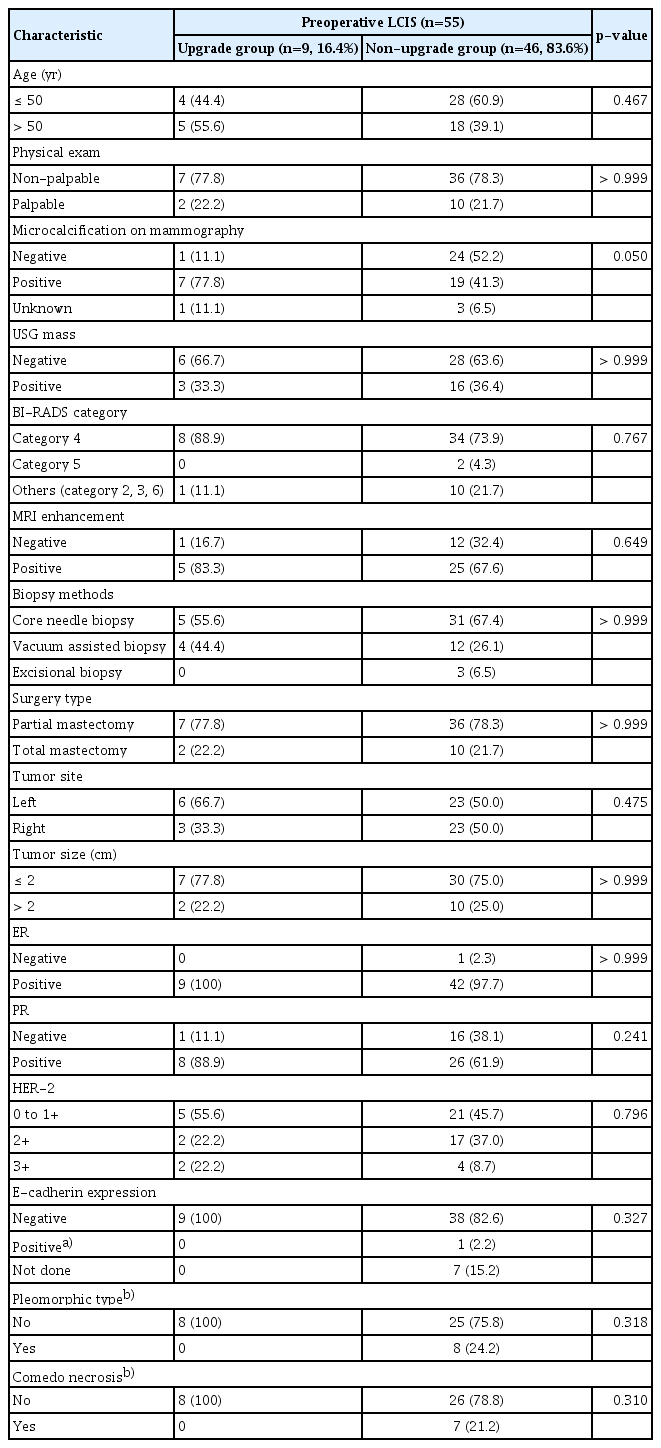
Clinicopathologic features were compared between the upgrade group and the non-upgrade group (Table 1). There was no significant difference between the two groups in terms of age; physical examination findings; ultrasonographic findings; Breast Imaging-Reporting and Data System (BI-RADS) category; MRI findings; biopsy methods; surgical methods; tumor site; tumor size; and the status of ER, PR, HER-2, and E-cadherin expression at baseline. Microcalcification on mammography was seen in 41.3% (19/46) of patients in the non-upgrade group and in 77.8% (7/9) of patients in the upgrade group, which indicated a marginally significant difference (p=0.05). In the multivariate analysis, microcalcification on mammography and PR positivity were significantly associated with the risk of upgrade of preoperative LCIS (odds ratio [OR], 14.155; p=0.023 and OR, 10.621; p=0.044) (Table 2).
Pleomorphism of LCIS was analyzed by preoperative biopsy (Table 1). The rates of pleomorphic LCIS and comedo necrosis were 19.5% and 17.1%, respectively. Based on preoperative biopsy findings, there were no cases of pleomorphic LCIS in the upgrade group, whereas there were eight cases of pleomorphic LCIS in the non-upgrade group. There were only seven cases of comedo necrosis in the non-upgrade group. There were no significant differences between the two groups in terms of pleomorphism and comedo necrosis (p=0.318 and p=0.310, respectively).
Discussion
The current study demonstrated that the upgrade rate of preoperative LCIS was 16.4%. A relatively significant proportion of the patients with preoperative LCIS had hidden invasive cancer that might be missed if only core needle biopsy is used as a definitive diagnostic tool. A previous study reported an 8.4%–9.3% rate of upgrade for LCIS, which was considerably higher than the acceptable target for surveillance, and the authors suggested excision of preoperative LCIS confirmed by a core needle biopsy [5]. Li et al. [7] suggested that LCIS might be a precursor of invasive carcinoma, and localized treatment for LCIS is warranted. Cheng et al. [8] also suggested that lumpectomy is the most appropriate management for LCIS. In this study, all upgrade groups were diagnosed by core needle or vacuum assisted biopsy, not by excisional biopsy (Fig. 2B). This result suggested that excision could be considered in case of LCIS for reliable tissue confirmation.
Nevertheless, there is controversy about the treatment of LCIS diagnosed on core needle biopsy, because of the varying upgrade rates mentioned in the literature (Table 3). Schmidt et al. [9] reported that the true upgrade rate of LCIS was 4% on excluding pleomorphic LCIS and image-discordant lesions. They suggested that surgery for classic LCIS is unnecessary, and careful radio-pathological correlation during initial biopsy is critical. Wen and Brogi [10] suggested that classic LCIS on core needle biopsy with concordant imaging does not require surgical resection. However, our study showed a relatively high rate of upgrade of LCIS, regardless of the presence of pleomorphism. There was no association between the upgrade rate and presence of pleomorphic LCIS or comedo necrosis in this study. Even though the pathologic slides of all cases of LCIS were reviewed by a specialized pathologist, no pleomorphism or comedo necrosis was detected in the upgrade group. Pleomorphic LCIS is considered to be an aggressive type of LCIS associated with high-grade DCIS and invasive cancer [11,12]. In the previous studies, the upgrade rates of pleomorphic LCIS were relatively high, at 20%–100% [9,13,14]. Hence, the NCCN guidelines recommend surgical excision for pleomorphic LCIS [1]. The difference in the upgrade rate of pleomorphic LCIS between the previous and current study might be due to the small sample size of each study and inter-observer variability in the pathological evaluations.
The definition of concordant images and pathologic results is variable [18]. Youk et al. [18] summarized five categories of radio-pathological correlation in a sonography-guided core needle biopsy of a breast lesion: concordant malignancy, discordant malignancy, concordant benign, discordant benign, and borderline or high-risk. Before the AJCC system 8th edition was published, LCIS was considered a malignant lesion; thus, when core needle biopsy for a BI-RADS category 4a lesion reveals classic LCIS, it could be considered either as discordant benign or borderline. When the radio-pathological correlation is borderline, a multidisciplinary approach or surgical excision to identify the hidden malignancy could be adopted. However, after the AJCC system 8th edition was published, when core needle biopsy for a category 4a lesion revealed classic LCIS, it was considered as concordant benign and not borderline or high-risk. In such cases, surgical excision should not be routinely recommended according to the NCCN guidelines. Since most previous studies used data obtained before the AJCC system 8th edition was published, the definition of the radio-pathological correlation for classic LCIS was considered to be either discordant benign or borderline. When we reviewed a previous study, the exact definition of the radio-pathological correlation was not specified [19,20]. Since the general recommendation of close follow-up for classic LCIS is based on ambiguous or arbitrary definition of the radio-pathological correlation for classic LCIS, it is difficult to routinely follow the revised guidelines for classic LCIS. A more detailed definition of the radio-pathological correlation for LCIS should be developed to avoid miscommunication among physicians, radiologists, pathologists, and surgeons.
The current study found that microcalcification on mammography and the expression of PR were significant in predicting the likelihood of upgrade of LCIS. A previous study reported a similar association between the upgrade rate of LCIS and mammographic calcification [5]. This result was similar to that observed in our study. Therefore, when cases of LCIS diagnosed on preoperative biopsy involve mammographic microcalcification and PR positivity, hidden invasive cancer or DCIS might be discovered after surgical excision (Fig. 3).

A case of the upgrade group with definite microcalcification on mammography without USG and MRI findings and the PR positivity. (A) Increased extent and amount of grouped microcalcification in lower central portion of the left breast on mammography. (B) Multiple probable benign enhancement without localized suspicious enhancements in both breasts on MRI. (C) Increased benign looking lesions in the both breasts on USG. MRI, magnetic resonance imaging; PR, progesterone receptor; USG, ultrasonography.
There were some limitations to this study. First, we analyzed a small number of cases. Few cases of preoperative LCIS were evaluated for calculating the upgrade rate. Second, when we reviewed the pathologic data, including data on pleomorphism, there were some missing data that might have affected the accuracy of the analysis. In the future, multi-center, large-scale, and long-term research on LCIS is necessary. Furthermore, IHC for core needle biopsy at preoperative pathologic evaluations is not always available in many institutions; thus, there is a limitation of generalization of the result of the current study. A multidisciplinary approach for evaluation of upgrading LCIS would be valuable for patients with LCIS in preoperative diagnosis. Finally, our research has a limitation of retrospective studies, selection bias. Nevertheless, the current study has valuable implications for clinical practice. Our study focused only on LCIS and not on lobular neoplasia or atypical lobular hyperplasia. We found that the ambiguity in the definition of radio-pathological correlation in the previous studies might have weakened the evidence of the current guidelines. In the current study, multivariate analysis found two significant predictors of the upgrade of preoperative LCIS.
This study showed a relatively high rate of upgrade to DCIS or invasive cancer in cases of preoperative LCIS. The presence of microcalcification on mammography and PRs can be potential predictors of upgrade. Surgical excision of LCIS during core needle biopsy could be considered as a management option to identify a hidden malignancy.
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of Severance Hospital, Yonsei University Health System (4-2020-0716). The informed consent was waived by IRB because of the retrospective design of the study.
Author Contributions
Conceived and designed the analysis: Park HS.
Collected the data: Lee J, Ku GY, Lee H, Park HS, Ku JS, Kim JY, Park S, Park BW.
Contributed data or analysis tools: Lee J, Ku GY, Lee H, Park HS, Ku JS, Kim JY, Park S, Park BW.
Performed the analysis: Lee J, Ku GY, Lee H, Park HS.
Wrote the paper: Lee J, Ku GY, Lee H, Park HS.
Revised and approved the submitted version of the manuscript: all authors.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.