Adjuvant Imatinib Treatment for 5 Years versus 3 Years in Patients with Ruptured Localized Gastrointestinal Stromal Tumor: A Retrospective Analysis
Article information
Abstract
Purpose
Three years of adjuvant imatinib is the standard treatment for resected gastrointestinal stromal tumors (GISTs) with rupture, but the recurrence rate is prominently high. We aimed to investigate the efficacy and safety of 5-year adjuvant imatinib compared with 3-year treatment in patients with a ruptured GIST following surgical resection.
Materials and Methods
A total of 51 patients were included in the analysis. The assessment of GIST rupture was based on Nishida’s classification. Twenty patients who were diagnosed before November 2013 were treated with 5 years of imatinib, and 31 patients who were diagnosed after November 2013 were treated with 3 years of imatinib. We retrospectively compared the clinical outcomes of the two groups.
Results
Baseline characteristics and the incidence of the adverse events were generally comparable between the two groups. During a median follow-up duration of 43.8 months and 104.2 months in the 3- and 5-year group, 8 and 9 patients had a disease recurrence, respectively. The 5-year group showed better recurrence-free survival (RFS) than the 3-year group. In multivariate analysis, low mitotic index was a significant independent favorable prognostic factor for RFS, while 5-year imatinib treatment was marginally associated with a favorable RFS.
Conclusion
Five years of adjuvant imatinib treatment in patients with ruptured GIST was associated with favorable survival outcomes with manageable toxicity profiles. Our findings warrant validation and confirmation in future studies.
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract [1]. Most GIST harbors an activating oncogene mutation in KIT (60%–70%) or platelet-derived growth factor receptor α (PDGFRA) (10%–15%) [2–4]. In the past two decades, the introduction of tyrosine kinase inhibitors (TKIs) has markedly improved the outcome of GIST. Imatinib mesylate, one of the selective TKIs that targets KIT and PDGRFA currently plays a crucial role in the management of GIST, both in the metastatic and adjuvant setting [5].
Because GIST is a soft, highly vascularized, and fragile tumor, it may rupture spontaneously or during surgical manipulation [6]. GIST rupture may cause spillage and dissemination of tumor cells into the intra-abdominal cavity, making it a significant adverse risk factor for tumor recurrence [5,7]. Currently, patients with GIST rupture are classified as a high-risk group for recurrence after resection according to the modified National Institutes of Health (NIH) Consensus Criteria [8].
Presently, 3-year adjuvant imatinib therapy is standard-of-care after resection for high-risk GIST patients including GIST rupture [5,9,10] based on the results of the Scandinavian Sarcoma Group (SSG) XVIII/Arbeitsgemeinschaft Internistische Onkologie (AIO) study [11]. However, the optimal duration of adjuvant therapy remains controversial [12]. Recently, the PERSIST trial, which evaluated the efficacy and tolerability of 5-year adjuvant imatinib in patients with resected GIST, reported the estimated 5-year recurrence-free survival (RFS) was 90% and suggested the role of long-term adjuvant therapy in preventing recurrence in GIST patients after resection [13]. Considering that the recurrence rate of patients with GIST rupture has been reported to be substantially higher than that of non-rupture patients after completion of 3 years of adjuvant imatinib therapy (55% vs. 14%) [14], an extended duration of adjuvant imatinib beyond 3 years could be considered to reduce the recurrence rate in patients with GIST rupture, but there has been a lack of evidence supporting this strategy.
At our institution, before the SSG XVIII/AIO study’s results were reported, patients with GIST rupture were considered as metastatic and treated with imatinib with palliative intent for at least 5 years. When there was no evidence of gross lesions after 5 years of imatinib treatment, the patients discontinued imatinib treatment and went into active surveillance. Since the approval and reimbursement of 3-year adjuvant imatinib based on the results of the SSG XVIII/AIO study in Korea in November 2013, 3-year adjuvant imatinib has been applied thereafter to patients with GIST rupture following surgical resection. Therefore, we had an opportunity to assess the clinical outcomes according to different durations of imatinib treatment in patients with GIST rupture.
Materials and Methods
1. Study patients
Between 2006 and 2018, a total of 1,409 patients who underwent macroscopically complete resection for localized non-metastatic GIST were identified from the GIST registry of Asan Medical Center, Seoul, Korea. We defined tumor rupture or perforation according to the Nishida classification through a comprehensive review of the preoperative radiologic findings of computed tomography (CT) and operative reports [15]. The Nishida classification defines GIST rupture as one of the following features: tumor spillage or fracture, blood-stained ascites, gastrointestinal perforation, microscopic infiltration into an adjacent structure, piecemeal resection, or intralesional dissection and incisional biopsy. Mucosal defect, intraluminal tumor perforation or gastrointestinal bleeding, microscopic peritoneal penetration of tumor cells or iatrogenic peritoneal damage, or R1 resection were not regarded as tumor rupture. According to the definition, a total of 53 patients who were documented as rupture or perforation were identified. After excluding two patients (one patient participated in a clinical trial that investigated adjuvant imatinib for 2 years (NCT00278876), and the other patient had concurrent metastatic gastric cancer), 51 patients were included as the study population.
2. Adjuvant imatinib treatment
To assess the efficacy and safety according to the different durations of adjuvant therapy, we divided the patients into two groups according to the treatment duration: the 3-year group (n=31) and the 5-year group (n=20) (Fig. 1). In principle, patients were started with 400 mg of imatinib once daily, and the dose was modified based on the grade of toxicity. Patients were followed-up at 4 weeks after initiating imatinib treatment to evaluate the tolerability of the treatment. Thereafter, regular physical examination and laboratory assessments, CT scans of the abdomen and pelvis and chest radiographs were conducted every 3 months during the treatment duration and the first 2 years after stopping treatment, and every 6 months for the next 3 years if disease recurrence was not documented. Adverse events were identified by retrospective medical records review and evaluated according to the Common Terminology Criteria for Adverse Events, ver. 5.0. For the non-hematologic toxicities, only grade 3 or higher adverse events were analyzed since there was a limitation in the complete analysis of toxicities of a lesser degree in this clinical practice setting.
3. Statistical analysis
The chi-squared test or Fisher’s exact test was used to analyze the categorical variables. RFS was defined as the time from the date of the operation to the date of the first radiologically documented disease recurrence or death from any cause, whichever occurred first. Overall survival (OS) was defined as the time from the date of the operation to the date of death from any cause or the last follow-up. The Kaplan-Meier method was used to estimate the OS and RFS, and a two-sided log-rank test was used to compare the treatment groups. Multivariate analysis using Cox’s proportional hazards model was performed to evaluate the prognostic value of the risk factors, including age, location of the tumor, largest tumor diameter, mitotic count, gene mutation type, and duration of treatment. A p-value < 0.05 was considered statistically significant. All data analysis was performed using R statistical software ver. 4.0.1 (R Core Development Team, Vienna, Austria).
Results
1. Characteristics of the study patients and tumor rupture
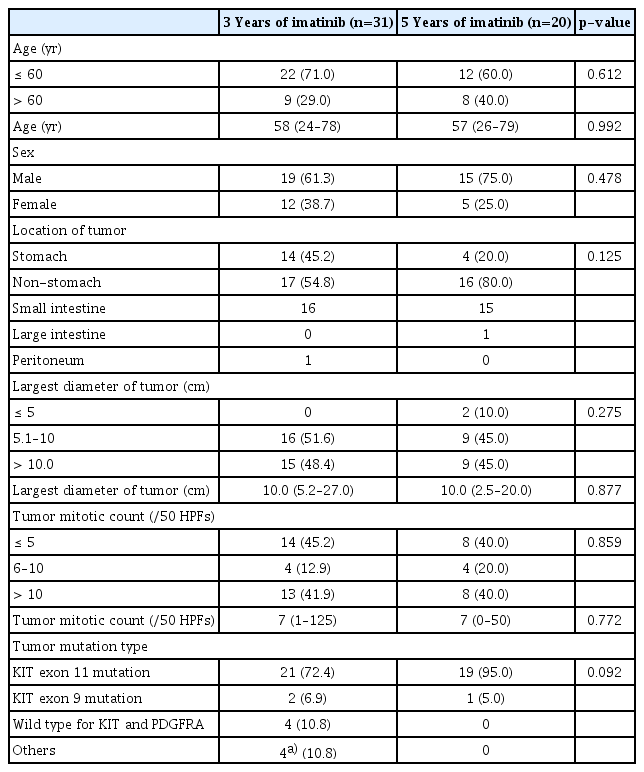
Table 1 shows the baseline characteristics of the study patients. All patients were Korean. Thirty-four patients (66%) were male, and the median age was 58 years (range, 24 to 79 years). There were no significant differences in the baseline characteristics between the 3-year group and the 5-year group, although the proportion of patients who had a non-stomach primary tumor tended to be higher in the 5-year group (80%) than the 3-year group (54.8%) (p=0.125). All patients were included in the high-risk group, according to the modified NIH criteria due to tumor rupture. According to the Armed Forces Institute of Pathology risk criteria, most of the patients were classified into the 6a group (S1 Table) [16]. The most common genotype was a KIT exon 11 mutation: 72% in the 3-year group and 95% in the 5-year group. There was no patient with the PDGFRA D842V mutation.
The features related to GIST rupture according to Nishida’s classification are summarized in S2 Table. The most common type was tumor fracture and/or tumor spillage (n=13, 41%) and gastrointestinal perforation through the tumor (n=9, 45%) in the 3- and 5-year groups, respectively.
2. Treatment profiles
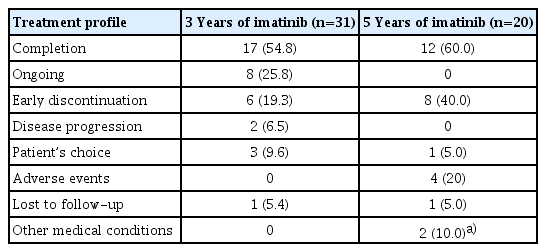
The median follow-up duration of the survivors was 43.8 months (range, 16.0 to 95.7 months) and 104.2 months (range, 60.0 to 149.3 months) in the 3- and 5-year group, respectively. Overall, 17 (54%) and 12 (60%) patients completed the scheduled treatment, and six (19.3%) and eight (40%) patients discontinued treatment early in the 3- and 5-year group, respectively (Table 2). The median adjuvant duration of the 5-year group was 60.0 months (range, 2.3 to 60.0 months), and the median adjuvant duration of the 3-year group except those with ongoing adjuvant treatment was 36.0 months (range, 0.78 to 36.0 months).
In the overall study population, disease recurrence was documented in 17 patients (9 in the 3-year group; 8 in the 5-year group) including two patients whose disease recurred while receiving adjuvant imatinib. Among them, three patients showed a locoregional recurrence (2 in the 3-year group and 1 in the 5-year group), and 14 patients showed distant recurrence (7 patients in each group). The most common distant recurrence site was the peritoneum (6 in the 3-year group and 4 in the 5-year group) followed by the liver (1 in the 3-year group and 3 in the 5-year group).
Most of the patients were treated with 400 mg imatinib once daily for first-line treatment after recurrence, except two patients whose disease recurred while they were receiving adjuvant imatinib. Of these two patients, one had a KIT exon 17 mutation and was treated with sunitinib, and the other had a KIT exon 11 mutation and was treated with 600 mg of imatinib.
3. Survival outcomes
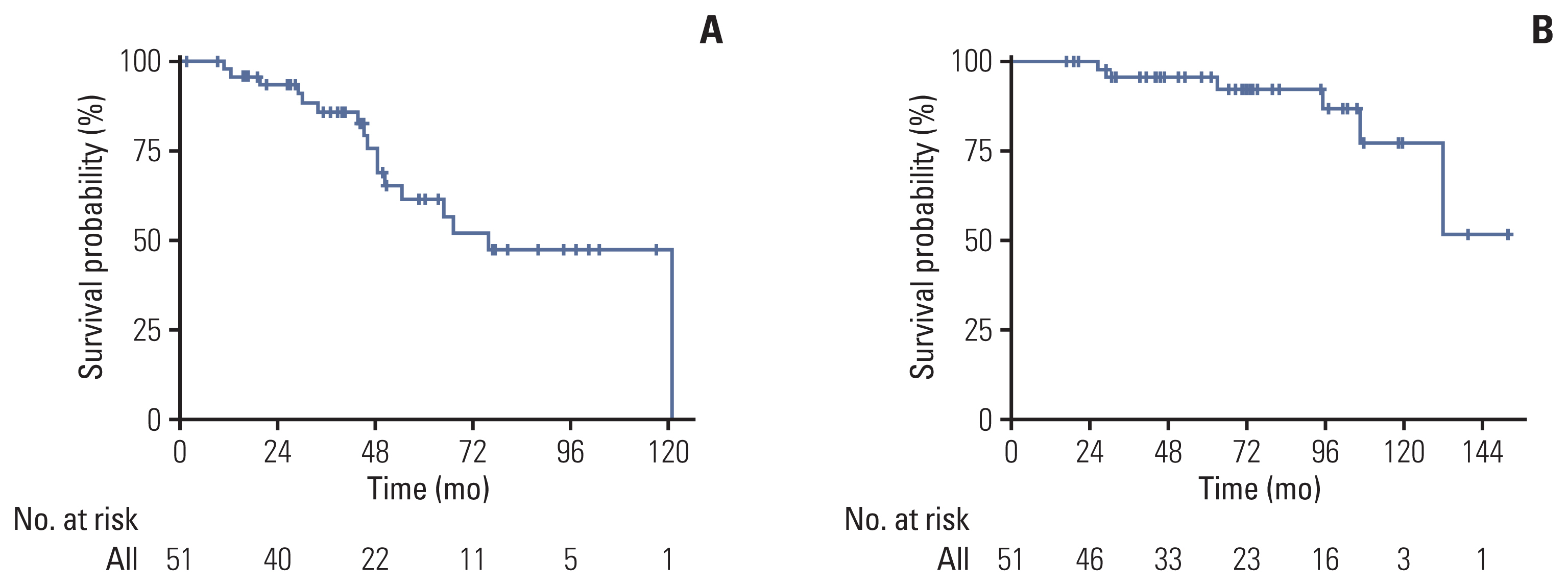
The 5-year RFS and OS of all patients were 61.4% (95% confidence interval [CI], 46.4 to 81.2) and 95.7% (95% CI, 89.0 to 100), respectively (Fig. 2). In the survival analysis according to treatment duration, the 5-year group showed better 5-year RFS than the 3-year group (78% vs. 30%, p=0.042) (Fig. 3A), while OS was comparable between the two groups (Fig. 3B). Among patients who had a KIT exon 11 mutation (n=40), the 5-year group still showed a trend toward a better RFS (p=0.089; 4-year RFS, 83.3% vs. 61.5%, respectively) (S3 Fig.).

Comparison of RFS (A) and OS (B) according to adjuvant imatinib treatment duration. CI, confidence interval; OS, overall survival; RFS, recurrence-free survival.
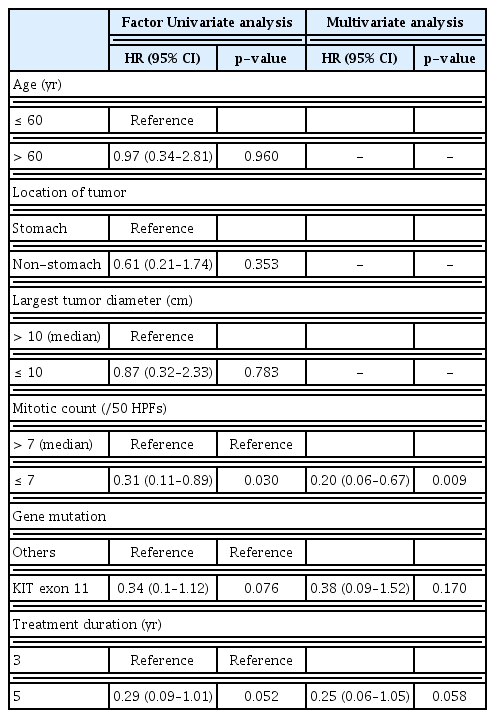
In the multivariate analysis, low mitotic count (≤ median) was independently associated with favorable RFS (hazard ratio [HR], 0.20; 95% CI, 0.06 to 0.67; p=0.009), while the 5-year imatinib treatment was marginally associated with a favorable RFS (HR, 0.25; 95% CI, 0.06 to 1.05; p=0.058) (Table 3).
4. Toxicity profiles and dose modification
Detailed toxicity profiles are described in S4 Table. There were no statistically significant differences in the frequency of toxicity between the 3-year and 5-year groups. Permanent dose modification (300 mg/day or 200 mg/day) was required in four patients (12%) in the 3-year group, and three patients (15%) in the 5-year group. None of the patients in the 3-year group discontinued treatment due to toxicity; however, four patients in the 5-year group eventually discontinued treatment prematurely due to intolerable toxicity despite dose modification (n=3) or steroid therapy (n=1) (Table 2). All of the toxicities leading to treatment discontinuation in the 5-year group occurred within the first year of imatinib initiation; grade 3 skin toxicity (at 6 months), grade 3 nausea and vomiting (at 2 months), grade 3 neuropathy (at 9 months), and grade 3 fatigue (at 12 months).
Discussion
In this registry-based retrospective study, we analyzed the safety and efficacy profiles of 5 years of imatinib compared to the standard 3 years of imatinib for patients with GIST rupture following surgical resection. The 5-year imatinib treatment exhibited favorable RFS (78% vs. 30% at 5 years, p=0.042) with an association with a reduced risk of recurrence in multivariate analysis (HR, 0.25; p=0.058). Furthermore, the frequency of adverse events in the 5-year group was not significantly different from that of the 3-year group. To our knowledge, this is the first study to highlight the feasibility of extended imatinib therapy based on the systematic definition of GIST rupture proposed by Nishida et al. [15], which may provide evidence and support for conducting future prospective studies.
Joensuu et al. [17] showed that even among patients with the same high risk according to the modified NIH risk classification, the 10-year recurrence rate varied widely from 30% to 100%; the authors also showed that the recurrence rate of patients with rupture was higher than that of patients without rupture, regardless of tumor size, location, and mitosis count. Accordingly, a recent report on the real-world clinical outcomes of high-risk patients treated with adjuvant 3-year adjuvant imatinib showed that patients with rupture had a higher recurrence rate than did those without rupture even after adjuvant treatment [14]. These findings suggest that the prognosis of patients with GIST rupture differs from that of high-risk GIST patients without rupture, and that patients with GIST rupture may need more intensive or longer treatment to reduce the risk of recurrence.
While our study suggested the potential benefit of prolonging the duration of adjuvant imatinib from 3 to 5 years, the follow-up analysis of the SSG XVIII/AIO trial showed that 3-year imatinib treatment was not significantly associated with a reduced risk over 1-year adjuvant imatinib treatment in patients with GIST rupture [18]. Therefore, we assume that while 3-year administration of imatinib may not be sufficient to reduce the recurrence risk relative to 1-year treatment, prolonged adjuvant imatinib over 3 years appears to lead to a reduced risk of recurrence. Currently, two randomized clinical studies are ongoing to evaluate the clinical utility of prolonged adjuvant imatinib (NCT02413736, NCT02260505); however, these studies did not focus specifically on patients with GIST rupture. Given the low incidence of GIST tumor rupture and the associated difficulty of conducting a prospective study focusing on GIST tumor rupture patients [19,20], our results provide valuable insights into the optimal duration of adjuvant imatinib for patients with GIST rupture.
Generally, it is well known that the benefits of adjuvant imatinib are greater in patients with a KIT exon 11 mutation than in patients with a KIT exon 9 mutation [21], and there is no established strategy of adjuvant imatinib treatment for those with non-exon 11 mutations or wild-type GIST [1]. In this study, to avoid the potential bias caused by different genotypes, additional subgroup analysis was conducted only on patients who had KIT exon 11 mutation, and it revealed the same trend of RFS benefit from 5-year imatinib.
There are several concerns with prolonged imatinib treatment. First, long-term exposure to imatinib would have the potential to increase the frequency of adverse events. In our study, all toxicities leading to imatinib discontinuation occur-red in the 5-year group. However, since all of the patients discontinued treatment due to toxicity within a year from the initiation of imatinib administration, the enrichment of patients who discontinued imatinib due to adverse events in the 5-year group does not appear to be attributable to prolonged imatinib administration. Besides, in our institution, there have been recent advances in the management of imatinib toxicities and improvement of treatment outcomes associated with them [22,23]. Considering that patients in the 5-year imatinib group were treated about 5 years earlier than those in the 3-year imatinib group, the 3-year group might have received better management for imatinib toxicities, and it could be another reason for differences in the proportion of early discontinuation between the two groups.
Patient medication adherence is an essential issue because it is highly associated with a successful treatment outcome [24]. In the PERSIST-5 trial, nearly 50% of patients discontinued imatinib earlier than the scheduled 5-year period; 20% of them discontinued treatment due to the patient’s choice [13]. Therefore, to maintain medication adherence and obtain optimal treatment effects of adjuvant therapy, attending physicians should focus on educating patients about their disease status, the benefits of adjuvant therapy, and how to manage the side effects of imatinib.
One of the important aspects of our study is that we comprehensively applied the systematic definition of ruptured GIST by Nishida et al. [15]. Most of the previous studies of GIST rupture have not clearly described how they defined GIST rupture, which may have contributed to the varying incidence of GIST rupture [6,15,17,25,26]. Future studies should be performed based on a systematic definition of GIST rupture such as Nishida’s classification to accurately define the patient subgroup with rupture and avoid misclassifications.
Some limitations of this analysis should also be considered. First, because our study was retrospective and based on a single-center experience, our results may be subject to selection bias. In particular, the patient classification of our study was not prospectively determined, which may limit the interpretation and generalization of our findings. Another limitation is the small sample size. Indeed, the small number of study patients appears to be the main reason for the difference in RFS not reaching statistical significance in multivariate analysis. Nevertheless, considering the rarity of ruptured GIST, the number of patients included in our study was relatively larger than that of the previous studies of ruptured GIST [15,20,27,28]. Finally, we were not able to show an OS difference according to the treatment duration. Whether the RFS benefit of 5-year imatinib treatment could be translated into an OS benefit should be further confirmed with a longer follow-up.
In conclusion, 5 years of imatinib treatment following surgical resection of ruptured GIST may be feasible and associated with favorable survival outcomes with manageable toxicity profiles. Our findings warrant validation and confirmation in future studies.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of Asan Medical Center, Korea (approval number: 2021-1489), and the study was conducted according to the principles of the Helsinki declaration. Due to the nature of this retrospective study, the requirement of obtaining informed consent was waived by the IRB.
Author Contributions
Conceived and designed the analysis: Kang YK.
Collected the data: Ryu MH, Bang YH, Kim HD, Lee HE, Kang YK.
Contributed data or analysis tools: Kang S, Ryu MH, Bang YH, Kim HD, Kang YK.
Performed the analysis: Kang S, Ryu MH, Kim HD, Lee HE, Kang YK.
Wrote the paper: Kang S, Ryu MH, Kim HD, Kang YK.
Conflicts of Interest
Nothing directly related to this work. Outside of this work, YKK has served as a consultant for ALX Oncology, Zymeworks, Amgen, Novartis, Macrogenics, Daehwa, Blueprint, Surface Oncology, BMS, and Merck (MSD).