A Single-Arm, Prospective, Phase II Study of Cisplatin Plus Weekly Docetaxel as First-Line Therapy in Patients with Metastatic or Recurrent Salivary Gland Cancer
Article information
Abstract
Purpose
Salivary gland cancers (SGCs) are relatively rare but comprise various histologic subtypes, which complicates design of prospective trials. Systemic chemotherapy plays a limited role in treatment of SGCs, but cisplatin and docetaxel showed efficacy in a previous preclinical study. Here, we conduct a prospective, phase II study to evaluate the efficacy and toxicities of cisplatin plus weekly docetaxel in patients with metastatic or recurrent SGC.
Materials and Methods
We included patients with histologically confirmed SGCs of the following subtypes: mucoepidermoid carcinoma, adenocarcinoma, ductal carcinoma, or adenoid cystic carcinoma. Patients had no prior systemic chemotherapy for metastatic or recurrent tumors and at least one measurable lesion. Patients were treated with docetaxel 35 mg/m2 (D1, 8) and cisplatin 70 mg/m2 (D1) every 21 days.
Results
Forty-one patients were enrolled between April 2014 and October 2020. The median age was 58 years (range, 32 to 73 years). The most common histologic subtype was adenoid cystic carcinoma (63.4%), followed by ductal carcinoma (24.4%). The most common metastatic site was the lung (75.6%). The median treatment cycle was 5.5 (range, 3 to 8), and the objective response rate was 46.3%, with three complete responses. The median duration of response was 6.8 months (interquartile range, 4.0 to 10.2). The progression-free survival and overall survival were 9.4 months (95% confidence interval [CI], 8.4 to 10.5) and 28.2 months (95% CI, 22.7 to 33.6), respectively. There were no treatment-related deaths. The most common grade 3/4 adverse events were neutropenia (4.9%) and fatigue (4.9%).
Conclusion
Cisplatin plus weekly docetaxel is effective and tolerable with manageable toxicity as first-line therapy in patients with metastatic or recurrent SGC.
Introduction
Salivary gland cancers (SGCs) are relatively rare, accounting for 1%–6% of all cancers of the head and neck [1]. They have various histologic patterns, with 23 histologic subtypes according to the 2017 World Health Organization classification. The prognosis and behavior of these tumors vary considerably according to subtype. In previous studies, adenoid cystic carcinoma (ACC) had a 10-year survival rate of 52%–65%, whereas mucoepidermoid carcinoma (MEC) and adenocarcinoma had 5-survival rates of about 75% and 40%, respectively [2–4]. In some cases, patients suffer rapid progression and die due to their cancers, highlighting the need for effective therapies. However, because of the rarity, indolent nature of disease, and heterogeneity among subtypes, it is difficult to conduct prospective clinical study in patients with SGCs [5,6].
Although cisplatin and cisplatin-based regimens have been explored most frequently, the response rates to these protocols have been modest and their effects on survival were impossible to discern [7,8]. In a preclinical study, paclitaxel and docetaxel demonstrated activity against SGCs [9,10]. A phase II trial of single-agent paclitaxel was conducted by the Eastern Cooperative Oncology Group (ECOG) in 45 patients with advanced SGC, among whom eight of 31 patients with MEC or adenocarcinoma achieved partial response (PR). However, responses were not identified in 14 patients with ACC [9]. In another phase II study, Catimel et al. [10] reported antitumor activity of docetaxel in head and neck cancer. Based on its impressive antitumor activity, Raguse et al. [11] evaluated the activity of docetaxel in four patients with high-grade MEC of the major salivary glands. The treatment was well tolerated, and two patients achieved complete response (CR) while the others exhibited PR [11]. The combination chemotherapy of docetaxel and cisplatin has proven efficacy in patients with non–small cell lung cancer and recurrent or metastatic head and neck cancer [12,13]. However, myelosuppression is one of serious concerns with every 3-week schedule of docetaxel administration [13]. In a previous study of cisplatin plus weekly docetaxel in recurrent or metastatic nasopharyngeal cancer, we observed a high response rate and modest toxicities, suggesting that weekly docetaxel plus cisplatin is a reasonable therapeutic option [14].
Here, we present a prospective phase II study of cisplatin plus weekly docetaxel to evaluate the efficacy and toxicities in patients with metastatic or recurrent SGCs.
Materials and Methods
1. Eligibility criteria
Eligible patients were 18 years of age or older, with an ECOG performance status of 0–1. Patients had to be diagnosed with histologically confirmed SGCs of the head and neck or other sites and with one of the following histologic subtypes: MEC, adenocarcinoma, ductal carcinoma, or ACC. Eligible patients had stage IV disease or recurrent tumor incurable by surgery or radiotherapy and at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Given the indolent nature of ACC, only patients who experienced progressive disease were eligible to participate in this study. Disease progression was defined as one of the following occurring within 6 months of study entry: an at least 20% increase in radiologically or clinically measurable disease, appearance of new lesions, or deterioration in clinical status. These exclusion criteria were applied only for ACC but not for non-ACC. Patients had to be expected to survive for approximately 12 weeks or longer, and prior systemic chemotherapy was not allowed, except adjuvant chemotherapy or chemotherapy completed more than 6 months prior. Major surgery or radiotherapy had to be completed at least 4 weeks before enrollment. Other requirements were adequate organ function defined as absolute neutrophil count (ANC) ≥ 1,500/mm3, hemoglobin ≥ 10 g/dL, platelets ≥ 100,000/mm3, total bilirubin ≤ 1.5×upper normal limit (UNL), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5×UNL (if liver metastases present, AST and AST ≤ 5.0×UNL), and creatinine clearance (CCr) ≥ 50 mL/min or serum creatinine ≤ 1.5×UNL. Exclusion criteria included uncontrolled hypertension, unstable or severe angina, history of myocardial infarction within the last 12 months, uncontrolled arrhythmia, or congestive heart failure with New York Heart Association classification III or IV. Patients with uncontrolled systemic disease such as diabetes mellitus, thyroid disease, or active infection were also excluded. Symptomatic central nervous system malignancies were excluded, except metastases completely resected or irradiated by whole brain radiation therapy or stereotactic radiosurgery. Patients with alcohol abuse were excluded.
2. Treatment plan
Eligible patients were treated with docetaxel 35 mg/m2 over 1 hour on days 1 and 8 and cisplatin 70 mg/m2 over 1 hour on day 1, with the doses repeated every 21 days. Tumor response was evaluated every 6 weeks until disease progression. Response was evaluated using computed tomography (CT) or magnetic resonance imaging according to RECIST 1.1 criteria. Treatment was continued until disease progression, unacceptable toxic effects, or withdrawal of informed consent. Dose reduction and treatment delay owing to adverse events (AEs) were allowed. However, if the patients required second dose reduction or could not receive the study treatment within 42 days of the beginning of the previous cycle, they discontinued the study permanently.
3. Treatment modifications
All toxicities were graded according to the Common Terminology Criteria for Adverse Events scale, ver. 4.0. Patients were treated with full or −1 level dose, which was 25% dose reduction (26 mg/m2 of docetaxel and 53 mg/m2 of cisplatin). For subsequent cycles, ANC ≥ 1,500/mm3 and platelets ≥ 100,000/mm3 were required. However, the wash-out period should not exceed 2 weeks. If myelotoxicity was recovered partially after the 2-week wash-out period (ANC ≥ 1,500/mm3 or platelets ≥ 100,000/mm3), both drugs were given at −1 level dose. If there was febrile neutropenia or ANC < 500/mm3, chemotherapy was postponed until ANC ≥ 1,500/mm3 and platelets ≥ 100,000/mm3 and both drugs were administered at −1 level dose.
In cases of abnormal renal function (CCr < 50 mL/min), docetaxel was administered as planned, but dose reduction in cisplatin occurred after a one-week delay. If CCr was ≥ 50 mL/min in the next week, cisplatin was administered at the previous dose. If CCr was not recovered within 3 weeks, patients were dropped out from the study. Dose reductions in both drugs were made for grade 2 peripheral neuropathy and grade 3 or 4 mucositis including esophagitis. If grade 3 or 4 neuropathy occurred, treatment was discontinued. If there were mild symptoms including localized cutaneous reaction such as mild pruritus, flushing, or rash, the infusion rate was decreased until recovery from symptoms. In cases of grade 2 hypersensitivity, diphenhydramine 50 mg intravenously with or without dexamethasone 10 mg intravenously was administered with monitoring until resolution of symptoms. Docetaxel infusion was resumed after recovery of symptoms. Depending on the physician’s assessment of the patient, docetaxel infusion should be resumed at a slower rate and then increased incrementally to the initial planned rate. Depending on the intensity of the reaction observed, additional oral or intravenous per-medication with an antihistamine should be given for the next cycle of treatment, and the infusion rate should be decreased initially and then increased to the recommended rate. For severe symptoms or anaphylaxis, such as bronchospasm, generalized urticaria, systolic blood pressure under 80 mm Hg, or angioedema, docetaxel infusion was discontinued immediately and diphenhydramine 50 mg intravenously with or without dexamethasone 10 mg intravenously and/or epinephrine were administered as needed while monitoring patients until resolution of symptoms. These patients would be taken off the study.
4. Statistical analyses
The primary endpoint was response rate, and the secondary endpoints included overall survival (OS), progression-free survival (PFS), and safety and toxicity profiles. PFS was defined as the time from the date of treatment to the date of documented disease progression, the date of death from any cause, or the last follow-up, whichever came first. OS was defined as the time from the date of treatment to the date of death from any cause and was censored at the date of the last follow-up visit. Response rate for SGCs after docetaxel single therapy was reported previously to be 0% to 12% according systemic review [15]. Assuming a response rate of 0.20, target error of 0.05, and power of 90%, we required 38 patients according to SWOG Cancer Research And Biostatistics (CRAB). Considering a 10% dropout rate, the total recruitment needed to include 42 patients. The total study period was expected to be about 48 months (36 months for accrual of patients and 12 months for additional follow-up). This time period could be shortened or prolonged depending on patient accrual rate. Survival analysis was performed using the Kaplan-Meier method and compared by the log-rank test. p-values less than 0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS statistics ver. 27 (IBM Corp., Armonk, NY).
Results
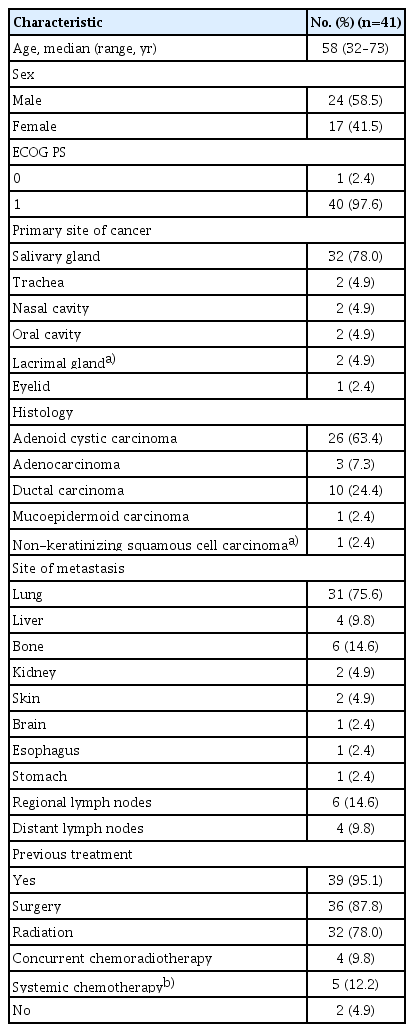
1. Patient characteristics
Between April 16, 2014, and October 13, 2020, a total of 41 patients was enrolled in this study from Samsung Medical Center. The median age was 58 years (range, 32 to 73 years), and the male to female ratio was 58.5% to 41.5%. Forty patients had ECOG performance status of 1. The most common histological subtype was ACC (63.4%), followed by ductal carcinoma (24.4%). Two patients were treatment-naïve, and the other 39 patients had received treatment for tumors, including surgery (87.8%), radiation (78.0%), concurrent chemoradiotherapy (9.8%), and systemic chemotherapy (12.2%). The most common site of metastasis was the lung (n=31, 75.6%), followed by bone (n=6, 14.6%) and regional lymph nodes (n=6, 14.6%) (Table 1). Four patients with SGCs and one patient with nasal cavity cancer previously had been treated with systemic chemotherapy. Among them, three patients had been given neoadjuvant (n=1) or adjuvant (n=2) chemotherapy, and the other two had been given palliative chemotherapy, which was completed 6 months or longer before enrollment in this study.
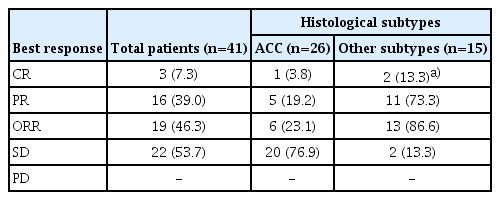
2. Efficacy and survival analysis
Median treatment duration was 3.9 months (interquartile range [IQR], 3.2 to 4.7) and median number of treatment cycles was 5.5 (range, 3 to 8). The objective response rate (ORR) was 46.3% (n=19/41; 95% confidence interval [CI], 38.4 to 68.9), with three CR (7.3%) and 16 PR (39.0%). According to histologic subtype, 23.1% of patients (n=6/26) with ACC achieved PR including 1 CR, whereas 86.6% (n=13/15) of patients with other subtypes showed PR including two CR (Table 2). In patients with ductal carcinoma or adenocarcinoma, ORR was 79% with one CR (7.7%) and nine PR (69.2%). The best response in each patient is depicted in Fig. 1 and the CT imaging of the patient who achieved CR is presented in Fig. 2.
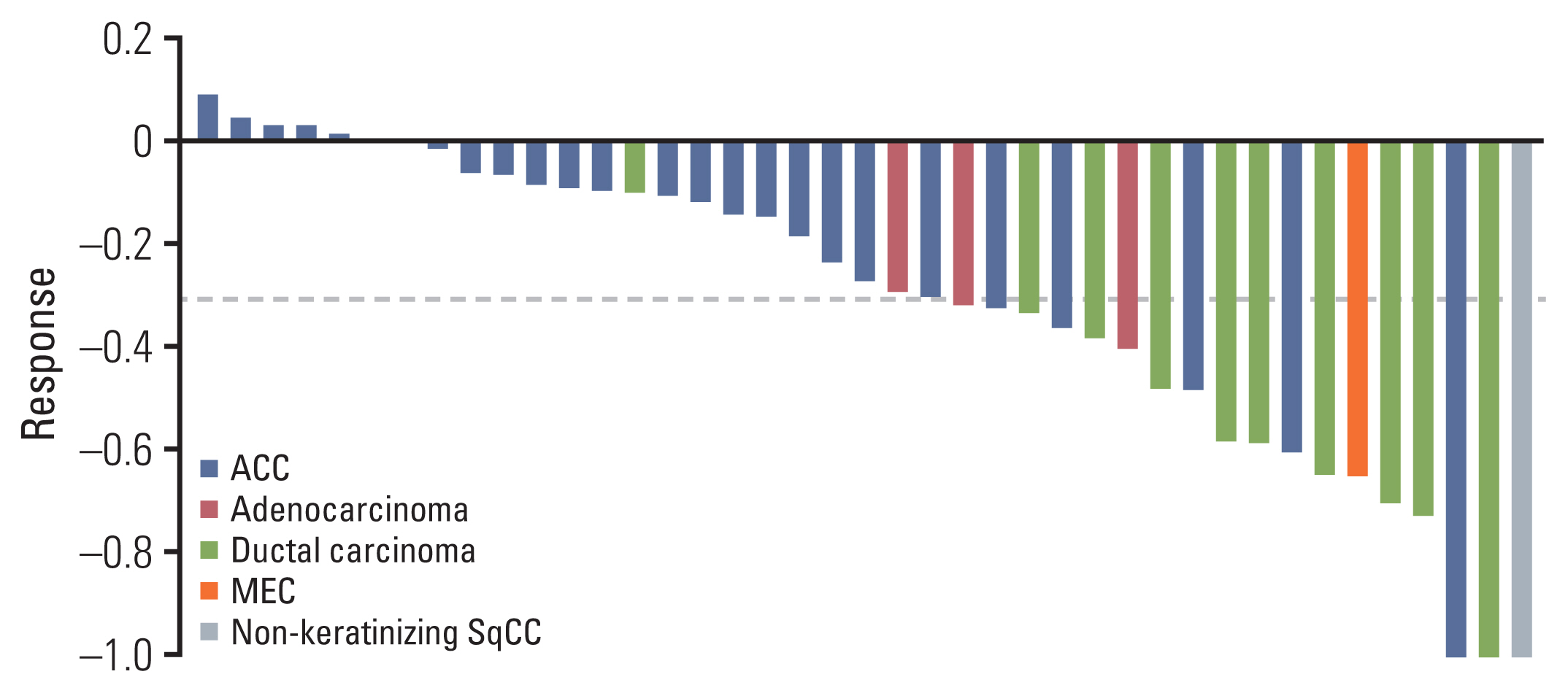
Waterfall plot presenting response to cisplatin plus weekly docetaxel in each patient. The patient who experienced first progression around 4 months achieved stable disease (decreased size about 10%) and then progressed. ACC, adenoid cystic carcinoma; MEC, mucoepidermoid carcinoma; SqCC, squamous cell carcinoma.
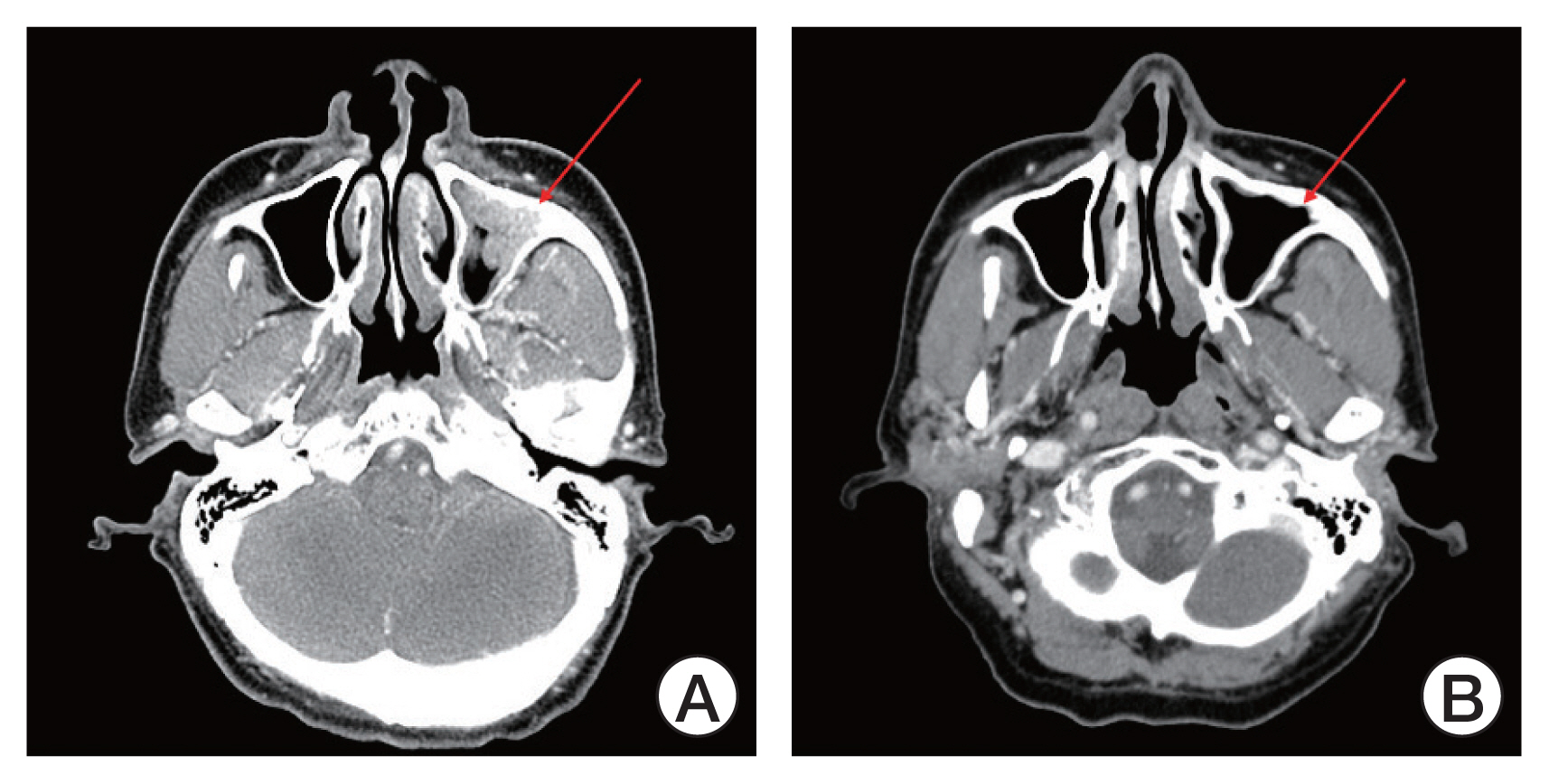
Computed tomography (CT) imaging of patient achieving complete response (CR). (A) CT imaging at the time of screening (August 2018). (B) CT imaging at the time when the patient achieved CR (April 2019). The red colored arrows indicate the lesion before and after treatment.
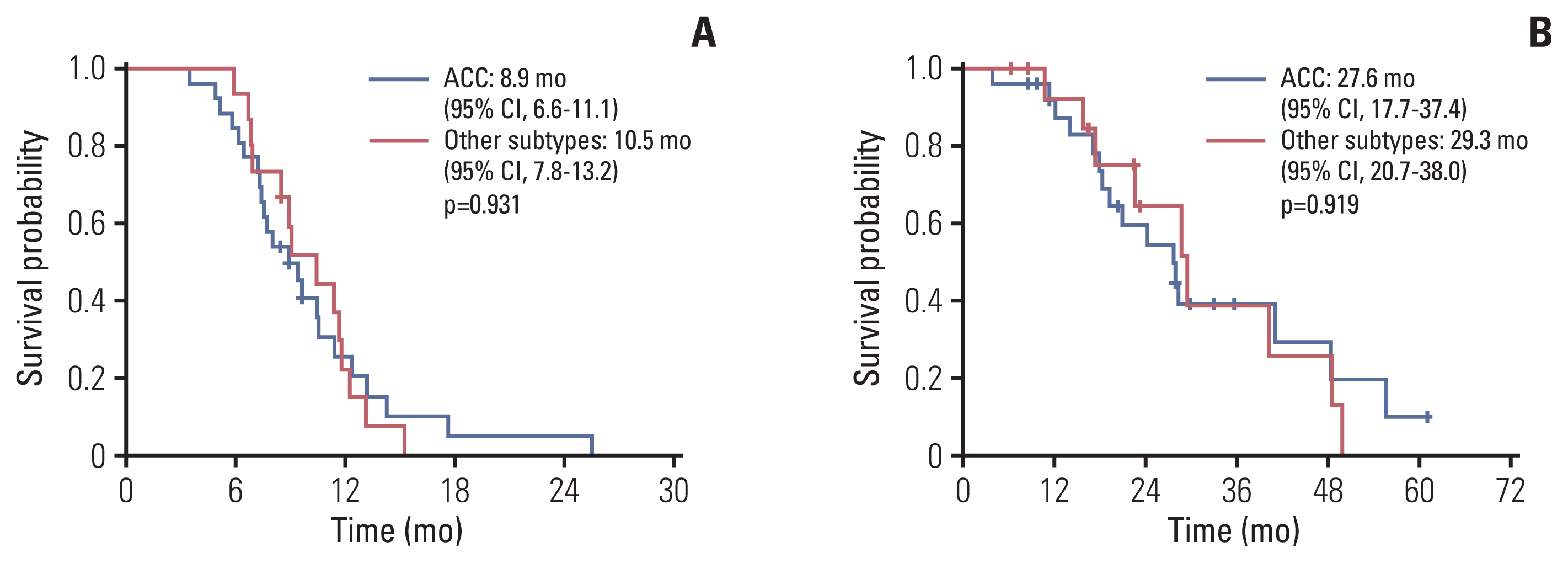
The median follow-up duration was 18.8 months (range, 3.8 to 60.7 months). In all patients, the median PFS was 9.4 months (95% CI, 8.4 to 10.5) (Fig. 3A), the median duration of response was 6.8 months (IQR, 4.0 to 10.2), and the median OS was 28.2 months (95% CI, 22.7 to 33.6) (Fig. 3B). According to histological subtype, the median PFS and OS were 8.9 months (95% CI, 6.6 to 11.1) and 27.6 months (95% CI, 17.7 to 37.4), respectively, in patients with ACC and 10.5 months (95% CI, 7.8 to 13.2) and 29.3 months (95% CI, 20.7 to 38.0) in patients with other subtypes. These were not significantly different between subtypes (p=0.931 and p=0.919, respectively) (Fig. 4A and B). In patients with ductal carcinoma or adenocarcinoma (n=13), the median PFS and OS were 9.1 months (95% CI, 6.7 to 11.4) and 29.3 months (95% CI, 15.4 to 43.3), respectively. There was no significant difference compared to the results in all patients (p=0.521 and p=0.545).
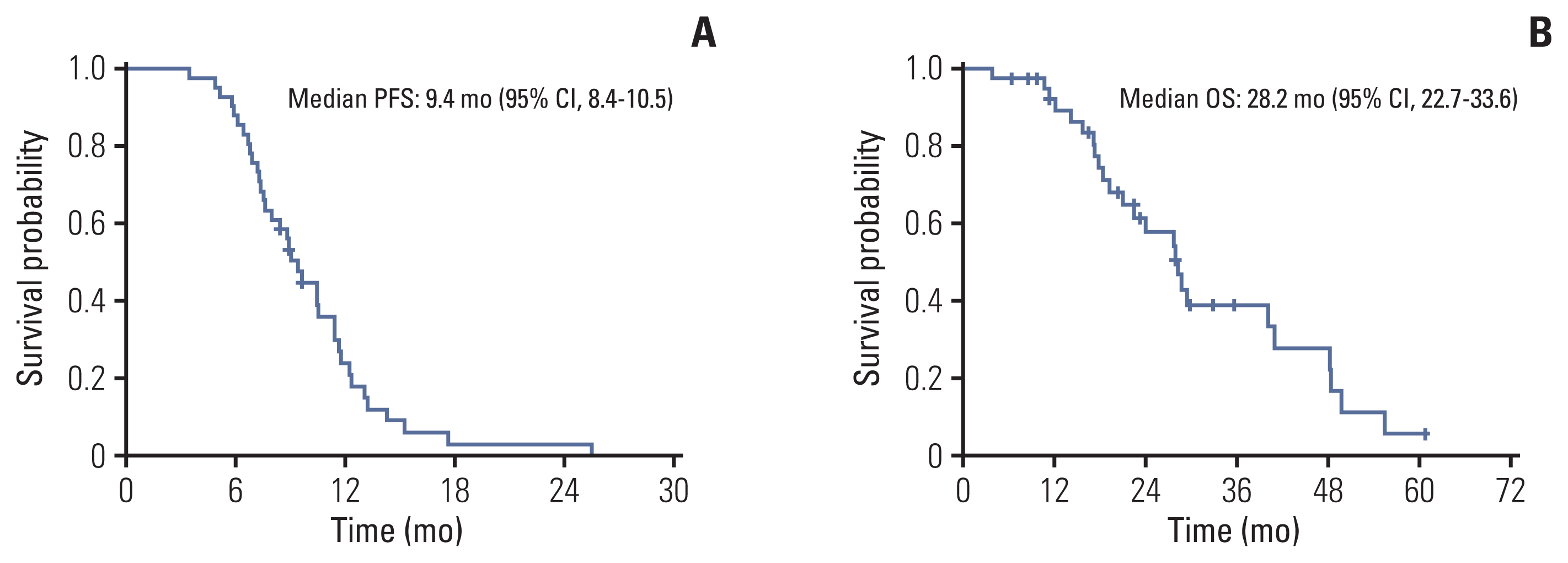
Kaplan-Meier curves of survival analysis: progression-free survival (PFS) (A) and overall survival (OS) (B) of total population. CI, confidence interval.
3. Safety and toxicity profiles
There were no treatment-related deaths in this study. The most common AE of any grade was alopecia (61.0%), followed by anorexia (43.9%) and diarrhea (31.7%). Grade 3 or 4 AEs included neutropenia (4.9%) and fatigue (4.9%) (Table 3). Treatment schedule was delayed in 16 patients (39.0%) and dose modification was required in 14 patients (34.1%) due to toxicity. Neutropenia (19.5%) was the leading cause of treatment delay, and diarrhea (4.9%) and mucositis (4.9%) were the most common causes of dose reduction (Table 4).
Discussion
It is a challenge to conduct prospective clinical trials in patients with metastatic SGC due to the indolent nature of the disease course and heterogeneous histologic subtypes. In this prospective study, cisplatin plus weekly docetaxel demonstrated a high ORR (46.3%) and long PFS (median 9.4 months) regardless of histological subtype. Further, this combination therapy was tolerable and associated with low incidence of neutropenia and gastrointestinal toxicities.
While surgery and/or radiotherapy are recommended to treat localized SGC, systemic chemotherapy is reserved for palliative treatment in patients with symptomatic locally recurrent and/or metastatic disease that is not suitable for treatment by further surgery or radiation [6]. However, given the lack of standard therapy in patients with metastatic SGC, there are unmet medical needs for development of novel treatment strategies [9,11,16]. In general, most patients with metastatic ACC experience slow progression; due to lack of effective systemic chemotherapy, wait and see is the most commonly used approach. To minimize bias related to the indolent nature of ACC, we enrolled only patients who experienced progressive disease, in whom progression occurred within 6 months of study entry, with an at least 20% increase in radiologically or clinically measurable disease, appearance of new lesions, or deterioration in clinical status. It is promising that 23% of our patients achieved PR with 1 CR and PFS 8.9 months. Intriguingly, in patients with other histologic subtypes, the ORR was 86.6% with 2 CR and median PFS was 10.5 months. Considering the dismal prognosis in patients with salivary ductal carcinoma, the results of this combination therapy are encouraging. However, to confirm our results, large, randomized studies are needed in both ACC and salivary ductal carcinoma.
Docetaxel usually is administered at a dose of 60–75 mg/m2 on day 1 every 21 days and is associated with high incidence of mucositis, other gastrointestinal toxicities, and myelosuppression, which can be increased when administered in combination with cisplatin. Previously, we reported that cisplatin with weekly docetaxel showed high response rate and modest toxicities in metastatic or recurrent nasopharyngeal cancer [14]. In the current study, grade 3 or 4 neutropenia was found only in 4.9% of patients, and dose reduction was required in 4.9% due to diarrhea and 4.9% due to neutropenia, suggesting that weekly docetaxel combination therapy is tolerable without compromising efficacy.
Recently, in the era of personalized therapy, drugs such as target agents have been investigated actively in the treatment of SGCs. The epidermal growth factor receptor (EGFR) is expressed in approximately 25% to 90% of SGCs, with approximately 85% of ACC expressing EGFR [16,17]. Thus, agents targeting EGFR deserve study as monotherapy or in combination with traditional chemotherapies in SGCs. For example, cetuximab, a monoclonal antibody targeting EGFR, showed ORR > 40%, and the median PFS and OS were 13 and 24 months, respectively, when combined with cisplatin for patients with metastatic ACC [18]. Human epidermal growth factor receptor 2 (HER2; also known as ERBB2) is another target in the treatment of several malignancies, including breast cancer and gastric cancer [19,20]. In a meta-analysis, SGCs were heterogeneous with respect to HER2 positivity, ranging from 0% up to 43%, with the highest prevalence in salivary ductal carcinoma [21]. Although the antitumor effect of HER2 directed monotherapy in patients with HER2-positive SGCs was at best modest, HER2 remains an important potential target in treatment for SGCs [22,23]. Recently, several strategies have been attempted for treatment of salivary ductal carcinoma, including anti-HER2 agents combined with chemotherapy and dual HER2 blockage with trastuzumab and pertuzumab [24–26]. Further, T-DXd is an antibody-drug conjugate composed of an anti-HER2 antibody, cleavable tetrapeptide-based linker, and membrane-permeable topoisomerase I inhibitor payload. Subgroup analysis of a phase I study of T-DXd for salivary ductal carcinoma demonstrated ORR of 47% (8/17) and PFS of 14.1 months, which is promising antitumor activity with durable response [27]. In contrast, other evidence suggested that overexpression of the vascular endothelial growth factor in SGCs, especially ACC, is related to higher stage and worse disease-specific survival [28]. Given these results, several antiangiogenic agents have been studied in treatment of ACC, but the results have been disappointing, with either no response or only modest activity [29,30]. Thus, there is no targeted agent approved by the Food and Drug Administration for treatment of SGCs.
This study has several limitations. First, this is a single-arm study without a control group. Even though stringent criteria were adopted for patients with ACC, potential bias cannot be avoided, and the role of this treatment combination for improving survival cannot be demonstrated without a randomized study. Secondly, given the heterogeneity in histological subtypes, the statistical power might not be sufficient to demonstrate efficacy in each subtype. Nevertheless, considering the rarity of SGCs and lack of standard first-line treatment, challenges of large number of patients recruitment, cisplatin plus weekly docetaxel resulted in high response rates and durable response in both ACC and salivary ductal carcinoma, suggesting that this combination is a reasonable treatment option.
Cisplatin plus weekly docetaxel is effective and tolerable with manageable toxicity as first-line therapy in patients with metastatic or recurrent SGC.
Notes
Ethical Statement
This study was reviewed and approved by our institutional review board (IRB number: 2013-07-149) (ClinicalTrial.gov Identifier: NCT05008237). All patients provided written informed consent before enrollment in accordance with the Declaration of Helsinki.
Author Contributions
Conceived and designed the analysis: Lee SJ, Ahn MJ.
Collected the data: Kim HR, Lee SJ, Park S, Jung HA, Lee SH, Jeong HS, Chung MK, Ahn MJ.
Contributed data or analysis tools: Kim HR, Lee SJ, Park S, Jung HA, Lee SH, Jeong HS, Chung MK, Ahn MJ.
Performed the analysis: Kim HR.
Wrote the paper: Kim HR, Ahn MJ.
Conflicts of Interest
This study was supported in part by Dong-A pharmaceutical company.