Long-Term Outcomes and Sequelae Analysis of Intracranial Germinoma: Need to Reduce the Extended-Field Radiotherapy Volume and Dose to Minimize Late Sequelae
Article information
Abstract
Purpose
We aimed to refine the radiotherapy (RT) volume and dose for intracranial germinoma considering recurrences and long-term toxicities.
Materials and Methods
Total 189 patients with intracranial germinoma were treated with RT alone (n=50) and RT with upfront chemotherapy (CRT) (n=139). All cases were confirmed histologically. RT fields comprised the extended-field and involved-field only for primary site. The extended-field, including craniospinal, whole brain (WB), and whole ventricle (WV) for cranial field, is followed by involved-field boost. The median follow-up duration was 115 months.
Results
The relapses developed in 13 patients (6.9%). For the extended-field, cranial RT dose down to 18 Gy exhibited no cranial recurrence in 34 patients. In CRT, 74 patients (56.5%) showed complete response to chemotherapy and no involved-field recurrence with low-dose RT of 30 Gy. WV RT with chemotherapy for the basal ganglia or thalamus germinoma showed no recurrence. Secondary malignancy developed in 10 patients (5.3%) with a latency of 20 years (range, 4 to 26 years) and caused mortalities in six. WB or craniospinal field rather than WV or involved-field significantly increased the rate of hormone deficiencies, and secondary malignancy. RT dose for extended-field correlated significantly with the rate of hormone deficiencies, secondary malignancy, and neurocognitive dysfunction.
Conclusion
De-intensifying extended-field rather than involved-field or total scheme of RT will be critical to decrease the late toxicities. Upfront chemotherapy could be beneficial for the patients with complete response to minimize the RT dose down to 30 Gy. Prospective trials focused on de-intensification of the extended-field RT are warranted.
Introduction
Intracranial germinomas are relatively rare tumors, accounting for about 3% of primary pediatric brain tumors worldwide, but in East Asia show a higher proportion of 10% [1]. Although the traditional cure rate is over 95% with radiotherapy (RT) [2], RT could produce late complications, such as hormone insufficiency, secondary malignancy, and neurocognitive dysfunction. For concern over the toxicities, the omission of RT was previously attempted, but caused a high rate of failure and survival compromise [3]. Thus, previous studies have tried to decrease the dose and volume of RT over concerns with RT complications [4–15]. However, the optimal intensity of RT is still under debate, and various patterns of treatments, including craniospinal, whole brain (WB), whole ventricle (WV), involved-field RT alone, and with or without chemotherapy are applied in the clinics [16].
In the present study, we retrospectively analyzed 189 cases of intracranial germinomas that had been pathologically proven. For 25 years, our policy of treatment in intracranial germinomas has changed over time from craniospinal RT alone, to modified RT with reduced field and upfront chemotherapy. From the cohort of intracranial germinomas in this institute, we could analyze the pattern of failures and toxicities according to the intensity of RT. The present study aimed to find the optimal volume and dose of RT to secure oncological safety and minimize late toxicities in intracranial germinomas.
Materials and Methods
1. Patients
The medical records of 195 patients with intracranial germinoma who underwent RT from January 1981 to December 2015 were retrospectively reviewed. Inclusion criteria were as follows: (1) intracranial germinoma, newly diagnosed by histologic exam; and (2) RT alone or CRT (radiotherapy with upfront chemotherapy). Of these 195 patients, 189 met the inclusion criteria. The reasons for exclusion were non-germinomatous elements on pathologic examination (except for nonmalignant teratoma) (n=3), refusal or incompletion of radiotherapy (n=2), and incompletion or insufficient documentation (n=1).
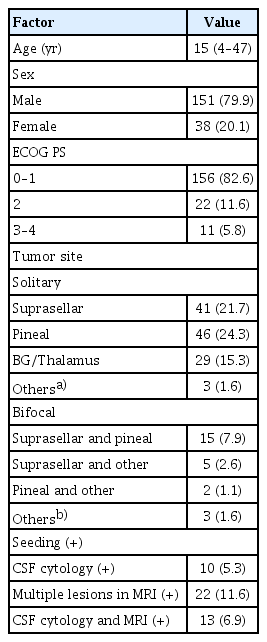
The median age was 15 years (range, 4 to 47 years) (Table 1). Male patients were 151 (79.9%), while females were 38 (20.1%). The number of patients with Eastern Cooperative Oncology Group score (0–1), 2, and (3–4) was 156, 22, and 11, respectively. Presenting signs and symptoms were diabetes insipidus (n=64, 33.9%), visual impairment or diplopia (n=59, 31.2%), headache (n=40, 21.2%), and vomiting (n=26, 13.8%).
The primary tumors in the patients without seeding were most frequently located in the suprasellar region (n=61) or pineal region (n=63). Also, germinomas arising only in basal ganglia or thalamus were found in 29 patients.
Solitary disease was defined as solitary lesion of germinoma without evidence of dissemination (negative craniospinal fluid [CSF] cytology and negative imaging). Bifocal disease was defined as radiological detection of bifocal tumors in the pineal or the suprasellar or basal ganglia/thalamus regions with negative CSF cytology and imaging. Initial positive seeding was defined as the presence of an intracranial multiple lesions (except bifocal disease), spinal metastases, or tumor cells in CSF. According to this definition of disease, of 189 patients, 119 had solitary disease, and 25 had bifocal disease. Initial positive seeding in the ventricle or spine was detected in 45, as determined by magnetic resonance imaging (n=22), cerebrospinal fluid cytology (n=10), and both (n=13) (Table 1).
The tumor marker level was unavailable in three patients. The serum human chorionic gonadotropin level was undetectable in 133 patients, ≤ 50 mIU/mL in 46 patients, and > 50 mIU/mL in seven patients. The α-fetoprotein (AFP) level was considered normal when it was ≤ 11 ng/mL; however, the AFP level was greater or fluctuating in patients with chronic hepatitis. The AFP level was ≤ 5 ng/mL in 177 patients, 7–11 ng/mL in six patients, and 22–30 ng/mL in three hepatitis B virus carrier.
2. Treatment
Of the 189 patients, 50 underwent RT alone, and 139 underwent CRT. RT alone was applied to 40 patients bet-ween 1982 and 1999, and CRT was introduced from 1992. CRT was applied to 11 patients between 1992 and 1999. After 1999, the main treatment policy had been changed to CRT (n=136). However, 10 patients (20% of RT alone) received RT alone after 1999 due to comorbidities (n=3), patients’ refusal of chemotherapy (n=5) or other reasons (n=2).
In the RT alone group (n=50), craniospinal RT was followed by involved-field RT boost, except for five patients with poor performance (Table 2). The craniospinal axis dose was 24 Gy (range, 20 to 30 Gy) for those with initial negative seeding, but 30 Gy (range, 24 to 36 Gy) for those with initial positive seeding. The primary tumor dose was 54 Gy (range, 45 to 57 Gy).
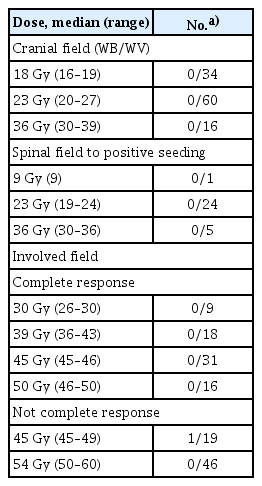
In the CRT group (n=139), upfront chemotherapy was undertaken with two cycles (range, one to four) of bleomycin, etoposide, and cisplatin or etoposide and cisplatin for 66 patients > 18 years old, five cycles (range, 1 to 5) of cisplatin, etoposide, cyclophosphamide, and vincristine (CCG 9921A or 9931A) for 67 patients, and “8-in-1” (solumedrol, vincristine, lomustine, procarbazine, hydroxyurea, cisplatin, cytosine arabinoside, and cyclophosphamide) for four patients. In the CRT group (n=139), extended-field RT, including craniospinal RT, WB RT, and WV RT, was followed by involved-field RT boost, of which the extent depended on the initial tumor extent or preferences of physicians (Table 2). Involved-field RT alone without extended-field RT after upfront chemotherapy was performed in 28 patients. Of the 28 patients, three patients had initial positive seeding in the periventricular area, although after upfront chemotherapy, the seeding lesions were disappeared in the magnetic resonance imaging. Extended-field dose was 23 Gy (range, 16 to 39 Gy). The radiation dose to the primary tumor depended on the response to upfront chemotherapy. To the primary tumor, patients showing complete response (CR) to upfront chemotherapy received 45 Gy (range, 25 to 50 Gy), and patients not showing CR received 50 Gy (range, 45 to 55 Gy) (p < 0.001). The patients in the RT-alone group had a significant tendency to undergo craniospinal RT, rather than cranial RT or involved-field RT alone, and high dose of both the extended-field and involved-field RT (p < 0.001, p < 0.001, and p < 0.001, respectively).
Germinoma located only in the basal ganglia or thalamus without initial seeding were found in 29 patients. Of the 29 patients, six patients underwent RT alone with craniospinal RT, and 23 patients underwent CRT with involved-field RT alone (n=7), WV RT (n=10), WB RT (n=4), and craniospinal RT (n=2).
3. Toxicities
Patients with initial hormone deficiency at diagnosis (n=67) were excluded from counting the number of patients requiring hormone-replacement therapy for the analysis of toxicities. Patients with insufficient follow-up duration lower than 36 months (n=10) were excluded from counting the number of patients in the analysis of the secondary malignancy. For the analysis of school activity, we excluded 69 adult patients and counted the number of patients who could return to their own grade of school without flunking after the completion of radiotherapy among the school-aged child or adolescent.
Neurocognitive dysfunction was defined as a subjective report of having a severe disability in learning memory, attention, processing, and executive functioning limiting school performance at school-age, or occupational activities at adult age. Neurocognitive dysfunction was assessed regularly with subjective questionnaire in the outpatient clinics of the department of radiation and pediatric oncology or the pediatric clinic of long-term survivors. We excluded 10 patients in pre-school age, 18 patients inaccessible to the medical records of regular neurocognitive questionnaire, and 11 patients with poor performance, Eastern Cooperative Oncology Group (ECOG) 3–4 at diagnosis.
4. Statistical analysis
Analyses were performed using SPSS ver. 18 (SPSS Inc., Chicago, IL). We used the chi-square test for categorical variables, and a Student’s t test for continuous variables. Overall survival (OS) and recurrence-free survival (RFS) were calculated as the interval from the date of diagnosis to the date of death, and to the date that death or progression was detected, respectively. Survival curves were generated using the Kaplan-Meier method, and univariate survival comparison was performed using the log-rank test. A value of p < 0.05 was considered statistically significant.
Results
1. Response to RT and upfront chemotherapy
In the RT-only group, 30 patients (60.0%) had a CR and 20 (40.0%) a partial response (PR) at 3 months after RT. All the PR patients had a CR by 21 months, and no progression was observed. In the CRT group, the response to upfront chemotherapy was a CR in 74 patients (56.5%), a PR in 57 (43.5%), and stable/progressive disease in eight patients, according to an imaging study before radiotherapy. After radiotherapy following chemotherapy, all patients except one were transited to a CR at 3 months after RT. One case of a delayed CR was observed at 9.5 months.
2. Failures and survival patterns
The median follow-up duration was 116 months (range, 3 to 358 months), from the date of diagnosis. In the RT alone group, the median follow-up duration was 112 months (range, 3 to 358 months), while in the CRT group, 117 months (range, 8 to 308 months) (p=0.470). The relapses developed in five patients (10.0%) in the RT alone, and eight patients (5.8%) in the CRT group (Table 2). Regarding the RT field, the out-field recurrence was in two patients in the RT alone group, who did not receive spinal irradiation. The other three patients of recurrence after RT alone showed in-field recurrence at the periventricular area. Otherwise, in the CRT group, all of the eight patients showing recurrence experienced out-field recurrence. Except for one patient of spinal recurrence, seven patients of eight received the involved-field RT alone, without any extended-field RT.
Regarding the radiation dose in CRT group, low dose down to 18 Gy to cranial field exhibited no cranial recurrence (Table 3). For positive seeding, low dose down to 23 Gy showed no spinal recurrence. For the involved-field boost after the upfront chemotherapy, low dose down to 30 Gy (range, 26 to 30 Gy) in the patients with CR to the upfront chemotherapy showed no primary recurrence (n=9).
Low dose down to 45 Gy in the patients with partial or stable response showed one recurrence of 19 patients. However, the recurrence of one patient extended from multiple intracranial recurrences to the primary site after the involved-field RT alone.
The OS rate at 10 years was 85.2% in the RT alone, and 92.8 % in the CRT group (p=0.066). The RFS at 10 years was 79.5% in the RT alone group, and 89.3 % in the CRT group (p=0.083). OS and RFS were not affected by any clinical parameter.
After the development of recurrence in 13 patients, 10 patients received salvage treatment, and three with poor performance did not. Of the 10 patients, five underwent CRT, four underwent chemotherapy only, and one patient underwent radiotherapy only. All radiotherapy for salvage aim was performed with craniospinal irradiation. Salvage treatment rescued five patients (50%) with disease-free status. All rescued patients received re-irradiation as a salvage treatment.
3. Basal ganglia and thalamus
Of 29 patients who had germinoma at the basal ganglia or thalamus, relapses developed in three patients among a total seven patients who underwent CRT with involved-field RT alone. Two of the three recurrences were located in the periventricular area. Another recurrence developed widely from the primary site of the basal ganglia to the periventricular region in the ipsilateral frontal and temporal lobe. No relapse developed in the other 22 patients who underwent extended-field RT, including RT to craniospinal axis (n=2), WB (n=4), or WV (n=10) after upfront chemotherapy and craniospinal RT alone (n=6). The OS and RFS rates at 10 years were 86.8% and 84.3%, respectively.
4. Late toxicities and treatment factors
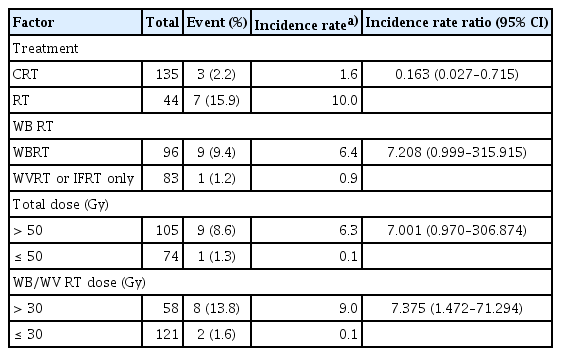
The frequency of hormone-replacement therapy was significantly correlated to WB RT rather than WV or involved-field RT alone (p=0.003), and the high RT dose over 30 Gy for extended-field RT (p=0.036) (Table 4). Secondary malignancy developed in 10 patients (5.3%) with a latency period of 20 years (range, 4 to 26 years) and caused mortalities in six of the 10 patients (Table 5). Solid cancer in the nine patients was located inside the RT field. Otherwise, another patient experienced lymphoma at the distal femur outside of the RT field. Glioblastoma was the most common type of secondary malignancy (n=5). The incidence rate ratio of secondary malignancy was increased to 7.208 (95% confidence interval, 0.999 to 315.915) in the larger RT volume of extended-field with WB RT or craniospinal RT, and 7.375 (95% confidence interval, 1.472 to 71.294) in the higher RT dose of extended-field (Table 5). The rate of neurocognitive dysfunction was significantly related to high dose of extended-field RT (p=0.027). RT alone rather than upfront chemotherapy followed by RT was significantly related to the rates of late toxicities including hormone deficiency, school activity, neurocognitive dysfunction, and secondary malignancy.
Discussion
The optimal RT fields and dose are still controversial, and discussion of this issue should consider both maintaining tumor control and minimizing late toxicities. The present study of 189 patients with pathologically proven germinoma has confirmed that radiotherapy with or without upfront chemotherapy results in excellent tumor control and survival, comparable to previous studies [4–13]. Excellent tumor control could be sustained when RT covered the initial disease extent with craniospinal RT for patients with initial positive seeding, and WV RT for patients with initial negative seeding. However, the minimum coverage of RT could not be further decreased to below the initial disease extent, whether chemotherapy is combined or not. We experienced high rates of periventricular recurrence in the brain as the out-field recurrences after the involved-field RT alone with chemotherapy, similar to the previous studies using the involved-field RT alone after chemotherapy or chemotherapy alone [3–5,7,9,14]. Chemotherapy could not sterilize the disease spread along the CSF. Otherwise, without chemotherapy, two of five patients with initial negative seeding showed spinal recurrence after cranial RT alone. Although Yen et al. [17] and Byun et al. [4] reported fairly good outcomes after the omission of spinal RT without chemotherapy, they both reported one case of spinal failure. Upfront chemotherapy might be beneficial for patients with initial negative seeding to omit spinal irradiation safely. Therefore, with upfront chemotherapy, the pattern of recurrences will conservatively support WV for initial negative seeding, and craniospinal RT for initial positive seeding.
Moreover, the volume and dose of the extended-field RT were also important for the development of late toxicities. To the best of our knowledge, the present study is the first report to show the statistical correlation between the RT factors and late toxicities in patients with intracranial germinoma. We found that WB RT, rather than WV or involved-field RT alone, significantly increased the rate of hormone deficiencies and secondary malignancy, and the high dose of extended-field RT to the brain increased the rate of hormone deficiencies, secondary malignancy, and neurocognitive dysfunction. In particular, the patients receiving low dose under 30 Gy to the brain showed quite good quality of life. Moreover, median 18 Gy to cranial field in the CRT group showed excellent in-field control in 34 patients of our studies, which were also observed in the previous studies using low-dose of cranial RT ranging 19 to 24 Gy [5,8,18]. Our study further supported low-dose cranial RT with WV RT, rather than WB RT, to deal with late toxicities, as well as tumor control.
Adequate RT field in the germinoma originating from the basal ganglia or thalamus, a distinct entity located in the brain parenchyma nearby ventricle, has also been a critical issue [5,19]. For the patients, the involved-field RT alone with upfront chemotherapy resulted in high rates of intracranial recurrences outside the RT field. In contrast, the extended-field with WB or WV, followed by involved-field boost, showed no recurrence in or outside the RT field. The previous studies also showed insufficient disease control in the involved-field RT alone, and no recurrence with WB or craniospinal RT [5,19]. Notably, we employed WV RT as the extended-field RT after upfront chemotherapy in 10 patients, and experienced no recurrence. Modern magnetic resonance imaging and RT technique enable delineation of the tumor location, and safely covered the possible tumor infiltration with RT [20]. Also, considering the toxicities related to WB RT in our study, WV RT with focal boost after upfront chemotherapy might be adequate for the germinoma in the basal ganglia or thalamus.
Concerning the total RT dose to the involved-field, the present study showed that median 30 Gy ranging 26 to 30 Gy can prevent in-field recurrence after CR to upfront chemotherapy. In contrast, one patient among 19 patients showing partial or stable response to chemotherapy showed in-field recurrence after median 45 Gy (range, 36 to 49 Gy). For CRT, Aoyama et al. [13] previously reported that 24 Gy WV RT without further involved-field RT boost resulted in no failure in six patients who showed CR to upfront chemotherapy. For RT alone, Cho et al. [8] previously suggested that RT dose to the involved site can be decreased to 39.3 Gy with RT alone. Although the optimal RT dose is still controversial, the present study suggests that dose adaptation according to the response of chemotherapy could decrease the RT dose low to 30 Gy in the subgroup showing CR to upfront chemotherapy. Moreover, in the future, further optimization of the RT dose should be followed to test the minimal limit of the RT dose.
However, this study is not free from limitations. First, in this retrospective study, no systematic and scoring assessment of neurocognitive function had been performed. To minimize the uncertainty of assessing neurocognitive function, we narrowed the criteria to severe limitation in neurocognitive function of school activity under 18 years, and occupational activity in the adult. Systematic assessment of neurocognitive function will be necessary to define the RT factor affecting specific function in the patients with germinoma. Second, although the number of patients in this study is relatively large, we confirmed the pattern of relapse in a small-sized subgroup, especially around the low margin of RT dose. Prospective evaluation of oncologic safety should be verified in future clinical trials. Third, although the CRT group showed the lower rate of late toxicities than RT lone in the analysis of late toxicities, the intensity of extended-field radiotherapy in CRT group were significantly lower than RT alone group. Therefore, from the results of this study, the combination of chemotherapy alone cannot guarantee the lower toxicities without taking care of extended-field RT. The potential benefit of upfront chemotherapy will be the oncologically safe de-intensity of extended-field RT without compromising the tumor control rate.
De-intensifying extended-field rather than involved-field or total scheme of RT will be critical to decrease the late toxicities. Upfront chemotherapy could be beneficial for patients with negative seeding to omit the spinal RT and for patients with CR to minimize the RT dose down to 30 Gy. WV RT with upfront chemotherapy can be recommended for intracranial germinoma arising from basal ganglia or thalamus. Prospective trials focused on de-intensification of the extended-field RT are warranted.
Notes
Ethical Statement
All of data collection and analysis were performed after approval from the institutional review board (IRB No. 1904-010-1023) in accordance with the declaration of Helsinki. The written informed consent was waived in this retrospective study.
Author Contributions
Conceived and designed the analysis: Wang KC, Kim IH.
Collected the data: Lee JH, Eom KY, Phi JH, Park CK, Kim SK, Cho BK, Kim TM, Heo DS, Hong KT, Choi JY, Kang HJ, Shin HY, Choi SH, Lee ST, Park SH, Wang KC, Kim IH.
Contributed data or analysis tools: Lee JH, Kim IH.
Performed the analysis: Lee JH, Eom KY, Wang KC, Kim IH.
Wrote the paper: Lee JH, Wang KC, Kim IH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2018R1D1A1A02085487, NRF-2020M2D9A-2092373 to J.H.L) and by the SNUH Research Fund (04-2019-0830 to J.H.L.) funded by Seoul National University Hospital.