Double Tract Reconstruction Reduces Reflux Esophagitis and Improves Quality of Life after Radical Proximal Gastrectomy for Patients with Upper Gastric or Esophagogastric Adenocarcinoma
Article information
Abstract
Purpose
The aim of the present study was to compare the difference between double tract reconstruction and esophagogastrostomy.
Materials and Methods
Patients who underwent radical proximal gastrectomy with esophagogastrostomy or double tract reconstruction were included in this study.
Results
Sixty-four patients were included in this study and divided into two groups according to reconstruction method. The two groups were well balanced in perioperative safety and 3-year overall survival (OS). The rates of postoperative reflux esophagitis in the double tract reconstruction group and esophagogastrostomy group were 8.0% and 30.8%, respectively (p=0.032). Patients in the double tract reconstruction group had a better global health status (p < 0.001) and emotional functioning (p < 0.001), and complained less about nausea and vomiting (p < 0.001), pain (p=0.039), insomnia (p=0.003), and appetite loss (p < 0.001) based on the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire. Regarding the EORTC QLQ-STO22 questionnaire, patients in the double tract reconstruction group complained less about dysphagia (p=0.030), pain (p=0.008), reflux (p < 0.001), eating (p < 0.001), anxiety (p < 0.001), dry mouth (p=0.007), and taste (p=0.001). The multiple linear regression analysis showed that reconstruction method, postoperative complications, reflux esophagitis, and operation duration had a linear relationship with the global health status score.
Conclusion
Double tract reconstruction could better prevent reflux esophagitis and improve quality of life without scarifying perioperative safety or 3-year OS.
Introduction
Gastric cancer is a severe threat worldwide, especially in the eastern Asian area. It has been reported that gastric cancer is the fifth most common cancer and the third leading cause of cancer-related death globally [1]. In China, gastric cancer is the third leading cause of cancer-related death, and its incidence ranks second among all malignancies. There are more than 410,000 new cases and 290,000 cancer-related deaths each year [2,3].
In recent years, the incidence of proximal gastric cancer has steadily increased [4]. With the advancements in diagnostic techniques, an increasing number of patients with early gastric cancer are being diagnosed. According to the Japanese Gastric Cancer Treatment Guidelines, proximal gastrectomy is suitable for patients with upper gastric adenocarcinoma at an early stage or those with adenocarcinoma of the esophagogastric junction with a tumor size less than 4 cm [5]. As a result, the number of patients needing proximal gastrectomy has increased. Moreover, perioperative safety and postoperative quality of life after proximal gastrectomy have gained increasing attention [6-8]. At present, a major problem of proximal gastrectomy is the high incidence of reflux esophagitis. Although partial function of the stomach is preserved, quality of life is impaired. Therefore, several reconstruction methods have been attempted to solve this problem. According to the Japanese Gastric Cancer Treatment Guidelines, double tract reconstruction and esophagogastrostomy are two of the recommended reconstructions for proximal gastrectomy [5]. Esophagogastrostomy is the traditional and most widely performed reconstruction method after proximal gastrectomy [9]. The main deficiency of esophagogastrostomy might be the high incidence of reflux esophagitis. It has been reported that the incidence of reflux esophagitis after esophagogastrostomy ranges from 9.1% to 35.3% [10,11]. Conversely, some studies have reported that double tract reconstruction might reduce the incidence of reflux esophagitis after proximal gastrectomy [12,13]. However, double tract reconstruction is more complicated than esophagogastrostomy, and there is a lack of high-level evidence to support the advantages of double tract reconstruction [4].
Therefore, the best reconstruction method after proximal gastrectomy is still controversial. Moreover, postoperative quality of life after proximal gastrectomy with esophagogastrostomy or double tract reconstruction is rarely investigated or reported. Thus, the purpose of the present study is to compare the differences between double tract reconstruction and esophagogastrostomy after proximal gastrectomy, and to identify the superiority of double tract reconstruction.
Materials and Methods
1. Patients
The patients’ clinical and pathological data were retrospectively collected from a prospectively maintained database at our hospital. Between December 2014 and May 2019, patients who underwent radical proximal gastrectomy with esophagogastrostomy, or double tract reconstruction were included in the present study. All patients were diagnosed with upper-third gastric adenocarcinoma at an early clinical stage or adenocarcinoma of the esophagogastric junction with a tumor size less than 4 cm. The diagnosis and clinical stage were confirmed by endoscopic biopsy analysis, ultrasound endoscopy, chest X-ray, abdominopelvic computed tomography scans, and laparoscopic exploration. The terminology was defined based on the Japanese classification of gastric carcinoma [14]. The clinical and pathological stages were classified based on the 8th edition Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) TNM staging system [15].
2. Surgical procedure
The surgical procedure in our department was performed in accordance with the Japanese Gastric Cancer Treatment Guidelines [16]. All patients underwent laparoscopic exploration and open proximal gastrectomy. Radical proximal gastrectomy included resection of the proximal stomach and part of the abdominal esophagus and preserved more than half of the stomach. The reconstruction method was discussed and determined by both the patients and surgeons before surgery. The degree of lymph node dissection (LND) was also performed in accordance with the Japanese treatment guidelines [16]. D1 or D1+ lymphadenectomy was chosen according to the disease stage. Esophagogastrostomy reconstruction was performed by an end-to-side anastomosis with a circular stapler between the esophagus and remnant stomach. The anastomosis was located on the anterior wall of the remnant stomach. For double tract reconstruction, the jejunum 25 cm distal to the Treitz ligament was transected, distal limb of the jejunum was lifted to prepare the esophagojejunostomy. An end-to-side esophagojejunostomy was performed with a circular stapler, and the jejunal stump was closed with a linear stapler. Next, a side-to-side gastrojejunostomy was performed 15 cm below the esophagojejunostomy. Finally, a side-to-side jejunojejunostomy was performed 15-20 cm below the gastrojejunostomy.
3. Clinical and pathological characteristics
The clinical and pathological characteristics of patients collected from the database included age, sex, body mass index (BMI), tumor location, proximal and distal margins, degree of differentiation, Lauren classification, tumor size, pT category, pN category, pTNM category, number of harvested lymph nodes (LNs), degree of LND, lymphovascular invasion (LVI), perineural invasion (PNI), and adjuvant chemotherapy. The intraoperative and postoperative parameters included postoperative hospital stay, blood loss volume, operation duration, postoperative complications, 30-day reoperation rate, and 30-day mortality rate. Complications were defined according to the Clavien-Dindo classification system [17]. The indication of adjuvant chemotherapy for patients with gastric cancer was pT2N0M0 with high-risk features. High-risk features included poorly differentiated or high-grade cancer, lymphovascular invasion, neural invasion, or < 50 years of age. Meanwhile, patients with pT3, pT4, or pN+ were also recommended to receive adjuvant chemotherapy.
4. Follow-up
All patients were recommended to go to the outpatient clinic to receive re-examinations every 3 months in the first 3 years after the operation, every 6 months in the next 2 years and every year thereafter. The re-examinations mainly included physical examination, laboratory blood tests, and computed tomography scans. Endoscopy was recommended once a year after the operation. Evaluation of reflux esophagitis was conducted using endoscopy at 12 months after the operation. The Los Angeles classification was applied to evaluate the degree of reflux esophagitis [18]. Patient quality of life was evaluated by the validated Chinese Mandarin edition of the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 ver. 3.0 questionnaire and EORTC QLQ-STO22 questionnaire. Permission to use these questionnaires in the present study was obtained from the EORTC Quality of Life Group. The patients were invited to complete these two questionnaires at 12 months after the operation. The last follow-up date was November 2020. The follow-up assessments were conducted mainly through telephone interviews, email communication, or outpatient examinations.
5. Statistical analysis
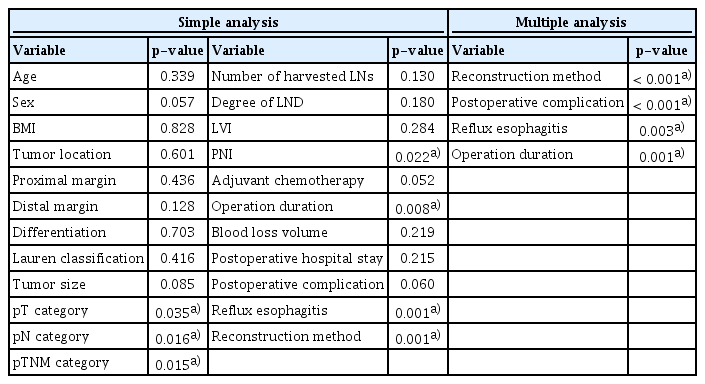
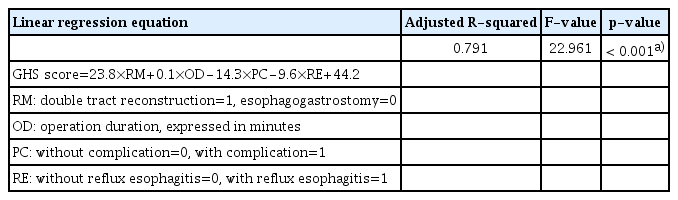
All statistical analyses were performed using IBM SPSS Statistics ver. 26.0 software (IBM Corp., Armonk, NY). Continuous variables with normal or skewed distributions are expressed as the mean±standard deviation or median with interquartile range, respectively. Student’s t test or Mann-Whitney U test was used to compare variables with normal or skewed distributions among groups, respectively. Categorical variables are represented by the number of cases with percentage and were compared by the chi-square test or Fisher exact test. Kaplan-Meier survival analysis and the log-rank test were used to compare overall survival (OS) between the two groups. Univariate analysis and multivariate logistic regression analysis were conducted to identify the risk factors for reflux esophagitis. Simple and multiple linear regression analyses were applied to identify factors that might affect the global health status score in the EORTC QLQ-C30 questionnaire. A linear regression equation was established to describe the factors related to global health status. p < 0.05 (two-sided) was considered significant in the statistical analysis.
Results
1. Clinical and pathological characteristics
Altogether, 64 patients were included in this study. Thirtynine patients underwent esophagogastrostomy reconstruction and 25 patients underwent double tract reconstruction. Age, sex, BMI, tumor location, proximal and distal margins, differentiation, Lauren classification, number of harvested LNs, and LVI were comparable between the two groups (Table 1). The proportion of patients with early-stage disease was higher in the double tract reconstruction group than in the esophagogastrostomy group (p=0.031). The tumor size was smaller in the double tract reconstruction group than in the esophagogastrostomy group (1.5 cm vs. 3.0 cm, p=0.010). The proportion of D1+ lymphadenectomy was lower in the double tract reconstruction group than in the esophagogastrostomy group (56.0% vs. 79.5%, p=0.045). The rate of PNI was lower in the double tract reconstruction group than in the esophagogastrostomy group (12.0% vs. 38.5%, p=0.044). Fewer patients received adjuvant chemotherapy in the double tract reconstruction group than in the esophagogastrostomy group (32.0% vs. 59.0%, p=0.035).
2. Perioperative parameters and survival
The two groups were well balanced in terms of length of postoperative hospital stay, blood loss volume, and postoperative complications (Table 2). The operation duration in the double tract reconstruction group was longer than that in the esophagogastrostomy group (240 minutes vs. 195 minutes, p=0.001). The 30-day reoperation rate and 30-day mortality rate were comparable between the two groups.
As of November 2020, the median follow-up time was 39.8 months (range, 11.4 to 67.2 months). The 3-year OS rates in the esophagogastrostomy group and double tract reconstruction group were 79.9% and 90.9%, respectively (p=0.066) (Fig. 1).
3. Reflux esophagitis
Reflux esophagitis occurred in 12 patients (30.8%) in the esophagogastrostomy group and two patients (8.0%) in the double tract reconstruction group (p=0.032) (Table 2). Of the twelve patients who were diagnosed with reflux esophagitis in the esophagogastrostomy group, nine patients were classified as level A and three patients were classified as level B according to the Los Angeles classification. In the double tract reconstruction group, both patients were classified as level A according to the Los Angeles classification.
To identify the risk factors for postoperative reflux esophagitis, a univariate analysis was performed (Table 3). Then, all factors with a p-value less than 0.1 were included in the multivariable logistic regression analysis. Finally, the reconstruction method was found to be the only independent risk factor for reflux esophagitis (p=0.004) (Table 3).
4. Quality of life
The scores of the EORTC QLQ-C30 ver. 3.0 questionnaire were compared between the esophagogastrostomy and double tract reconstruction groups (Table 4, Figs. 2 and 3). The global health status score was higher in the double tract reconstruction group than in the esophagogastrostomy group (83.3 vs. 50.0, p < 0.001). Compared with patients in the esophagogastrostomy group, patients in the double tract reconstruction group had better emotional functioning (p < 0.001), and complained less about nausea and vomiting (p < 0.001), pain (p=0.039), insomnia (p=0.003), and appetite loss (p < 0.001). The results of the EORTC QLQ-STO22 questionnaire were also compared between groups (Table 4, Fig. 4). Patients in the double tract reconstruction group complained less about dysphagia (p=0.030), pain (p=0.008), reflux (p < z0.001), eating (p < 0.001), anxiety (p < 0.001), dry mouth (p=0.007), and taste (p=0.001) than those in the esophagogastrostomy group.
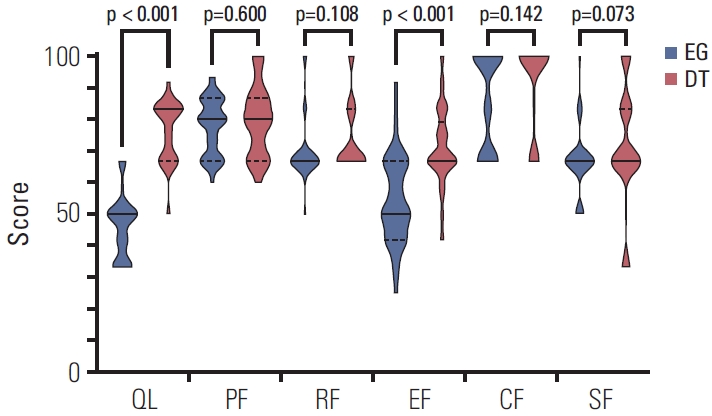
Violin plots of global health status and functional scales of the EORTC QLQ-C30 questionnaire. Solid lines represent medians and dotted lines represent quartiles. A high score for global health status represented a high quality of life. A high score for a functional scale represented a healthy level of functioning. The patients in the DT group had better global health status (p < 0.001) and emotional functioning (p < 0.001) than those in the EG group. CF, cognitive functioning; DT, double tract reconstruction; EF, emotional functioning; EG, esophagogastrostomy; EORTC, European Organization for Research and Treatment of Cancer; PF, physical functioning; QL, global health status; RF, role functioning; SF, social functioning.
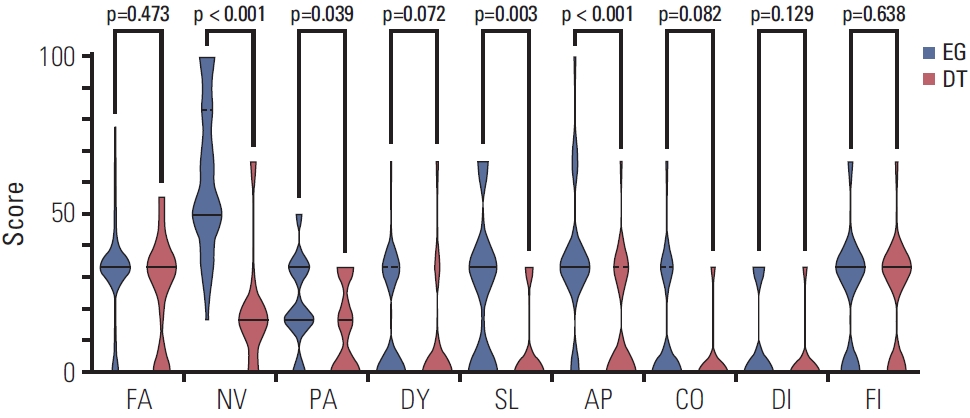
Violin plots of the symptom scales of the EORTC QLQ-C30 questionnaire. Solid lines represent medians and dotted lines represent quartiles. A higher score represented worse symptoms. Patients in the DT group complained less about nausea and vomiting (p < 0.001), pain (p=0.039), insomnia (p=0.003), and appetite loss (p < 0.001) than those in the EG group. AP, appetite loss; CO, constipation; DI, diarrhea; DT, double tract reconstruction; DY, dyspnea; EG, esophagogastrostomy; EORTC, European Organization for Research and Treatment of Cancer; FA, fatigue; FI, financial difficulties; NV, nausea and vomiting; PA, pain; SL, insomnia.

Violin plots of the symptom scales of the EORTC QLQ-STO22 questionnaire. Solid lines represent medians and dotted lines represent quartiles. A higher score represented worse symptoms. Patients in the DT group complained less about dysphagia (p=0.030), pain (p=0.008), reflux (p < 0.001), eating (p < 0.001), anxiety (p < 0.001), dry mouth (p=0.007), and taste (p=0.001) than those in the EG group. ANX, anxiety; BI, body image; DM, dry mouth; DT, double tract; DYS, dysphagia; EAT, eating; EG, esophagogastrostomy; EORTC, European Organization for Research and Treatment of Cancer; HAIR, hair loss; PAIN, pain; RFX, reflux; TA, taste.
In the simple linear regression analysis of global health status, the results showed that pT category (p=0.035), pN category (p=0.016), pTNM category (p=0.015), PNI (p=0.022), operation duration (p=0.008), reflux esophagitis (p=0.001), and reconstruction method (p=0.001) were all correlated with global health status (Table 5). All variables with a p-value less than 0.1 were included in the multiple linear regression analysis. A linear regression equation was established, and the results showed a linear relationship between the global health status score and reconstruction method, postoperative complications, reflux esophagitis, and operation duration (Tables 5 and 6).
Discussion
Esophagogastrostomy reconstruction is a traditional reconstruction method after proximal gastrectomy. However, esophagogastrostomy is associated with a high incidence of postoperative reflux esophagitis. To address this disadvantage, several modified reconstruction methods, including double tract reconstruction, have been attempted.
The safety and feasibility of double tract reconstruction after proximal gastrectomy have been of great concern in recent years. In the present study, the results showed that there was no significant difference in blood loss volume, length of postoperative hospital stay or postoperative complications between esophagogastrostomy and double tract reconstruction. The operation duration was longer in the double tract reconstruction group than in the esophagogastrostomy group in this study. Considering that the double tract reconstruction procedure is more complex than the esophagogastrostomy procedure, it is reasonable that the operation duration of double tract reconstruction was longer than that of esophagogastrostomy. On the other hand, whether double tract reconstruction causes a high rate of complications because of the technical complexity and increased number of anastomoses has been questioned [10,11]. In the present study, postoperative complications, blood loss volume and length of postoperative hospital stay were all comparable between the two reconstruction methods. These results suggest that the complexity of the surgical procedure dose not increase the risk of the operation.
The anti-reflux function of double tract reconstruction after proximal gastrectomy has been investigated in previous studies [12,13]. However, most of the results are based on studies with exceedingly small samples. In the present study, the rates of reflux esophagitis in the esophagogastrostomy group and double tract reconstruction group were 30.8% and 8.0%, respectively (p=0.032). Moreover, multivariate logistic regression analysis showed that the reconstruction method was the only independent risk factor for reflux esophagitis (p=0.004). Double tract reconstruction is a protective factor against postoperative reflux esophagitis after proximal gastrectomy. Considering the reconstruction procedure, it is possible that the mechanism of anti-reflux function is mainly attributed to the lifted jejunum between esophagojejunostomy and gastrojejunostomy [19,20].
Many studies have reported nutritional status after proximal gastrectomy [13,21]. However, postoperative quality of life after proximal gastrectomy has rarely been reported before. Thus, the present study compared quality of life after proximal gastrectomy with the EORTC QLQ-C30 ver. 3.0 and QLQ-STO22 questionnaires between esophagogastrostomy and double tract reconstruction. According to the EORTC scoring manual, a higher score represented a better level of functioning, or a worse level of symptoms [22]. In the present study, double tract reconstruction had obvious advantages over esophagogastrostomy based on the EORTC QLQ-C30 questionnaire, including in global health status, emotional functioning, nausea and vomiting, pain, insomnia, and appetite loss (Table 4). For the EORTC QLQ-STO22 questionnaire, symptoms including dysphagia, pain, reflux, eating, anxiety, dry mouth, and taste were much milder in the double tract reconstruction group than in the esophagogastrostomy group (Table 4). The mechanism of the anti-reflux function of double tract reconstruction has been discussed in the previous paragraph of this section. Furthermore, previous studies reported that the prevention of reflux esophagitis might improve the psychological, physical, and social functioning of patients [23]. Moreover, the prevention of reflux esophagitis could also relieve symptoms such as nausea, vomiting, pain, insomnia, appetite loss, dysphagia, eating, anxiety, and dry mouth [24-26]. Therefore, the better scores in the double tract reconstruction group might be due to the superiority of the surgical procedure itself in preventing reflux esophagitis after proximal gastrectomy.
Considering that the global health status score in the EORTC QLQ-C30 questionnaire can reflect overall quality of life, a linear regression analysis was performed to investigate the related factors [22]. The results showed that reconstruction method (p < 0.001), postoperative complications (p < 0.001), reflux esophagitis (p=0.003), and operation duration (p=0.001) had a linear relationship with the global health status score (Table 6). The mechanism of double tract reconstruction in preventing reflux esophagitis and improving quality of life has been discussed in the previous paragraph. Furthermore, some studies have reported that postoperative complications could induce physical and mental discomfort and impair quality of life [27]. Interestingly, operation duration was also identified in the equation. The operation duration could reflect how elaborate the operation was. Theoretically, an elaborate operation could protect the nerves better, and better preserve organ function [28,29]. Therefore, a longer operation duration might improve quality of life after the operation.
The present study also has some limitations. First, selection bias was difficult to avoid because this was a retrospective study. The pathological stage was not comparable between the two groups, resulting in differences in tumor size, degree of LND, PNI, and adjuvant chemotherapy. This phenomenon was mainly caused by the different choices of the patients. In our hospital, the reconstruction method was discussed and determined by both the patients and surgeons. Patients with early-stage disease were more likely to choose double tract reconstruction after learning about its superiority because these patients might pay more attention to quality of life after the operation. Thus, the shared decision-making method might be the cause of the bias. However, multivariable logistic regression analysis and multiple linear regression analysis could neutralize the confounding effects of these factors. Second, the sample size of the present study was not large enough, which might make the results of this study less convincing. Further large-scale, prospective, randomized controlled trial is needed to validate the results of the study.
In conclusion, double tract reconstruction after proximal gastrectomy was comparable with esophagogastrostomy in terms of perioperative safety and 3-year OS. Double tract reconstruction could better prevent reflux esophagitis and improve quality of life. The reconstruction method was the only independent risk factor for reflux esophagitis. There was a linear relationship between global health status score and reconstruction method, reflux esophagitis, postoperative complications, and operation duration.
Notes
Ethical Statement
This study was conducted in accordance with the Declaration of Helsinki and had been authorized by the Ethics Committee of Peking University Cancer Hospital (Reference No. 2018KT97) before beginning the study. Each patient in this study provided signed informed consent.
Author Contributions
Conceived and designed the analysis: Ji J.
Collected the data: Ji X, Jin C, Ji K, Zhang J, Wu X, Jia Z, Bu Z.
Contributed data or analysis tools: Ji X, Jin C, Ji K, Zhang J, Wu X, Jia Z, Bu Z.
Performed the analysis: Ji X, Jin C, Ji K, Zhang J, Wu X, Jia Z, Bu Z.
Wrote the paper: Ji X, Jin C, Ji K.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
This study was supported by the Research Incubation Project, Beijing Municipal Administration of Hospitals (PX2019039). The funder was not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.