Association among Body Mass Index, Genetic Variants of FTO, and Thyroid Cancer Risk: A Hospital-Based Case-Control Study of the Cancer Screenee Cohort in Korea
Article information
Abstract
Purpose
Obesity has been determined to be associated with fat mass and obesity-associated (FTO) gene and thyroid cancer risk. However, the effect of combined interactions between obesity and the FTO gene on thyroid cancer needs further investigation. This study aimed to examine whether interactions between body mass index (BMI) and the FTO gene are associated with an increased risk of thyroid cancer.
Materials and Methods
A total of 705 thyroid cancer cases and 705 sex- and age-matched normal controls were selected from the Cancer Screenee Cohort in National Cancer Center, Korea. A conditional logistic regression model was used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the measure of associations and the combined effect of BMI and FTO gene on thyroid cancer.
Results
BMI was associated with an increased risk of thyroid cancer in subclasses of overweight (23–24.9 kg/m2; adjusted OR, 1.50; 95% CI, 1.12 to 2.00) and obese (≥ 25 kg/m2) (adjusted OR, 1.62; 95% CI, 1.23 to 2.14). There were positive associations between the FTO genetic variants rs8047395 and rs8044769 and an increased risk of thyroid cancer. Additionally, the combination of BMI subclasses and FTO gene variants was significantly associated with thyroid cancer risk in the codominant (rs17817288), dominant (rs9937053, rs12149832, rs1861867, and rs7195539), and recessive (rs17817288 and rs8044769) models.
Conclusion
Findings from this study identified the effects of BMI on thyroid cancer risk among individuals carrying rs17817288, rs9937053, rs12149832, rs1861867, rs7195539, and rs8044769, whereas the effects of BMI may be modified according to individual characteristics of other FTO variants.
Introduction
The worldwide incidence of thyroid cancer has continuously increased in recent decades [1]. Thyroid cancer is the most common cancer in Korea [2], and its incidence rate is among the highest worldwide and has been increasing rapidly over the past decade [3]. This phenomenon of a thyroid cancer epidemic in Korea is mainly attributed to increased detection by thyroid ultrasonography screening and the easy accessibility of screening programmes [4].
In addition to the increased detection of thyroid cancer cases, multiple environmental factors may explain the increase in thyroid cancer incidence, such as lack of physical activity, oversupply of dietary iodine, and obesity [5–7]. The epidemiology of thyroid cancer is also affected by environmental variations, such as geographical area, ethnicity, and exposure to radiation [8].
In recent years, genetic factors that may play important roles in the molecular mechanisms underlying thyroid carcinogenesis have been investigated. Genome-wide association studies (GWASs) have reported loci in FOXE1, NKX2-1, DIRC3, and NRG1 [9,10] that may be related to susceptibility to thyroid cancer. The results of target sequencing studies indicate that certain genes, such as FAS, TP53, and XRCC1, may be associated with thyroid cancer risk [11]. Collectively, these findings suggest that the genetic variants of multiple genes should be investigated to identify candidates for susceptibility to thyroid cancer.
Several studies have suggested a strong link between obesity and the risk of thyroid cancer [12,13]. The fat mass and obesity-associated (FTO) gene, which is located on chromosome 16, is closely associated with obesity. Several GWASs have provided evidence that FTO is associated with body size, composition, adiposity, and metabolic disorders. Because FTO is associated with obesity and obesity is associated with thyroid cancer, a comprehensive investigation of the genetic-environmental interactions between FTO and obesity may provide important insights into the role of obesity in thyroid carcinogenesis and the variability of thyroid cancer trends in different environmental settings. Therefore, this study examined the independent association between Body mass index (BMI) and single nucleotide polymorphisms (SNPs) in FTO and thyroid cancer risk and their combined effect on modifying the risk of thyroid cancer.
Materials and Methods
1. Study selection and data collection
All eligible cases and controls were selected from the Cancer Screenee Cohort, which is a large hospital-based population of 41,109 subjects screened in National Cancer Center, Korea from August 2002 to July 2014. All the patients were asked to complete a self-administered questionnaire about their demographic characteristics and lifestyle factors.
The height and weight of each subject were measured using an Inbody 3.0 (Biospace, Seoul, Korea) body composition analyzer or an X-SCAN PLUS II Body Composition Analyzer (Jawon Medical, Gyeongsan, Korea). BMI was calculated as follows: weight (kg)/(height [m])2. The BMI values were stratified into three groups according to the Asian BMI Classification Index of the World Health Organization: normal (< 23 kg/m2), overweight (23–24.9 kg/m2), and obese (≥ 25 kg/m2 ) [14].
2. Case and control selection
Thyroid cancer cases were identified according to the International Classification of Diseases for Oncology (ICD-10). Patients with an ICD-10 Code of C73 were identified as thyroid cancer cases. The classification data were obtained from the 2013 Korea Central Cancer Registry database, which provides information on cancer incidence according to case classifications in Korea. Thyroid cancer cases were not analyzed in subgroups according to histological types because most cases (97%) were papillary thyroid cancer (PTC) (data not shown).
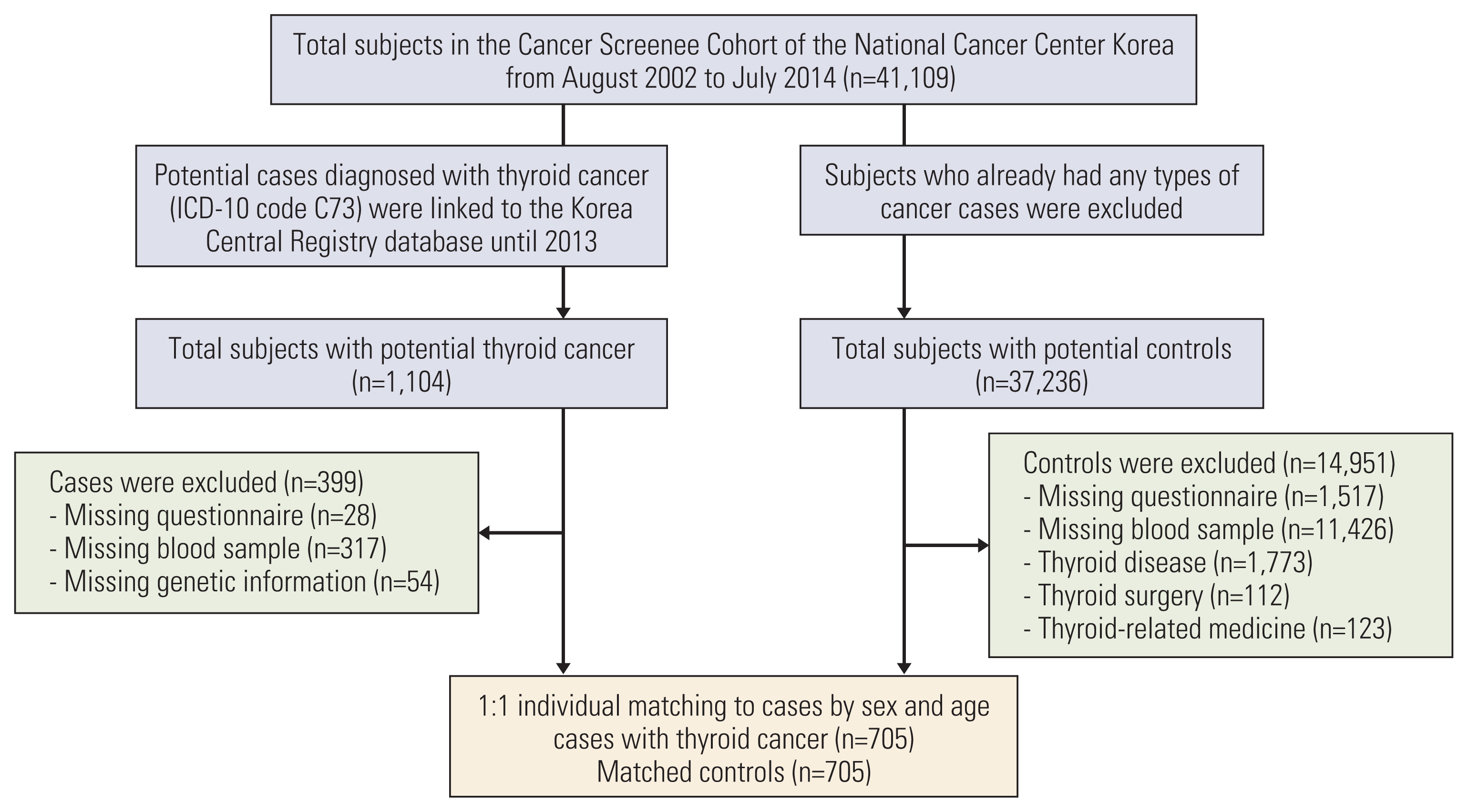
Among the 41,109 subjects in the Cancer Screenee Cohort, 1,104 thyroid cancer cases were eligible for enrollment. A total of 705 subjects were enrolled after the subjects who were missing questionnaires (n=28), blood samples (n=317), and genetic information (n=54) were excluded (Fig. 1). Control patients who were not diagnosed with any cancer, including thyroid cancer, were selected from the same cohort. Among the 37,236 potential controls, participants with missing questionnaires, missing blood samples, a history of thyroid-related disease, surgery, medicine usage, and a diagnosis of any type of cancer were excluded from the study. Controls were selected by 1:1 case matching for sex and age. The final analysis included 705 thyroid cancer cases and 705 controls.
3. Genetic analysis
Given that several complex mechanisms for the link between BMI and thyroid cancer have been proposed, including insulin resistance, hyperinsulinemia, and abnormalities in hormone biosynthesis and hormonal pathways, we searched for up-to-date potential variants in the FTO gene for BMI, diabetes, polycystic ovary syndrome, and thyroid functions [7]. The keywords to search PubMed for publications until October 5, 2020 were used as follows: “(variants OR polymorphism OR GWAS OR genome-wide association studies) AND (obesity OR body mass index OR diabetes OR polycystic ovary syndrome OR hyperandrogenemia OR thyroid) AND FTO,” and limited to titles and abstracts. As a result, 36 candidate SNPs of the FTO gene were selected for genetic analysis: rs8047395, rs9939609, rs8044769, rs8053740, rs1421085, rs17817499, rs1121980, rs8050136, rs13405728, rs9930506, rs7195539, rs62048379, rs56094641, rs7185735, rs3751812, rs11642015, rs62048402, rs62033400, rs9940128, rs55872725, rs1558902, rs7202116, rs17817288, rs11642841, rs1477196, rs1861867, rs763967273, rs759031579, rs141115189, rs9926289, rs76804286, rs8043757, rs1861868, rs9937053, rs9930333, and rs12149832.
To determine genetic variations in FTO, study participants’ DNA was extracted from isolated peripheral blood leukocytes obtained from whole-blood samples. Genotyping was performed using an Infinium OncoArray-500K BeadChip (Illumina Inc., San Diego, CA) with 499 170 SNPs. After applying the quality-control criteria, which included monomorphic variants, minor allele frequencies < 0.01, call rates < 95%, and deviations from Hardy-Weinberg equilibrium (p < 1×10−6), a total of 345,675 SNPs were available for further analysis. Among the 345,675 tags that passed the quality-control criteria, 27 SNPs were selected for further analysis. The linkage disequilibrium (LD) and tagging SNPs were accessed by using Haploview [15].
4. Statistical analysis
All statistical analyses were performed using R software ver. 3.6.0 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). T tests and chi-square tests were performed to compare the distribution of continuous and categorical variables in cases and controls, respectively, among the total participants and subgroups of males and females. The association between FTO variants and BMI was examined by conducting a genome association analysis for variants in chromosome 16 in the log-additive model. To evaluate the association of covariates, BMI, and FTO variants with thyroid cancer risk, a conditional logistic regression model was used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs). The conditional logistic regression model was adjusted for first-degree family history of thyroid cancer, marital status, educational level, occupation, monthly income, smoking status, alcohol consumption, and BMI group. Additionally, the package ‘SNPassoc’ was used to identify variants associated with BMI and perform the haplotype analysis of tagging SNPs [16]. The codominant, dominant, and recessive models for the association between SNPs and thyroid cancer risk were applied to obtain robust findings.
Results
1. Demographic characteristics and their associations with thyroid cancer
Table 1 provides the sociodemographic characteristics of 705 cases and 705 controls from the Korean National Cancer Center Screenee Cohort. Because of the 1:1 frequency-matched design, the age and sex distributions were equal for the cases and controls. In the total population, the average BMI was slightly higher in the cases than in the controls (p=0.01). In addition, the proportion of individuals with a first-degree family history of thyroid cancer was higher in the cases than in the controls (p < 0.001). However, differences between the cases and controls were not observed for marital status, educational level, occupation, monthly income, alcohol consumption, or smoking status. When classified by sex, BMI (p < 0.001) was higher in the male cases than in the male controls, whereas female cases were more likely to have a family history of thyroid cancer than female controls (p=0.01).
In the multivariate conditional logistic regression analyses, associations were observed between the demographic characteristics and thyroid cancer risk (Table 2). A family history of thyroid cancer was associated with an increased risk of thyroid cancer in the total population (adjusted OR, 2.97; 95% CI, 1.60 to 5.55). In contrast, the odds of thyroid cancer in sales and service participants were significantly lower than those in professions, office workers, and housewives (adjusted OR, 0.69; 95% CI, 0.50 to 0.94), whereas the odds in those with moderate income were higher than in those with low income (adjusted OR, 1.51; 95% CI, 1.03 to 2.23). An association was not observed among family history of cancer, marital status, educational level, monthly income, smoking status, and alcohol consumption in the total population. When classified by sex, the male population did not show any associations between occupation and thyroid cancer (Table 2).
BMI was associated with an increased risk of thyroid cancer in the overweight group (adjusted OR, 1.50; 95% CI, 1.12 to 2.00) and the obese group (adjusted OR, 1.62; 95% CI, 1.23 to 2.14) compared with the risk in the normal group. When stratified by sex, BMI was also associated with an increased risk of thyroid cancer in males in the overweight group (adjusted OR, 2.66; 95% CI, 1.42 to 4.97) and the obese group (adjusted OR, 2.78; 95% CI, 1.57 to 4.92) when the normal group was used as a reference, but the association in the females was not significantly different.
2. Genetic characteristics and their associations with body mass index and thyroid cancer
Table 3 presents the summary statistics of 27 SNPs included in the analysis. Additionally, there were differences in the distribution of genotypes of rs1861868 (p < 0.001) and rs8044769 (p=0.04) between total cases and controls, whereas unequal distribution was observed for rs8044769 (p=0.02) in females only (Table 4).
Table 5 shows the association between single FTO variants and thyroid cancer risk in the codominant, dominant, and recessive models. Variant rs1861868 was found to be associated with a decreased risk of thyroid cancer, with adjusted ORs (95% CIs) of 0.73 (0.57–0.92) for A/G vs. G/G, 0.48 (0.25–0.95) for A/A vs. G/G, and 0.70 (0.56–0.89) for A/G-A/A vs. G/G. In contrast, variants rs8047395 and rs8044769 were found to be associated with an increased risk of thyroid cancer, with ORs (95% CIs) of 1.39 (1.02–1.89) and 1.44 (1.05–1.97) in the recessive model.
Genome association analysis revealed that none of the 27 SNPs included in the study were significantly associated with overweight and obesity (p > 0.1) (Fig. 2). Furthermore, Fig. 3 shows the LD block structure with the high correlation among 27 variants in the FTO gene. Then, Haploview identified seven tagging SNPs including rs1861868, rs8047395, rs56094641, rs1477196, rs9930506, rs11642841, and rs7195539. Compared to the haplotype GAAGACA, AAAGACA (adjusted OR, 0.55; 95% CI, 0.36 to 0.84), and GGAAACA (adjusted OR, 0.43; 95% CI, 0.25 to 0.72) were found to be associated with significantly lower odds of thyroid cancer in the total population and female subgroup, respectively (Table 6).

Linkage disequilibrium plot for single nucleotide polymorphisms within the fat mass and obesity-associated (FTO) gene in chromosome 16. The numbers in the squares are r2 values (×100) between the loci, with red shades indicating greater r2 values.

Genome association analysis for variants related to overweight (A) and obesity (B) in chromosome 16. Included variants in this study are presented in the red color.
3. Combined effect of body mass index and variants on thyroid cancer risk
The adjusted ORs and 95% CIs for the combined effect of BMI and SNP on thyroid cancer are presented in Tables 7–9. When using G/G carriers with normal BMI as the reference, overweight and obesity were significantly associated with thyroid cancer risks in all genotype groups of rs17817288, with adjusted ORs (95% CIs) of 1.77 (1.07–2.91), 1.66 (1.06–2.62), and 2.15 (1.18–3.95) for overweight participants carrying G/G, A/G, and A/A, respectively; and 2.68 (1.65–4.34), 1.67 (1.08–2.58), and 1.71 (1.01–2.90) for obese participants carrying A/A, G/A, and G/G, respectively, in the codominant model (Table 7). Neither overweight nor obesity was associated with thyroid cancer risks in all genotype groups of other variants.

Combined effect of body mass index and each polymorphism on thyroid cancer risk in the codominant model

Combined effect of body mass index and each polymorphism on thyroid cancer risk in the dominant model

Combined effect of body mass index and each polymorphism on thyroid cancer risk in the recessive model
In the dominant model, when using G/G-A/G carriers with normal BMI as the reference, overweight and obesity were significantly associated with thyroid cancer risks in A/G-A/A genotype carriers of rs9937053, rs12149832, and rs1861867 (Table 8). The adjusted ORs (95% CIs) for rs9937053 were 1.66 (1.04–2.67) and 1.71 (1.13–2.58) for overweight participants; and 2.20 (1.39–3.49) and 1.65 (1.12–2.45) for obese participants carrying G/G and A/G-A/A, respectively. The adjusted ORs (95% CIs) for rs12149832 were 1.81 (1.13–2.89) and 1.69 (1.11–2.57) for overweight participants and 2.30 (1.45–3.66) and 1.68 (1.13–2.49) for obese participants carrying G/G and A/G-A/A, respectively. The adjusted ORs (95% CIs) for rs1861867 were 1.40 (1.04–1.90) and 2.69 (1.06–6.83) for overweight participants and 1.56 (1.17–2.07) and 2.09 (1.02–4.27) for obese participants carrying G/G and A/G-A/A, respectively. When using A/A carriers with normal BMI as the reference, overweight and obesity were significantly associated with thyroid cancer risks in G/A-G/G genotype carriers of rs7195539, with ORs (95% CIs) of 1.70 (1.12–2.57) and 1.64 (1.09–2.47) for overweight participants and 1.90 (1.27–2.86) and 1.75 (1.19–2.56) for obese participants carrying A/A and G/A-G/G, respectively.
In the recessive model, when using G/G carriers with normal BMI as the reference, overweight and obesity were significantly associated with thyroid cancer risks in A/A genotype carriers of rs17817288 and rs8044769 (Table 9). The adjusted ORs (95% CIs) for rs17817288 were 1.64 (1.19–2.25) and 2.11 (1.21–3.67) for overweight participants and 1.95 (1.43–2.67) and 1.66 (1.04–2.65) for obese participants carrying G/G-A/G and A/A, respectively. The adjusted ORs (95% CIs) for rs8044769 were 1.53 (1.12–2.10) and 2.55 (1.35–4.81) for overweight participants and 2.30 (1.45–3.66) and 1.68 (1.13–2.49) for obese participants carrying G/G-A/G and A/A, respectively.
Discussion
Our study examined the main effects of BMI and FTO in thyroid carcinogenesis and the effect of their combined interactions on thyroid cancer susceptibility. Our findings suggest that high BMI subclasses are positively associated with thyroid cancer risk, which is consistent with the results of several epidemiologic studies. In a systemic review of 36 studies, BMI was positively associated with thyroid cancer risk in both male and female populations [17]. In another systematic review of 21 articles that included 12,199 thyroid cancer cases, the risk of thyroid cancer was increased by 25% in overweight individuals and by 55% in obese individuals compared with the risk in normal weight individuals [18]. The findings were similar in a Korean hospital-based case-control study, which observed a positive association between BMI and thyroid cancer [12]. A multicenter prospective cohort study from the Korean Cancer Prevention Study-II also reported that an increased risk of thyroid cancer was associated with BMI [13]. Our findings support the association between BMI and an increased risk of thyroid cancer and indicate that the role of obesity-related FTO in thyroid cancer risk should be further evaluated.
FTO is an obesity-susceptibility gene that was identified in a previous study [19]. In addition, the association was observed across populations with diverse ethnicities, and it was found to have the largest impact in young adulthood [13]. In a study of 38,759 Europeans performed to identify an obesity risk allele in FTO, carriers harboring one copy of the risk allele weighed on average 1.2 kg more than people without the allele. Adults who were homozygous for the risk allele weighed 3 kg more and had a 1.67-fold higher rate of obesity than those without the allele [20]. Although the FTO gene is not associated with changes in fetal growth, it has a critical impact in young adults and is associated with changes in BMI and obesity in children aged seven and older. Studies have suggested that SNPs located within the first intron of FTO are strongly associated with obesity [19]. In vitro studies have shown that reducing the functional activity of FTO in a mouse model resulted in reduced fat mass and body weight, elevated energy expenditure and unchanged physical activity, whereas the overexpression of FTO in a mouse model caused increased adiposity and did not impact the energy expenditure; this mouse model provides evidence that FTO directly affects fat mass and is linked with obesity [21]. However, the exact molecular mechanisms underlying the effect of FTO on obesity are not fully understood.
This study has shown that the genetic variants rs8047395 and rs8044769 in the FTO gene are associated with thyroid cancer. Based on our findings, individuals who carry the minor G/G allele of rs8047395 have a higher risk of thyroid cancer than those carrying the A/A and G/A alleles, and individuals who carry the minor A/A allele of rs8044769 have a higher risk of thyroid cancer than those carrying the G/G and A/G alleles. Studies have observed an association between FTO and an increased risk of different types of cancers, which suggests that FTO has a role in carcinogenesis [22,23]. A study examining the SNPs rs7206790, rs8047395, rs9939609, and rs1477196 on intron 1 of FTO found that all of the SNPs were associated with an increased risk of breast cancer [24]. Similarly, a study conducted in a German population showed a positive association between the rs8047395 SNP of the FTO gene and PTC [25]. These findings provide primary evidence that FTO may play an important role in carcinogenesis. Moreover, a study conducted in the United States found that nine of the 10 top-ranking SNPs (ptrend < 0.010) investigated for thyroid cancer risk were located in FTO; however, none of these SNPs were associated with thyroid cancer risk after controlling for the false discovery rate. This study showed an association between BMI and increased PTC risk (OR, 1.18; 95% CI, 1.02 to 1.37), although additional adjustments for BMI did not change the SNP-PTC association [26]. However, the association between rs8044769 and thyroid cancer risk has not been examined. Different findings for these associations between studies may be related to the complex role of FTO in the molecular mechanisms of carcinogenesis, which remain unknown. The diverse roles of FTO in adiposity, energy balance, type 2 diabetes and metabolism may also play a role in carcinogenesis, which would result in inconsistent associations [27,28].
This study investigated the effect of interactions between BMI and genetic variants of FTO on thyroid cancer risk using a gene-environment interaction analysis method. We found a positive effect of BMI subclasses on thyroid cancer in all groups of different genotypes of rs17817288 in the codominant model, rs9937053, rs12149832, rs1861867, and rs7195539 in the dominant model, and rs17817288 and rs8044769 in the recessive model. Although the mechanism of this interaction remains unclear, FTO may interact with BMI in several ways. First, the association could be explained by the role of FTO in regulating energy homeostasis, body size, and fat accumulation [29]. Thus, genetic variants of this gene are associated with obesity, diabetes, and metabolic syndromes, which are cancer risk factors [30]. Second, previous studies have claimed that FTO is associated with DNA methylation [25], and genetic polymorphisms of FTO may impact the normal pattern of DNA synthesis and methylation, which are factors in carcinogenesis [31] and affect both body mass and tumor suppression. For BMI, recent studies have suggested that adiposity may also influence DNA methylation. A recent GWAS showed that BMI is associated with widespread changes in DNA methylation, which is a key regulator of gene expression and molecular phenotypes [32]. Thus, the influence of BMI and FTO on DNA methylation may explain the combined interaction effect on susceptibility to thyroid cancer.
This study makes a significant contribution to our understanding of how genetic variants of FTO affect the risk of thyroid cancer. Only one other study of a German population has investigated the association between thyroid cancer and the FTO genetic variant rs8047395. However, that study population was exclusively Caucasian, and few studies have been conducted in Asian populations [25]. Another strength of this study is the analysis of both socioeconomic status and lifestyle risk factors for thyroid cancer, including smoking status, alcohol consumption, and reproductive factors. This study has several limitations. First, this study is a hospital-based case-control study, and the controls may not be sufficiently representative of the general population because their genetic variants or lifestyle habits may be similar or related to cancer cases. However, the large number of controls in this study should eliminate any potential problem with similarities in the characteristics of participants. Second, this study may have a recall bias because the demographic information was based solely on self-conducted questionnaires dependent on the memory of the participants. However, data collected from the questionnaire were not a crucial component of the information analyzed for this study and likely did not affect our findings.
In summary, the interactions observed in this study suggest that the effect of subclasses of BMI on thyroid cancer risk may differ according to individual genetic variants of FTO. Although the underlying mechanism requires further investigation, this study improves our understanding of the role of BMI and FTO as indicators of thyroid cancer risk and their role in thyroid cancer carcinogenesis. In the future, additional epidemiological studies with larger sample sizes should be performed to support and extend our findings on the role of genetic risk factors.
Notes
Ethical Statement
All participants provided written informed consent, and the Institutional Review Board of the National Cancer Center approved the study protocols (IRB No. NCC2016-0088).
Author Contributions
Conceived and designed the analysis: Hoang T, Song D, Lee J, Lee EK, Hwangbo Y, Kim J.
Collected the data: Lee J, Lee EK, Hwangbo Y, Kim J.
Contributed data or analysis tools: Lee J, Lee EK, Hwangbo Y, Kim J.
Performed the analysis: Hoang T, Song D.
Wrote the paper: Hoang T, Song D, Lee J, Lee EK, Hwangbo Y, Kim J.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgements
This research was supported by grants from the National Cancer Center in Korea (No. 1910330) and National Research Foundation funded by the South Korean Government (2018R1D1A1A090838-76).