Second Primary Ovarian Epithelial, Fallopian Tube, and Primary Peritoneal Cancers after Breast Cancer Diagnosis: Korea Central Cancer Registry
Article information
Abstract
Purpose
A prior history of breast cancer is a risk factor for the subsequent development of primary peritoneal, epithelial ovarian, and fallopian tubal (POFT) cancers. This study aimed to estimate the incidence of secondary POFT malignancy in breast cancer patients and the clinical outcomes of primary and secondary POFT cancer.
Materials and Methods
We searched the Korea Central Cancer Registry to find patients with primary and secondary POFT cancer who had breast cancer in 1999–2017. The incidence rate and standardized incidence ratio were calculated. Additionally, we compared the overall survival of patients with primary and secondary POFT cancer.
Results
Based on the age-standardized rate, the incidence of second primary POFT cancer after breast cancer was 0.0763 per 100,000 women, which increased in Korea between 1999 and 2017. Among the 30,366 POFT cancer patients, 25,721 were primary POFT cancer only, and 493 had secondary POFT cancer after a breast cancer diagnosis. Second primary POFT cancer patients were older at the time of diagnosis (55 vs. 53, p < 0.001) and had a larger proportion of serous histology (68.4% vs. 51.2%, p < 0.001) than patients with primary POFT. There were no differences between the two groups in tumor stage at diagnosis. The 5-year overall survival rates were 60.2% and 56.3% for primary and secondary POFT cancer, respectively (p=0.216).
Conclusion
The incidence of second primary POFT cancer after breast cancer increased in Korea between 1999 and 2017. Besides, second primary POFT cancer patients were diagnosed at older ages and had more serous histology.
Introduction
Patients who have primary cancer often develop metachronous or synchronous multiple malignancies. In 2018, breast cancer was the most common cancer among women and accounted for 24.2% of the cases in women worldwide [1]. Similarly, it was the most prevalent cancer and accounted for 20.3% of the cases in Korean women in 2017. The number of newly diagnosed female breast cancer patients is increasing every year, and the relative survival rates of breast cancer are steadily increasing in Korea [2]. Given the increase in the incidence of breast cancer, combined with earlier diagnosis and improved treatments, an increasing number of women are surviving breast cancer in Korea.
Survival rates for women with breast cancer have constantly improved; therefore, the risk of developing a subsequent primary malignancy such as ovarian cancer is renewed. Especially, in BRCA1 and BRCA2 mutation carriers, who are at an increased risk for breast and ovarian cancer, breast cancer typically presents earlier than ovarian cancer. Additionally, there is a significant probability that ovarian cancer will be diagnosed after breast cancer [3]. A prior history of breast cancer is a risk factor for the subsequent development of ovarian cancer. The estimated risk of developing ovarian cancer is increased by approximately 2-fold for patients with a history of breast cancer [4].
Epithelial ovarian cancer is the second most common cause of gynecological cancer deaths, and it is also related to the BRCA1 and BRCA2 gene. In Korea, where the prevalence of ovarian cancer is increasing, it is the second most prevalent gynecological malignancy [5]. Basically, epithelial ovarian cancer is the same disease entity as primary peritoneal cancer and fallopian tubal cancer. Accordingly, primary peritoneal, epithelial ovarian, and fallopian tubal (POFT) cancers are diagnosed and treated in similar ways [6]. However, there is a scarcity of information on second primary POFT cancers after breast cancer in Korea. Therefore, the objective of this study was to estimate the incidence and risk of second primary POFT malignancies in female breast cancer survivors. In addition, we also aimed to detect the differences in the clinical outcomes of primary and second primary POFT cancers.
Materials and Methods
This study used data from patients enrolled in the Korea Central Cancer Registry (KCCR), which collects information regarding approximately 98% [7] of the cancer cases in Korea since 1999. According to the cancer registration guidelines, the first date of diagnosis, diagnosis route, primary cancer site, histological diagnosis, the Surveillance, Epidemiology and End Results (SEER) summary stage, laterality, differentiation, diagnostic method, and treatment can be identified. We searched the KCCR to detect all patients with secondary POFT cancer which developed after the diagnosis of primary epithelial breast cancer. Primary peritoneal cancer (C48), epithelial ovarian cancer (C56.9), and fallopian tube cancer (C57.0) were defined on the basis of the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) [8]. The histological types were categorized into serous, endometrioid, clear cell, mucinous carcinoma, and other histologic types of tumor. Breast cancer (C50) was also defined based on the ICD-O-3. In addition, breast sarcoma, phyllodes tumor, and Paget disease were not included in this study.
We grouped the patients according to their age at diagnosis with ovarian cancer (< 30 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, and ≥ 80 years), the SEER summary stage [9], histologic type, and the treatment modality which was administered. We did not separate high grade serous carcinoma from low grade carcinoma because the two histologic types were registered in the KCCR without distinction. The KCCR follows coding guidelines based on the 2004 rules of the International Agency for Research on Cancer for multiple primary cancer. In addition, secondary POFT cancers diagnosed within 2 months of the index breast cancer diagnosis were excluded to minimize the incidence of misclassification of undetected synchronous cancers and metastases.
The age-standardized incidence rate (ASR) was calculated in patients diagnosed with POFT cancer using Segi’s world standard population and was expressed per 100,000 people. The standardized incidence ratio (SIR) of subsequent POFT cancers among patients with breast cancer was calculated to quantify relative risk compared with those among the general population. The Kaplan-Meier method was used to create survival curves, and the log-rank test was used to compare the survival difference.
ASR and SIR were analyzed using SAS ver. 9.4 (SAS Institute, Inc., Cary, NC) and SEER*Stat 8.3.6 (National Cancer Institute, Bethesda, MD), respectively. Survival analysis was performed using Stata ver. 16 (StataCorp LLC, College Station, TX). All statistical tests were considered statistically significant for p < 0.05.
Results
1. Characteristics of second primary POFT cancers
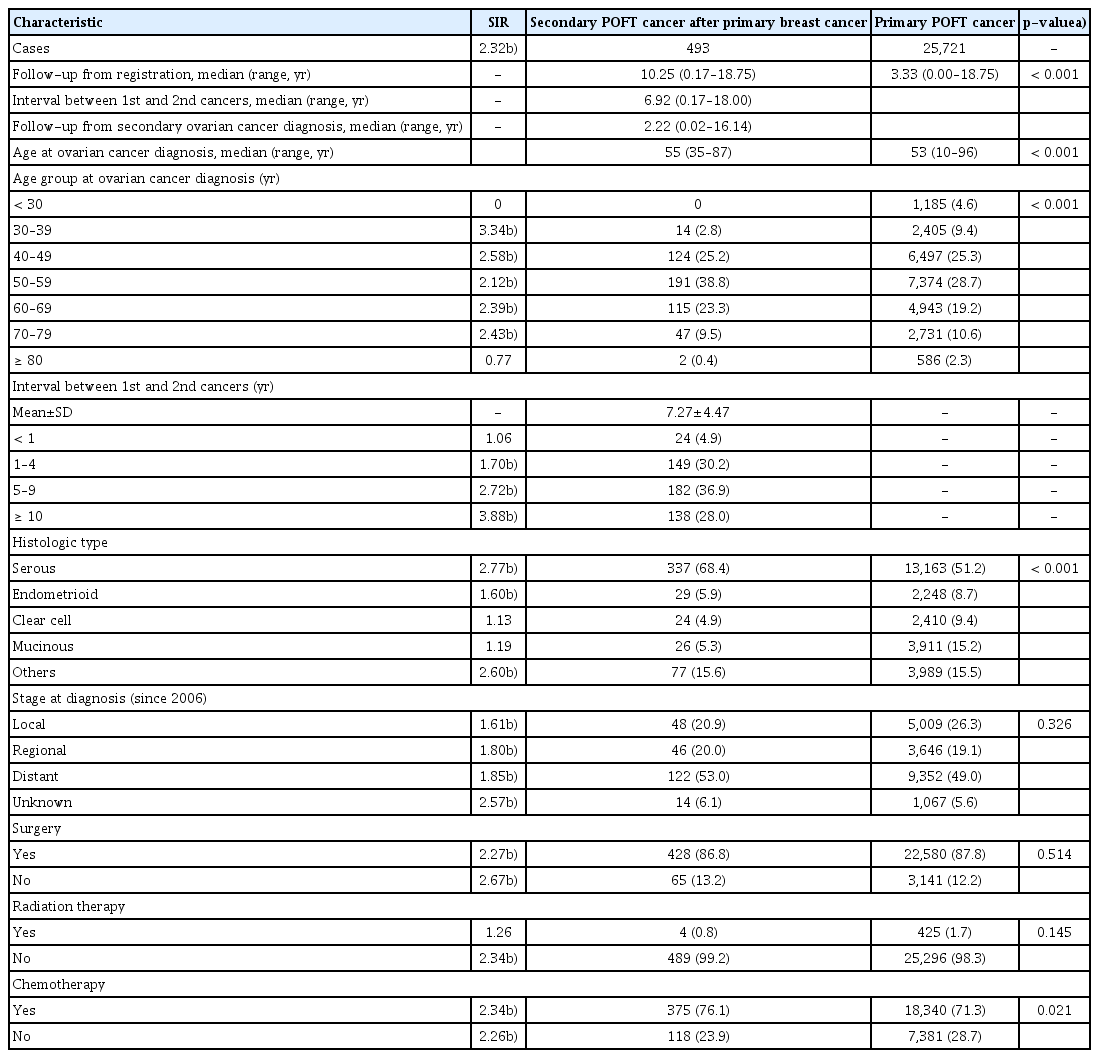
Between 1999 and 2017, the KCCR included 251,244 cases of breast cancer and 30,366 POFT cancer cases. Among the POFT cancer cases, 493 cases had second primary POFT cancer after primary breast cancer diagnosis. Based on the ASR, the incidence of total POFT cancer was 4.82 per 100,000 women. In addition, the incidence of second primary POFT cancers was 0.0763 per 100,000 women and that of second primary POFT cancer after breast cancer was 78.84 per 100,000 women in breast cancer survivors (Table 1).
The overall incidence of POFT cancer has been observed to increase steadily. In addition, despite slight fluctuations, the incidence of second primary POFT cancer has also been observed to increase. In 1999, there were 958 primary POFT cancer patients and no second primary POFT cancer patients. On the other hand, there were 2,478 primary POFT cancer patients and 77 second primary POFT cancer patients in 2017 (Table 1).
The median age of patients with primary and second primary POFT cancers was 53 years (range, 10 to 96 years) and 55 years (range, 35 to 87 years), respectively. In the case of second primary POFT cancer, the median age at diagnosis of breast cancer was 48 years (range, 25 to 80 years). Moreover, the median of the interval between previous breast cancer and second primary POFT cancer was 6.92 years (range, 0.17 to 18.00 years) (Table 2).
With respect to the entire group of breast cancer survivors, higher rates of second primary POFT cancers were observed to occur than in the general female population in KCCR (SIR, 2.32; 95% confidence interval [CI], 2.12 to 2.53). According to the age at second primary POFT cancer diagnosis, the SIR in the patients whose age at second primary POFT cancer was between 30 and 39 years was 3.34 (95% CI, 1.83 to 5.61) and most prominent. The SIRs also increased in all age groups except in those aged 80 or older.
The distribution of the patients’ age at diagnosis differed between primary and second primary POFT cancers and was significant (p < 0.001). The most common age group at the second primary POFT cancer diagnosis was 50–59 years (38.7%). However, there were 14 patients in the range of 30–39 years, accounting for only 2.84% (Table 2).
Depending on the histologic subtype, there were differences in the SIRs. In the serous type of POFT, the SIR was 2.77 (95% CI, 2.48 to 3.09). On the other hand, the SIR was 1.60 (95% CI, 1.07 to 2.29) in endometrioid type disease. The SIRs of clear cell type and mucinous type were 1.13 and 1.19, respectively, with no statistical significance.
By classifying the histologic subtype into serous, endometrioid, clear cell, mucinous, and others, the distribution pattern of each histologic subtype was significantly different between primary and second primary POFT cancers (p < 0.001). For instance, the differences stand out in serous cancer, in which the percentage of serous POFT cancers was 68.4% in second primary POFT cancers and 51.2% in primary POFT cancers.
In terms of the extent of the disease, there was no statistical difference in the disease stage distribution between primary and second primary POFT cancers (p=0.326). The SIRs were increased for the entire stage at diagnosis between 2006 and 2017. In the localized stage, the SIR was 1.61 (95% CI, 1.18 to 2.13). On the other hand, the SIR was 1.85 (95% CI, 1.53 to 2.20) in distant metastasis cases.
After the diagnosis of primary breast cancer, the SIRs for the occurrence of second primary POFT cancers were observed to increase with time. The SIRs of survivors for breast cancer were 1.70 (95% CI, 1.44 to 2.00), 2.72 (95% CI, 2.34 to 3.15), and 3.88 (95% CI, 3.26 to 4.59) in 1–4 years, 5–9 years, and over 10 years, respectively (Table 2).
2. Survival outcomes for second primary POFT cancers
The median follow-up period of primary POFT cancer cases was 3.33 years, and that of second primary POFT cancer cases was 10.25 years from the breast cancer diagnosis, 2.22 years from the secondary POFT cancer diagnosis (Table 2). The Kaplan-Meier survival analysis revealed a deteriorated survival curve in patients with second primary POFT cancers compared with that in patients with primary POFT cancers (Fig. 1). However, it was not significant using the log-rank test (p=0.216). The 5-year overall survival (OS) rates were 60.2% in primary POFT cancer patients and 56.3% in secondary POFT cancer patients.

Survival outcome of peritoneal, ovarian, and fallopian tube (POFT) cancer patients from the onset time of POFT cancer diagnosis in Korea.
In the patients with only POFT cancer (Fig. 2D–F), the younger the patients, the better the survival rate. On the other hand, the secondary POFT patients who were 40–49 years had a poorer survival time (median survival time, 72 months) than POFT patients who were 50–59 years (median survival time, 97 months) (Fig. 2A).
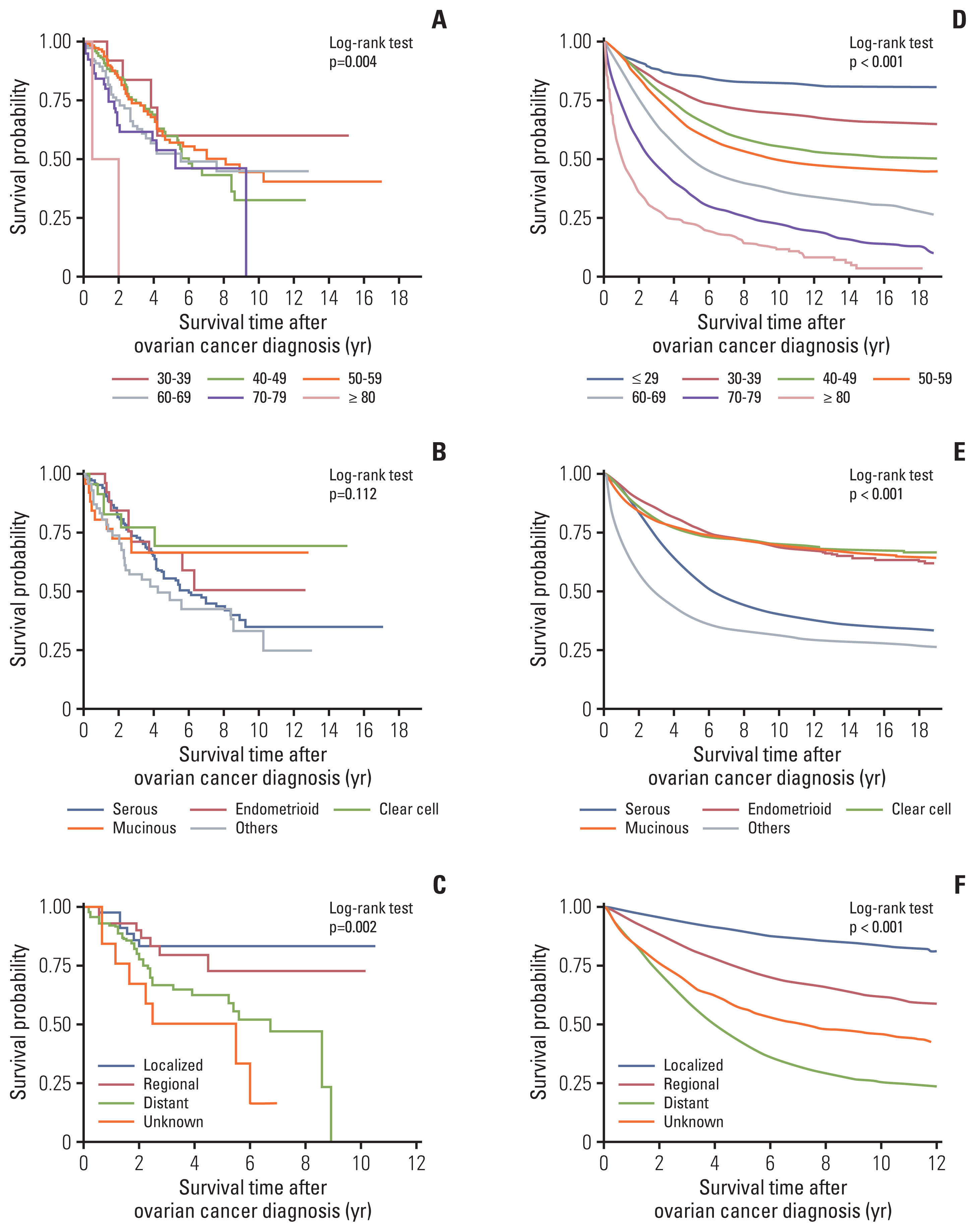
Survival outcomes from the onset time of peritoneal, ovarian, and fallopian tube (POFT) cancer diagnosis according to clinicopathologic characteristics (age, type, Surveillance, Epidemiology, and End Results stage). Women with second POFT cancer after breast cancer by age (A), histological type (B), and stage (since 2006) (C) and women with primary POFT cancer by age (D), histological type (E), and stage (since 2006) (F).
In the patients with second POFT cancer, the 5-year OS rate of patients with a serous histology was 55.7%, whereas the patients with endometrioid and clear cell histology had more favorable outcomes (66.6% and 69.7%, respectively), though not significant (p=0.112) (Fig. 2B). Localized POFT cancer was associated with 5-year OS rates of 83.6%, whereas distant metastases had a 5-year OS rate of 62.9% (p=0.002) (Fig. 2C).
Discussion
1. SIRs of second primary POFT cancers
This study reported that second primary POFT cancer accounted for 1.6% (493/30,366) of all newly diagnosed ovarian cancers in Korea, based on the KCCR data from 1999 to 2017. These patients have an increased risk compared to the patients without breast cancer. Moreover, we reported that the absolute number and the ratio of second primary POFT cancers to the total number of cancer cases are steadily increasing.
The incidence rates for second primary malignancies are usually expressed as a SIR, which is the ratio of the observed to expected malignancies. We reported higher rates of second primary POFT cancers (SIR, 2.32) in breast cancer. The increased overall SIR supports previous studies wherein elevated SIRs in ovarian cancer after primary breast cancer were found [10–13]. In this study, the patients with second primary POFT cancer diagnosed between the ages of 30 and 39 have the highest SIR (3.34). Similar to the results of this study, Schonfeld et al. [12] reported that higher rates of second primary ovarian cancers occurred among breast cancer survivors than in the general female population in SEER (SIR, 1.24; 95% CI, 1.2 to 1.3). Moreover, the SIRs also showed that the highest SIR was observed at the age of 40 years or younger (3.43; 95% CI, 2.9 to 4.0).
In this study, the incidence rate for second primary ovarian cancers increased in ASR from a low value of 0.0111 per 100,000 women in 2000 to a peak of 0.1801 per 100,000 women in 2017. It reflects the fact that the overall incidence of POFT cancer has steadily increased [14]. Ovarian cancers in the general population and breast cancer survivors are increasing in tandem, and we can speculate that this parallel trend may reflect shared risk factors such as decreased parity and festinated menarche in Korean women.
On the other hand, since it was in 1999 that we started thorough registration, for patients with secondary POFT after 1999 who were diagnosed with breast cancer before 1999, they might have not been included if the time since the breast cancer diagnosis had been too long.
2. Genetic predisposition of primary breast cancer and second primary POFT cancer patients
In breast cancer patients, it can be inferred that the second primary POFT cancer is due largely to the BRCA1/2 mutation. Germline mutations in the BRCA1 and BRCA2 genes are the strongest known genetic risk factors for both breast and epithelial ovarian cancer [15] and are found in 13.3% [16] to 15% [17] of women with epithelial ovarian cancer. In breast cancer, the prevalence rates were diverse from 5% to 20% according to population selection and frequency of founder mutations [18].
Individuals with a BRCA1 or BRCA2 mutation from hereditary breast cancer families have an elevated risk of ovarian cancer. Moreover, germline mutations in women with ovarian carcinoma have been recently identified in many of the previously identified breast cancer genes in the Fanconi anemia–BRCA pathway [19]. Metcalfe et al. [3] reported the 10-year actuarial risk of ovarian cancer after breast cancer in BRCA1 or BRCA2 pathogenic variant carriers. The risk was 12.7% for BRCA1 carriers and 6.8% for BRCA2 carriers (p=0.03) [3].
We could not infer the exact prevalence of BRCA1 and BRCA2 pathogenic variants because the registration data used in this study did not include BRCA status. In this study, 337 second primary POFT cancer patients (68.36%) had serous histology, and it is well known that the BRCA1 or BRCA2 pathogenic variant is associated with high grade serous type POFT cancers [20]. In addition, women from BRCA mutation negative and site-specific breast cancer families are not at an increased risk for second primary ovarian cancer [21].
Risk-reducing salpingo-oophorectomy (RRSO) can be used for overcoming the potential risk of secondary ovarian cancer, especially in BRCA1 and/or BRCA2 pathogenic variant carriers. The National Comprehensive Cancer Network guidelines also recommend RRSO for BRCA1 and/or BRCA2 pathogenic variant carriers.
3. Age at diagnosis of primary breast cancer and second primary POFT cancers in Korea
The peak age of breast cancer is 10 to 20 years younger in Korea than Western countries, where the incidence rate of breast cancer is the highest in the fifth decade, followed by the sixth and fourth decades [22]. Moreover, approximately 25% of invasive breast cancer cases in Korea occur in patients younger than 40 years [18]. The 5-year relative survival rate (RSR) of breast cancer diagnosed between 1993 and 1995 was 79.2%. However, due to continuous improvement, the 5-year RSR of breast cancer cases diagnosed between 2013 and 2017 was observed as 93.2% [2]. The treatment outcomes and OS in female breast cancer cases have improved because of increased mammography screening and the development of adjuvant chemotherapy, human epidermal growth factor receptor 2 therapy, and endocrine therapy. Therefore, breast cancer survivors are rapidly increasing in Korea. In this study, the mean age at diagnosis of the secondary POFT was 55.82±9.52, lower than previous reports from 66.3 [11]-67.8 [23]. The reason for the early age at diagnosis of the second primary POFT may be the reflection of a younger peak age of breast cancer. The SIR among patients younger than 40 years was 3.34, which was larger than that of older ages. However, the number of patients under the age of 40 was only 14 (2.84%); therefore, additional cases are needed to know the exact clinical outcomes.
This study had several strengths. We analyzed the incidence of POFT cancer after breast cancer diagnosis by utilizing the population-based cancer registry which contained approximately 98% of Korean cancer cases [7] and to our knowledge, this study is the first study in Korea to do so. However, our limitations include a lack of information about family history, medico-surgical history, BRCA1/BRCA2 gene status, and the hormone receptor status of breast cancer. There may have been an omission of secondary POFT cancer patients diagnosed with breast cancer before 1999. In addition, this study includes patients with a diagnosis interval of less than one year between breast cancer and POFT cancer. Sixty-nine cases of secondary POFT cancer occurring within 2 months were excluded from the data analysis. Of the remaining 493 cases, 24 of the secondary POFT patients (4.9%) had a diagnosis interval of less than 1 year. The median time to onset of secondary cancer in these cases was 6.5 months. Moreover, the present study did not describe the treatment history and subtypes of breast cancer. Further studies are needed to elucidate the precise influence of BRCA gene mutations on the development of POFT cancer in breast cancer survivors.
The overall incidence of second primary POFT cancer after breast cancer increased between 1999 and 2017 in Korea. In addition, second primary POFT cancer patients were diagnosed at older ages and had more serous histology. Secondary POFT cancer was associated with lower 5-year survival than primary POFT cancer; however, this was not statistically significant (56.3% vs. 60.2%, p=0.216).
Notes
Ethical Statement
This study was approved by the Institutional Review Board of the National Cancer Center, Korea (NCC2020-0176). The requirement for informed consent was waived because we analyzed de-identified data secondarily.
Author Contributions
Conceived and designed the analysis: Won YJ, Lim MC.
Contributed data or analysis tools: Lim J, Won YJ.
Performed the analysis: Lim J.
Wrote the paper: Ha HI, Lee EG, Jung SY, Chang YJ, Won YJ, Lim MC.
Interpretation, review: Ha HI.
Review and comment: Lee EG, Jung SY, Chang YJ.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This work was supported by the National Cancer Center Grant (NCC-1910132, 1911274).