Knockdown of EMMPRIN (OX47) in MRMT-1 Carcinoma Cells Inhibits Tumor Growth and Decreases Cancer-Induced Bone Destruction and Pain
Article information
Abstract
Purpose
Bone destruction and pain caused by cancer is one of the most devastating complications of cancer patients with bone metastases, and it seriously affects the quality of patients’ life. Extracellular matrix metalloproteinase inducer (EMMPRIN) is a cell adhesion molecule with increased expression in a variety of tumors. This study focused to clarify the specific function of EMMPRIN in bone metastasis of breast cancer.
Materials and Methods
Adenovirus with shRNA-EMMPRIN was transfected into MRMT-1 rat breast carcinoma cells, and the MRMT-1 cells with different expression levels of EMMPRIN were implanted into the bone marrow cavity of rat tibia. Next, the effect of down-regulation of EMMPRIN was evaluated as follows: bone damage was detected by X-ray radiological and tartrate-resistant acid phosphatase staining; the tumor burden was evaluated by hematoxylin and eosin staining; the test of pain-related behaviors was assessed used the bilateral paw withdrawal mechanical threshold; and the levels of secretory factors in tumor conditioned medium were determined by using enzyme-linked immunosorbent assay.
Results
We found that down-regulation of EMMPRIN in tumor cells can simultaneously reduce tumor burden, relieve cancer-induced bone destruction and pain.
Conclusion
EMMPRIN is expected to be a therapeutic target for relieving bone metastasis of breast cancer and alleviating cancer-induced bone destruction and pain. The method of targeting EMMPRIN may be a promising strategy for the treatment of cancer in the future.
Introduction
Osseous metastasis is a disease in which some malignant tumors originating from other tissues are transferred to bone tissue by blood. Bone damage and pain are the main manifestations in osseous metastasis. Bone tissue is one of the most common distant metastases in patients with breast cancer and other cancers including prostate, lung, kidney, thyroid, and sarcoma, etc. [1]. Over the past decade, the development of animal models has spurred our understanding of the mechanisms of how cancer causes bone destruction and pain [2]. It is widely believed that bone pain caused by cancer is associated with osteolytic lesions. Metastasis of malignant tumor cells into the bone cavity affects resident bone cells. Tumor cells produce tumor-associated factors including protons, enzyme, and cytokines, which change the microenvironment. The changes of the microenvironment thereby destroys the balance between bone formation of osteoblasts and bone-destroying osteoclasts, which ultimately leads to bone damage and further promote the development of cancer pain [3]. As the underlying mechanism is very complex and not fully understood, the treatment of cancer-induced bone destruction and pain is still a clinical challenge.
Matrix metalloproteinases (MMPs) are a family of more than 20 proteases participate in a variety of physiological and pathological events. MMPs play important roles in tumor progression. Studies have shown that MMPs promote metastasis of malignant tumors [4]. Extracellular matrix metalloproteinase inducer (EMMPRIN), a cell surface glycoprotein, plays a key regulatory function in promoting secretion of MMPs [5,6]. EMMPRIN is named differently among various species, including CD147 (human), basilin (mouse), and OX47 (rat). Previous studies have shown that the expression of EMMPRIN is extremely enriched in a variety of tumor cells [7], which play important roles in promoting angiogenesis and promoting tumor growth and metastasis [8,9]. In previous research, we knocked down the expression of EMMPRIN in murine Lewis lung carcinoma cell line, and compared the changes in the secretion profile of soluble factors using an antibody array. The results indicated that silencing EMMPRIN altered the expression of a wide range of genes, including chemokines, cytokines, growth/survival factors, tissue-remodeling factors, and enzymes involved in metabolism [10]. Among them, we noted that knocking down of EMMPRIN significantly reduced the secretion of osteocalcin and osteoactivin, and increased the secretion of bone morphogenetic proteins (BMP-5, -6, and -7). These above secreted factors play important roles in regulation of bone remodeling [11,12]. The presence of tumor cells in the bone microenvironment disrupts the balance between osteoblasts and osteoclasts, resulting in excessive bone loss or formation [13,14]. We speculate that EMMPRIN, as an important inducer of tumor progression, may potentially promote bone destruction when the tumor cells metastasized to the bone. Although both basic and clinical studies have shown that EMMPRIN is an indicator of poor prognosis in many tumors, the role of EMMPRIN in tumor invasion to bone has not been explored.
In this study, we used an animal model in which MRMT-1 rat breast cancer cells were inoculated into the tibia bone marrow cavity of female rats to study the effects of EMMPRIN on bone destruction and pain. We found that down-regulation of EMMPRIN expression in breast cancer cells could simultaneously reduce tumor burden, bone destruction as well as pain caused by tumors. Our results suggest that EMMPRIN had multiple roles in tumor progression, and it might be developed into a potential new target to control pain and bone destruction.
Materials and Methods
1. Cells culture and knockdown of EMMPRIN (OX47) expression
MRMT-1 rat breast carcinoma cells were purchased from the Jiniou Biological Technology Company (China, Guangzhou). Cells were cultured in medium containing RPMI 1640 (ThermoFisher, Waltham, MA) with 10% fetal bovine serum (Gibco), at 37°C under a mixture of 95% air and 5% CO2.
OX47 expression in MRMT-1 cells was knocked down by lentiviral transduction. The construction of shRNA-EMMPRIN and the control of lentiviral vectors have been characterized in our previous study [15]. The stable MRMT-1 cell line with OX47 knocked down was obtained during culturing in RPMI 1640 medium with 0.6 mg/mL neomycin (Sigma-Aldrich, St. Louis, MO) for 2 weeks. The silencing efficiency was verified by real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR) and western blot analysis as we described previously [15].
2. Implantation of MRMT-1 cells into the medullary cavity of the rat tibia
Female Sprague-Dawley rats weighing 180–200 g were provided by the Animal Center of School of Medicine, Xi’an Jiaotong University, and randomly divided into two groups, which were inoculated with control vector MRMT-1 cells or OX47 knockdown MRMT-1 cells.
Prior to implantation, these resuspended cells were counted and diluted to achieve a final concentration of 2×107 cells/mL. The surgery was performed as previously described and modified [2,16]. Rats were anesthetized with intraperitoneal injection of pentobarbital sodium (50 mg/kg). The left leg of the rat was scraped clean and the skin was sterilized with 75% v/v ethanol. A 12-gauge needle was inserted at the site of intercondylar eminence and pierced below the knee joint into the medullar cavity of tibia. Then a 10 μL micro-injector was attached to the needle and 5 μL cells (1×105 cells) were injected into the medullar cavity of tibia. After injection, the wound was closed with bone wax (Ethicon, Johnson and Johnson Medical NZ, Auckland, New Zealand) and sprinkled with penicillin antibiotic powder. The animals were put back into to their cages when they recovered consciousness.
3. The test of pain-related behaviors
All behavioral tests were performed using the double-blind method. Rats were placed in cages individually, the bilateral paw withdrawal mechanical threshold was assessed by the application of an electronic von Freydevice (2290 Electrovonfrey, IITC, Woodland Hills, CA) as previously described [16,17]. The specific method of operation is as follows: rats were tested regarding the withdrawal threshold of the plantar surface of the hind paw using von Frey filaments. The left and right paws were stimulated alternately, and each hind paw was stimulated 6 times in 5 minutes. The changes of behavioral signs were tested from day 4 postoperatively and were measured every other day until 30 days.
4. The detection of bone radiological
On the 7th, 14th, 21st, and 28th day after tumor cell inoculation, the tumor-bearing rats were anesthetized. Then, bone damage was detected by X-ray using the E-COM digital radiographer system (Guangdong E-COM Technology Co., Ltd., Guangdong, China).
5. Tissues collection and hematoxylin and eosin staining
The tumor-bearing rats were deeply anesthetized by intra-peritoneal injection of overdose sodium pentobarbital (60 mg/kg). 0.01 M cold phosphate buffer saline (PBS; pH 7.4) followed by a fix solution which contained 4% paraformaldehyde in 0.01 M PBS was perfused into the rat heart. Then, the bilateral tibial bones and L4 spinal cord were taken and fixed with paraformaldehyde overnight at 4°C. Decalcification was performed with 4% EDTA for 1 week at room temperature. Frozen sections of the tibia were prepared by conventional methods and stained with hematoxylin and eosin (H&E).
6. Tartrate-resistant acid phosphatase staining
Tartrate-resistant acid phosphatase (TRAP), a marker of activate osteoclasts, is currently the most widely used index to evaluate ability of bone resorption. Frozen sections of the tibia were stained using the TRAP kit (Genmed Scientific, Arlington, MA) according to the manufacturer’s protocol. The numbers of TRAP-positive multinucleated osteoclasts were counted in four random regions of each section.
7. Immunohistochemistry of c-Fos and glial-fibrillary acidic protein
In the study, immunohistochemical analyses of c-Fos and glial-fibrillary acidic protein (GFAP) on spinal cord were performed to examine neurochemical alterations in tumor-bearing rats. Frozen spinal cord tissues were cut into 10 μm on a freezing microtome and the gelatin-coated slides were collected and processed. For c-Fos and GFAP immunohistochemistry, the detailed methods of immunostaining have been characterized in our previous studies [15,18]. The stained sections were observed with a fluorescence microscope (Olympus BX51, Tokyo, Japan). Four sections were randomly selected for each marker of each animal for cell counting or immunofluorescence level measurement, and an average value was calculated to determine the value of each animal.
8. Enzyme-linked immunosorbent assay
The levels of MMP2 (Catalog #F16170, Westang, Shanghai, China), MMP9 (Catalog#orb381134, Biorbyt, Wuhan, China), osteocrin (Catalog#E00357, Wksubio, Shanghai, China), osteoactivin (Catalog#E00355, Wksubio), BMPs (Catalog#FK-L1802, Fanke, Shanghai, China) in tumor conditioned medium (TCM) were determined by using enzyme-linked immunosorbent assay (ELISA) assay kits. Experiments were carried out according to the manufacturer’s instructions. The absorbance value was acquired using a VersaMax Tunable MicroPlate Reader (Molecular Devices, Sunnyvale, CA) at 490 nm.
9. Statistical analyses
All data were expressed as means±standard error of mean. Student’s t test for random measures was performed followed by post hoc Fisher test to determine statistically significant differences. p < 0.05 was considered significant.
Results
1. Down-regulation of EMMPRIN (OX47) inhibited growth of MRMT-1 rat breast carcinoma cells in vitro and in vivo
To construct the OX47 knocked down MRMT-1 cell line and its control cell line, a lentivirus containing the target sequence of OX47 or scrambled sequence was transduced into MRMT-1 cells. The down-regulation of OX47 was confirmed by qRT-PCR (Fig. 1A) and Western blot analysis (Fig. 1B). The vitro cell viability assessment test showed that the proliferation of MRMT-1 cells transfected with OX47 shRNA virus was significantly inhibited compared to MRMT-1 cells transfected with control-shRNA virus (Fig. 1C).

Down-regulation of extracellular matrix metalloproteinase inducer (OX47) inhibited growth of MRMT-1 rat breast carcinoma cells in vitro and in vivo. Real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR) (A) and western blot (B) analyses of OX47 expression in MRMT-1 cells transfected with control-shRNA virus and OX47-shRNA virus. For qRT-PCR analyses, glyceraldehyde 3-phosphate dehydrogenase was used as an internal control; For western blot analyses, tubulin was used as an internal control. For qRT-PCR study, each experiment was repeated three times and the standard deviation is denoted using error bars. shRNA control vs. shRNA OX47; **p < 0.01. (C) Growth curve of MRMT-1 cells transduced with control-shRNA (solid circle) and OX47-shRNA (open circle) by MTT assay. Standard deviation is denoted using error bars (n=6). *p < 0.05. (D) Representative H&E staining results of the tumor-bearing tibia in sham operation group (left) (receiving implantation of heat-killed tumor cells), control-shRNA group (middle) and OX47-shRNA group (right) on the 28th day after MRMT-1 cells injections. (E) Statistical analysis of the percentage tumor area in tibia (n=6) were measured by examining six different visual fields under a microscope at 200× magnification in a blinded manner. Multiple comparisons were performed with one-way ANOVA, ****p < 0.0001.
MRMT-1 cells were implanted into the medullary cavity of rat tibia. The tumor burden level in the femur was quantified by H&E staining. As the tumor cells proliferated rapidly, after 28 days of injection, the bone marrow cavity of the control group was filled with a large number of tumor masses. Compared with the shRNA-control group, OX47 low-expression MRMT-1 cells grew slowly and the tumor burden area of rat was significantly reduced (Fig. 1D and E).
2. Down-regulation of EMMPRIN (OX47) alleviated cancer-induced bone destruction
In order to observe the changes in bone structure after tumor cell inoculation, the bone structure examination was performed on the rats by radiological examination system. On the 7th day after the injection of tumor cells, we did not observe any osteopenia and bone destruction. However, on the 14th day after the injection, some loss of medullary bone and obvious cortical bone erosion were observed. As the deterioration progressed, on the 21st day after the injection, a full-thickness cortical bone loss and displaced fracture were observed in the control group. The representative examples are shown in Fig. 2A. In contrast, we found that the progression of established osteolytic bone destruction as assessed by radiographic analysis in rats inoculated with OX47 low-expression MRMT-1 cells was markedly inhibited (Fig. 2A and B). Furthermore, histological examination demonstrated that inoculated control MRMT-1 cancer cells progressively destroyed rat bone with increased numbers of TRAP-positive osteoclasts. However, the number of TRAP-positive multinucleated osteoclasts was significantly reduced in the group of rats inoculated with OX47 low-expression MRMT-1 cells (Fig. 2C and D).
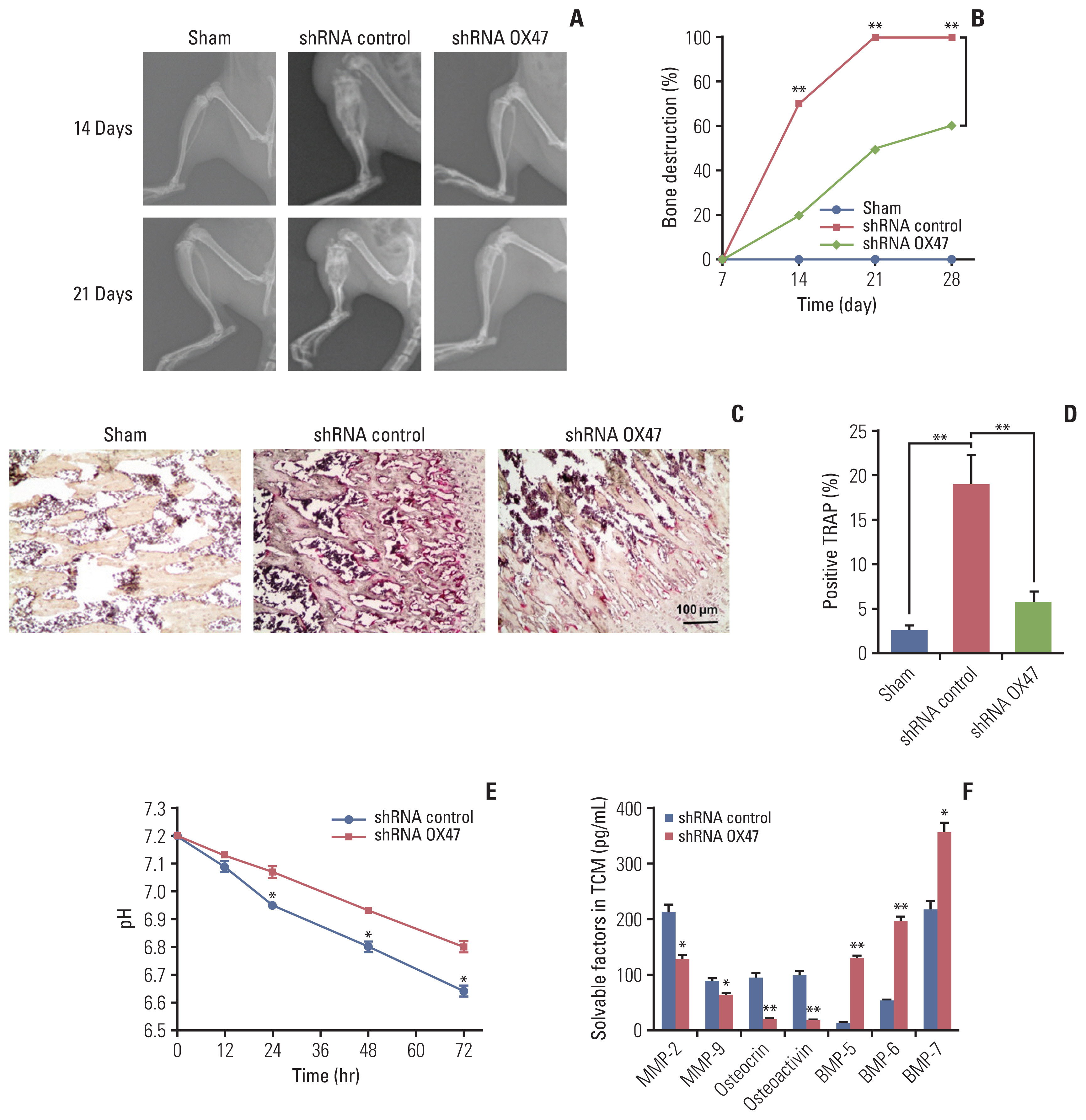
Down-regulation of extracellular matrix metalloproteinase inducer (OX47) alleviated cancer-induced bone destruction. Representative radiographs images (A) and quantification (B) of bone destruction in control-shRNA group and OX47-shRNA group on the 14th and 21st day after MRMT-1 cells injection, shRNA control vs. shRNA OX47; **p < 0.01. (C, D) Representative tartrate-resistant acid phosphatase staining photomicrographs (C) and quantification (D) of osteoclasts in control-shRNA group (n=6) and OX47-shRNA group (n=6) on the 21st day after MRMT-1 cells injection, shRNA control vs. shRNA OX47; **p < 0.01. (E) Knockdown of OX47 increased the pH value of tumor conditioned medium (TCM) which collected from MRMT-1 cells incubating for 12–72 hours, shRNA control vs. shRNA OX47; *p < 0.05. (F) Enzyme-linked immunosorbent assay quantitative results of solvable factors in TCM, shRNA control vs. shRNA OX47; *p < 0.05, **p < 0.01.
Our previous study reported that EMMPRIN was highly expressed in cancer cells and promoted tumor growth and metastasis by regulating the secretion of soluble factors, including MMPs, osteocrin, osteactivin, and BMPs [10]. To identify whether these factors are responsible for stimulating osteoclasts activation, we next investigated the effect of down-regulation of OX47 on the tumor microenvironment. We first studied the changes in H+ levels and found that knockdown of OX47 significantly increased the pH of TCM (Fig. 2E). Next, we measured the concentrations of MMP-2, MMP-9, osteocrin, osteoactivin, and BMPs using various ELISA kits. As can be seen from Fig. 2F, MMP-2, MMP-9, osteocrin, and osteocalcin were significantly decreased. What’s more, the levels of BMPs were significantly increased in the TCM of OX47 low-expression MRMT-1 cells.
3. Down-regulation of EMMPRIN (OX47) relieved cancer-induced pain
We used von Frey hair esthesiometer to assess the dynamic change of mechanical allodynia of rats a few days after MRMT-1 cell implantation. As can be seen from Fig. 3, the sham-operated rats showed no signs of mechanical allodynia. In contrast, the withdrawal threshold of all tumor-bearing rats received MRMT-1 cell injection decreased significantly from day 12. Then the allodynia progressed rapidly.
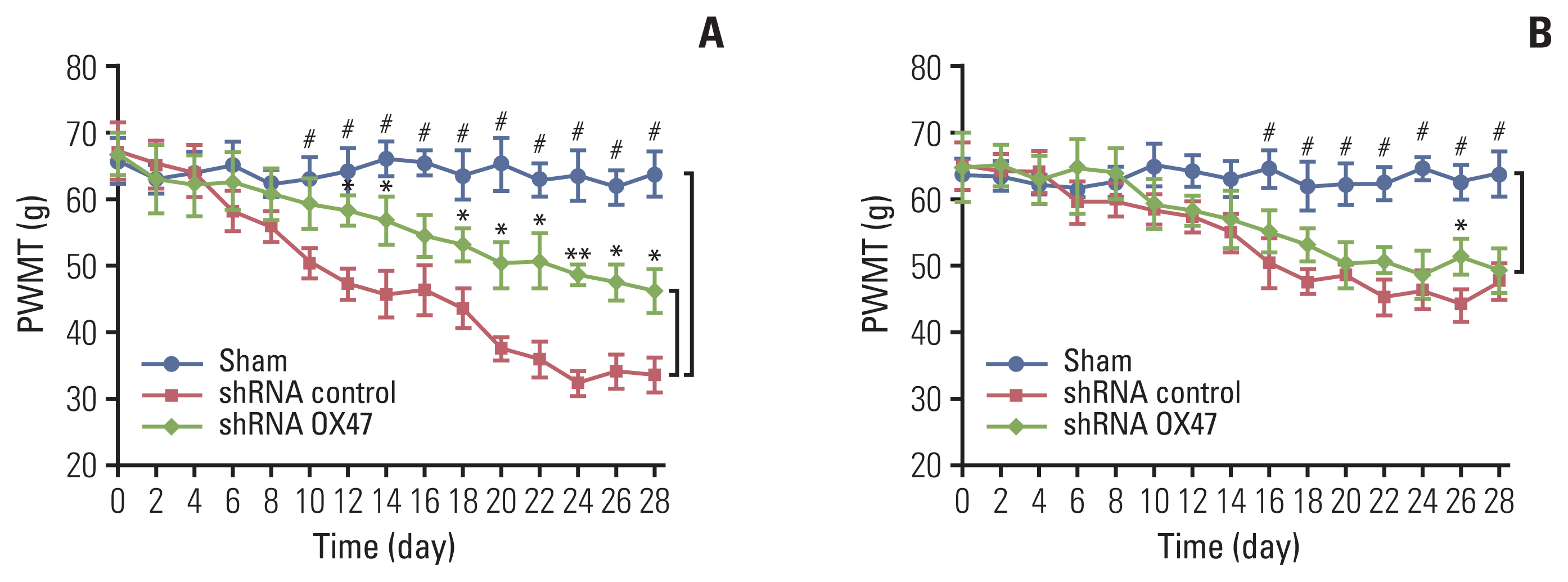
Down-regulation of extracellular matrix metalloproteinase inducer (OX47) relieved cancer-induced pain. Ipsilateral (A) and contralateral (B) hind paw withdrawal responses to von Frey hair stimulation in MRMT-1 cells (OX47 is knocked down or not) injected rats, shRNA control vs. shRNA OX47; *p < 0.05, **p < 0.01; shRNA control vs. Sham; #p < 0.01. PWMT, paw withdrawal mechanical threshold.
The reduction in the paw withdrawal threshold to von Frey hair stimulation occurred not only on the surgical side hind paw (Fig. 3A), but also on the contralateral side hind paw (Fig. 3B). However, the magnitude of the threshold reduction on both sides was different; the threshold of withdrawal of ipsilateral paws in rats inoculated with OX47 low-expression MRMT-1 cells was significantly higher than that of the control group.
In addition, we performed immunohistochemical analysis of c-Fos (an immediate-early gene for assessing sensory neuron activity) and GFAP (a glial marker). We found that compared with sham-operated rats, the number of c-Fos–positive neurons and GFAP-labeled glial cells in the ipsilateral dorsal horn of the rats increased significantly after inoculation of cancer cells. What’s more, knockdown of OX47 in the cancer cells significantly reduced staining of c-Fos and GFAP in the corresponding segments of the ipsilateral spinal cord (Fig. 4A and B).
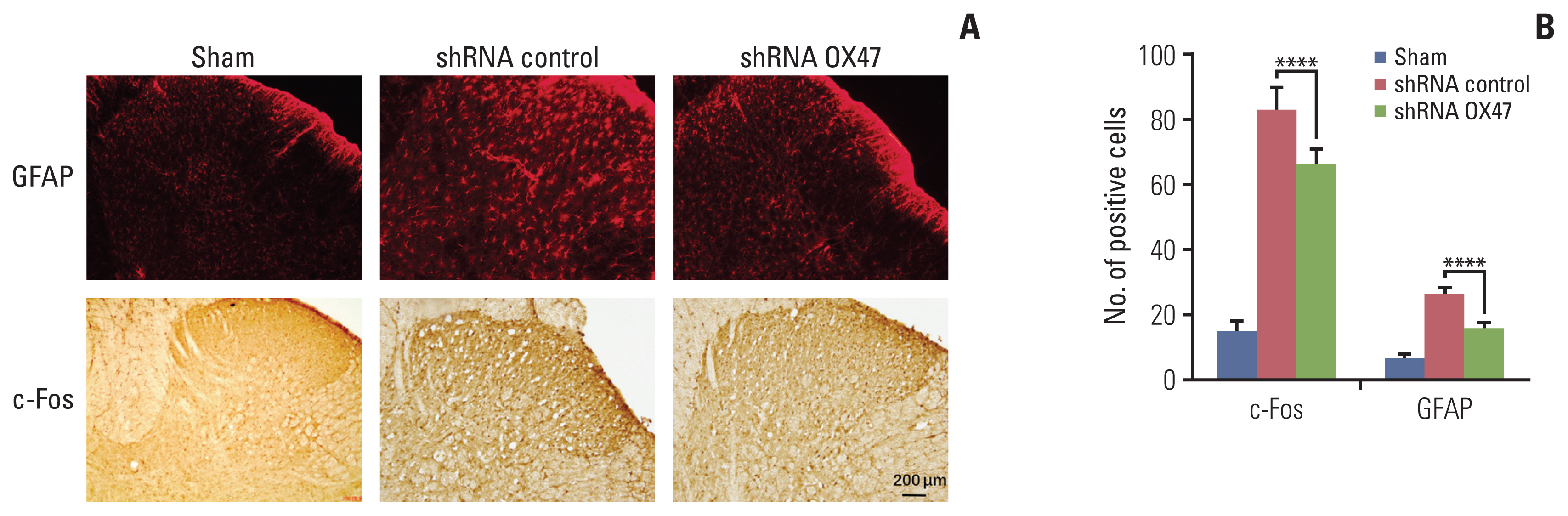
Immunohistochemical analysis of c-Fos (an immediate-early gene for assessing sensory neuron activity) and glial fibrillary acidic protein (GFAP; a glial marker). (A, B) Representative photomicrographs of c-Fos and GFAP immunostaining in the dorsal horn within spinal L4 segments of sham rats and tumor-bearing rats (A). GFAP and c-Fos positive cells were counted by examining six different visual fields under a microscope at 200× magnification in a blinded manner. Multiple comparisons were performed with one-way ANOVA, ****p < 0.0001 (B).
Discussion
Studies have shown that EMMPRIN is highly expressed in a variety of tumors. It plays important roles in promoting tumor growth, angiogenesis, invasion, and metastasis [19]. In this study, we used an in vivo bone metastasis model of breast cancer to study the role of EMMPRIN in driving bone destruction and cancer-induced pain. We found that after implantation, tumor cells infiltrated in the marrow space and led to extensive tumor-induced bone destruction. There existed persistent or motor-induced pain in tumor-bearing rats, as well as typical neurochemical changes in the spinal cord that reflected persistent pain states. Knockdown of EMMPRIN expression in the tumor cells significantly reduced tumor burden, bone destruction, and attenuated the development of cancer-induced pain. Further histological examinations and ELISA assays showed that EMMPRIN might be associated with changes of some factors in the tumor microenvironment, which regulated the activation of osteoblasts and neurons.
EMMPRIN affects adjacent local microenvironment by promoting the secretion of matrix metalloproteins (MMPs) [20,21], vascular endothelial growth factor [22], and insulin-like growth factor-I [23]. A lot of evidence has shown that both of MMPs and acidic substances could cause bone matrix degradation and pain [24]. Our results also indicated that knocking down of EMMPRIN in tumor cells reduced expression levels of osteocrin as well as osteoactivin, increased expression of BMP-5, -6, and -7. Although osteocrin has been shown to be a bone-specific secreted protein which regulates the osteoblast phenotype [13,25], osteoactivin had previously been reported to be a novel osteoclastic protein and played a key role in osteoclast differentiation [26]. Osteoactivin is expressed in aggressive human breast cancers and promotes breast cancer metastasis to bone [27,28]. BMP-5, -6, and -7 belong to the transforming growth factor β superfamily and perform functions in osteoblast differentiation and in cartilage/bone development. BMPs are secretory signaling molecules known for their ability to induce the formation of bone and cartilage [29]. These findings suggested that down-regulation of OX47 expression in tumor cells attenuated destruction of bone. It may be associated with secretory factors that induce phenotypic differentiation of osteoblasts and osteoclasts.
In our study, inhibition of EMMPRIN alleviated cancer pain. The relevant mechanism maybe it simultaneously inhibited cancer growth and bone destruction. In addition, we observed that inhibition of EMMPRIN also significantly reduced the number of c-Fos–positive neurons and GFAP-positive glial cells in the corresponding segments of the ipsilateral spinal cord. Previous studies have revealed a stereotypic set of neurochemical changes occured in the spinal cord in animals with cancer-induced pain, these areas receive sensory input from the tumor-bearing bone, and lead neurons activation and glial cells proliferation [30]. Inhibition of EMMPRIN expression attenuated both neurochemical changes suggesting EMMPRIN reduced central sensitization of spinal cord.
In summary, EMMPRIN plays significant multi-effect roles in the process of breast cancer cells metastasis to bone. Inhibition of EMMPRIN showed multifaceted inhibitory effects on cancer progression, including reducing tumor burden and bone destruction, as well as reducing pain. This information will help to develop new therapeutic strategies to synergistically improve the survival and quality of life for patients with cancer metastasized to bone.
Notes
Ethical Statement
Animal experiments were approved by the Animal Use Subcommittee of the Animal Protection Committee of Xi’an Jiao tong University, and all procedures were conducted under the guidance of the Animal Protection and Use Institutions Committee.
Author Contributions
Conceived and designed the analysis: Chen Y, Jiang T, Gou X.
Collected the data: Chen Y, Jiang T, Cai D, Sun C, Wang X, Zhao X, Gou X.
Contributed data or analysis tools: Chen Y, Jiang T, Cai D, Sun C, Wang X, Zhao X, Gou X.
Performed the analysis: Chen Y, Luan J, Jiang T, Cai D, Sun C, Wang X, Zhao X, Gou X.
Wrote the paper: Luan J.
Conflicts of Interest
Conflicts of interest relevant to this article was not reported.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81101744 to Yanke Chen), Projects of International Cooperation and Exchanges Natural Science Foundation of ShaanXi Province of China (2017KW-059 to Yanke Chen) and the Scientific Research and Sharing Platform Construction Project of Shaanxi Province (2018PT-09 to Yanke Chen). The data that support the findings of this study are available from the corresponding author upon reasonable request.